Abstract
Purpose
Tumor-infiltrating FoxP3+ T cells have been reported in various human tumors, which impaired cell-mediated immunity and promoted disease progression. However, its prognostic value for survival in patients with different gastrointestinal cancers [hepatocellular carcinoma (HCC), colorectal cancer (CRC), gastric cancer (GC)] remains controversial.
Methods
Relevant literature was searched using PubMed, Embase, Cochrane, Ovid Medline and Chinese wanfang databases. A meta-analysis was conducted to estimate pooled survival and recurrence ratios. The odds ratio (OR) and 95% confidence intervals (CI) were calculated employing fixed- or random-effects models depending on the heterogeneity of the included trials.
Results
For HCC and GC, the overall survival at 1, 3 and 5-year of high FoxP3+ T cells infiltration patients were lower than low FoxP3+ T cells infiltration patients (P<0.05). The recurrences at 1, 3 and 5-year of high FoxP3+ T cells infiltration patients were higher than low FoxP3+ T cells infiltration patients (P<0.001). But for CRC, the overall survival at 1, 3 and 5-year of high FoxP3+ T cells infiltration patients were higher than low FoxP3+ T cells infiltration patients (P<0.001). There were no differences in 1, 3 and 5-year recurrences between high and low FoxP3+ T cells infiltration patients (P>0.05).
Conclusions
Our findings suggested that tumor-infiltrating FoxP3+ T cells were a factor for a poor prognosis for HCC and GC, but a good prognosis for CRC.
Introduction
Immune cells that infiltrate tumors engage in an extensive and dynamic crosstalk with cancer cells and some of the molecular events that mediate this dialog have been revealed [1]. In the past decade, much effort has been devoted to finding the function of regulatory T cells (Tregs) in tumor. Tregs are a subgroup of CD4+ T helper cells with the function of suppressing T-cell immunity in both physiologic and disease statuses. Forkhead box protein P3 (FoxP3) is a transcription factor necessary and sufficient for induction of the immunosuppressive functions of Tregs, and it is now considered as the most specific marker for Tregs in tumors [2].
Abundance tumor-infiltrating FoxP3+ T cells are expected to be associated with an unfavorable prognosis, as expected from their capacity to inhibit antitumor immunity. However, this idea has been challenged by recent studies showing that, high tumor infiltration by FoxP3+ T cells is not always associated with a poor prognosis. On the contrary, it can improve survival in some tumors [3]–[5]. It was inconsistent with the initial hypothesis that FoxP3+ T cells inhibit antitumor immunity. Even in the same kind of tumor, this conclusion was not entirely consistent [4], [5]. The discrepancy was very obvious, especially in the gastrointestinal cancers such as hepatocellular carcinoma (HCC), colorectal cancer (CRC) and gastric cancer (GC) which all were considered as inflammation-associated cancers since with rich exogenous antigens.
To investigate this apparent discrepancy, we sought to conduct a meta-analysis to estimate the prognostic importance of tumor-infiltrating FoxP3+ T cells level for overall survival (OS) and disease-free survival (DFS) among patients with HCC, CRC and GC, aiming to gain insights into whether FoxP3+ T cells could provide useful guidance in the biological understanding and treatment of solid tumors.
Materials and Methods
Literature search
Relevant articles were identified by two reviewers via an electronic search of PubMed, EMBASE, Cochrane, Ovid Medline and Chinese wanfang databases using the following keywords: (FoxP3 or regulatory T cells), (hepatocellular carcinoma, colorectal cancer or gastric cancer) and “prognosis”. And the search time period of the electronic database was from inception to Feb 8th, 2014. Additionally, possible missing papers were searched in reference lists of selected papers and systematic review. A search for unpublished literature was not performed. Disagreement on article inclusion between the two reviewers was resolved via a third reviewer.
Inclusion and exclusion criteria
Inclusion criteria for this study were as follows: (1) patients were diagnosed clearly; (2) report of FoxP3+ T cells in tumor surgical specimens; (3) FoxP3+ T cells evaluation using immunohistochemical method; (4) association of high and low FoxP3+ T cells infiltration patients with overall survival (OS), and/or disease-free survival (DFS) and contained survival curves. (5) when the same author or group reported results obtained from the same patient population in more than one article, the most recent report or the most informative report was included.
Exclusion criteria for this study were as follows: (1) letters, reviews, case reports, conference abstracts, editorials, and expert opinion were excluded; (2) articles in which have no information on survival rates or survival curve; (3) Non-surgical treatment study; (4) non-primary cancer, such as metastatic cancer or recurrent cancer; (5) peripheral blood or peritumoral specimens.
Name of authors or journals of the articles did not influence our decision in excluding or including the articles.
Statistical analysis
Hazard ratio (HR) and its 95% confidence interval (CI) were used to estimate the association between FoxP3+ T cells and patients' prognosis. If a direct report of survival and recurrence ratios were not available, then the survival data read from Kaplan-Meier curves were read by Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/) as described previously [6]–[8]. This work was performed by two independent persons to reduce inaccuracy in the extracted survival rates.
All analyses were performed with Review Manager version 5 (RevMan, Cochrane Collaboration, Oxford, England). Statistical heterogeneity between trials was evaluated by χ2 test and was considered significant when P<0.05. In the absence of statistically significant heterogeneity, the Mantel-Haenszel method in the fixed-effect model was used for the Meta analysis. Otherwise, the DerSimonian and Laird method in the random-effect model was selected. The odds ratio (OR) with 95%CI was used to assess treatment efficacy. The combined result was an average OR and 95%CI weighted according to the standard error of the OR of the trial. P<0.05 was considered statistically significant. We used funnel plots to assess the publication bias, and tested for funnel plot asymmetry using Egger's test and Begg's test.
Results
Study selection and characteristics
For HCC, 13 eligible trials involving 1964 patients were ultimately identified in Table 1 [9]–[21]. For CRC, 10 eligible trials involving 2756 patients were ultimately identified in Table 2 [22]–[31]. For GC, 16 eligible trials involving 1873 patients were ultimately identified Table 3 [32]–[47]. FigureS1A (for HCC), FigureS1B (for CRC) and FigureS1C (for GC) illustrate the search process and the final selection of relevant studies.
Table 1. Main characteristics of studies about HCC included in the meta-analysis.
| Author | Year | Journal | Quality score | Number of cases | Marker | Antibody | Cutoff | Survival | |
| M/F | High/low | ||||||||
| Gao[9] | 2007 | J Clin Oncol | 6 | 260/42 | 147/155 | FoxP3 | Biolegend | Median | OS,DFS |
| Kobayashi[10] | 2007 | Clin Cancer Res | 6 | 113/34 | 73/74 | FoxP3/CD4 | Novocastra | Median | OS,DFS |
| Sasaki[11] | 2007 | Eur J Surg Oncol | 6 | 126/38 | 84/80 | FoxP3 | Abcan | Median | OS,DFS |
| Li[12] | 2008 | Zhonghua Zhong Liu | 7 | 54/9 | 20/43 | FoxP3 | Abcan | Other | OS |
| Shen[13] | 2009 | Can J Surg | 6 | 70/6 | 35/41 | FoxP3 | Abcan | Median | OS,DFS |
| Zhou[14] | 2009 | Int J Cancer | 7 | - | 36/49 | FoxP3 | Abcan | Median | OS,DFS |
| Lin[15] | 2010 | Chin J Cancer | 6 | 85/17 | 49/53 | FoxP3 | Abcan | Median | OS |
| Chen[16] | 2011 | PLoS One | 7 | - | 57/86 | FoxP3 | Abcan | Median | OS,DFS |
| Chen[17] | 2011 | Med Oncol | 7 | - | 70/71 | FoxP3 | Abcan | Median | OS,DFS |
| Huang[18] | 2012 | Digestion | 7 | 45/9 | 27/27 | FoxP3 | Abcan | Median | OS,DFS |
| Wu[19] | 2012 | J Gastroenterol Hepatol | 7 | 341/45 | 207/179 | FoxP3 | Abcan | Other | OS,DFS |
| Huang[20] | 2013 | J Gastroenterol Hepatol | 7 | 50/6 | 28/28 | FoxP3 | Abcan | Median | OS,DFS |
| Lin[21] | 2013 | Cancer Prev Res | 7 | - | 162/83 | FoxP3/CD4 | Abcan | Other | OS,DFS |
F, female; M, male; Quality score was assessed using the validated Jadad scale; High, high FoxP3+ T cells infiltration; Low, low FoxP3+ T cells infiltration.
Table 2. Main characteristics of studies about CRC included in the meta-analysis.
| Author | Year | Journal | Quality score | Number of cases | Marker | Antibody | Cutoff | Survival | |
| M/F | High/low | ||||||||
| Sinicrope[22] | 2009 | Gastroenterology | 7 | 84/76 | 101/59 | FoxP3 | Abcan | Other | DFS |
| Lee[23] | 2010 | Cancer | 7 | 29/34 | 39/24 | FoxP3 | eBioscience | Other | OS |
| Suzuki[24] | 2010 | Cancer Immunol Immunother | 6 | 53/41 | 30/64 | FoxP3 | Abcan | Mean | OS,DFS |
| Frey[25] | 2010 | Int J Cancer | 6 | - | 614/616 | FoxP3 | Abcan | Other | OS,DFS |
| Nosho[26] | 2010 | J Pathol | 6 | - | 384/384 | FoxP3 | BioLegend | Other | OS |
| Tosolini[27] | 2011 | Cancer Research | 5 | - | 18/38 | FoxP3 | Abcan | Other | DFS |
| Yoon[28] | 2012 | PLoS One | 7 | - | 78/78 | FoxP3 | Abcan | Median | OS |
| Suzuki[29] | 2013 | Clinical Immunology | 6 | 49/39 | 34/54 | FoxP3 | Abcan | Mean | OS.DFS |
| Zeestraten[30] | 2013 | Cancer Microenvironment | 6 | 44/36 | 38/38 | FoxP3 | Abcan | Median | OS,DFS |
| Kim[31] | 2013 | PLoS One | 7 | 37/28 | 27/38 | FoxP3 | Abcan | Mean | OS |
F, female; M, male; Quality score was assessed using the validated Jadad scale; high FoxP3+ T cells infiltration; Low, low FoxP3+ T cells infiltration.
Table 3. Main characteristics of studies about GC included in the meta-analysis.
| Author | Year | Journal | Quality score | Number of cases | Marker | Antibody | Cutoff | Survival | |
| M/F | High/low | ||||||||
| Mizukami[32] | 2008 | Br J Cancer | 7 | 56/24 | 40/40 | FoxP3 | eBioscience | Median | OS |
| Perrone[33] | 2008 | Eur J Cancer | 7 | 53/57 | 58/52 | FoxP3 | eBioscience | Median | OS,DFS |
| Haas[34] | 2009 | BMC Gastroenterol | 6 | 40/12 | 26/26 | FoxP3 | Abcan | Median | OS |
| Shen[35] | 2010 | J Cancer Res Clin Oncol | 7 | 89/44 | 66/67 | FoxP3 | Biolegend | Median | OS |
| Du[36] | 2011 | Cancer Sci | 6 | 131/48 | 87/92 | FoxP3 | Abcam | Median | OS,DFS |
| Kim[37] | 2011 | J Surg Oncol | 7 | 126/54 | 90/90 | FoxP3/CD4 | Abcan | Median | OS,DFS |
| Lu[38] | 2011 | J Surg Oncol | 7 | - | 30/30 | FoxP3 | Abcan | Median | OS |
| Shu[39] | 2011 | Zhonghua weichangwaike | 7 | - | 45/43 | FoxP3 | eBioscience | Median | OS |
| Wang[40] | 2011 | Ann Surg Oncol | 7 | 69/38 | 53/54 | FoxP3 | Abcan | Median | OS |
| Ishigami[41] | 2012 | Cancer Immunol Immunother | 7 | 99/42 | 76/65 | FoxP3 | Dako | Mean | OS |
| Kashimura[42] | 2012 | Gastric Cancer | 6 | 89/34 | 62/61 | FoxP3 | Abcan | Median | OS,DFS |
| Yoshii[43] | 2012 | Br J Cancer | 7 | 44/48 | 49/43 | FoxP3 | Abcan | Median | OS |
| Deng[44] | 2013 | PLoS One | 6 | 70/29 | 48/51 | FoxP3 | Abcan | Median | OS |
| Kim[45] | 2013 | Hum Pathol | 6 | 55/44 | 49/50 | FoxP3 | Abcan | Median | OS |
| Zhou[46] | 2013 | PLoS One | 7 | 89/44 | 87/46 | FoxP3 | Biolegend | Mean | OS |
| Ma[47] | 2014 | Br J Cancer | 7 | 132/65 | 24/173 | FoxP3 | Abcam | Other | OS |
F, female; M, male; Quality score was assessed using the validated Jadad scale; high FoxP3+ T cells infiltration; Low, low FoxP3+ T cells infiltration.
Meta-analysis for HCC
Survival during follow-up 1, 3, 5-year after surgical resection: The overall survival rate during follow-up 1-year was significantly lower in high FoxP3+ T cells infiltration patients (82.8%) than low FoxP3+ T cells infiltration patients (92.1%) with a combined OR of 0.38 (95%CI = 0.28–0.52, P<0.001. Figure 1A). The overall survival rate during follow-up 3-year was significantly lower in high FoxP3+ T cells infiltration patients (51.8%) than low FoxP3+ T cells infiltration patients (76.8%) with a combined OR of 0.30 (95%CI = 0.24–0.37, P<0.001. Figure 1B). The overall survival rate during follow-up 5-year was significantly lower in high FoxP3+ T cells infiltration patients (38.4%) than low FoxP3+ T cells infiltration patients (64.1%) with a combined OR of 0.31 (95%CI = 0.21–0.44, P<0.001. Figure 1C).
Figure 1. Forest plot of Hazard ratio (HR) for survival of HCC patients.
Fixed effect model of odds ratio for survival of follow-up 1(A), 3-year (B) and random effect model of odds ratio for survival of follow-up 5-year (C) of HCC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
Recurrence during follow-up 1, 3, 5-year after surgical resection: The recurrence rate during follow-up 1-year was significantly higher in high FoxP3+ T cells infiltration patients (32.9%) than low FoxP3+ T cells infiltration patients (19.0%) with a combined OR of 2.25 (95%CI = 1.79–2.83, P<0.001. Figure 2A). The recurrence rate during follow-up 3-year was significantly higher in high FoxP3+ T cells infiltration patients (60.2%) than low FoxP3+ T cells infiltration patients (33.8%) with a combined OR of 3.39 (95%CI = 2.22–5.17, P<0.001. Figure 2B). The recurrence rate during follow-up 5-year was significantly higher in high FoxP3+ T cells infiltration patients (69.6%) than low FoxP3+ T cells infiltration patients (49.6%) with a combined OR of 2.56 (95%CI = 2.09–3.13, P<0.001. Figure 2C).
Figure 2. Forest plot of HR for recurrence of HCC patients.
Fixed effect model of odds ratio for recurrence of follow-up 1(A), 5-year (C) and random effect model of odds ratio for recurrence of follow-up 3-year (B) of HCC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
Meta-analysis for CRC
Survival during follow-up 1, 3, 5-year after surgical resection: The overall survival rate during follow-up 1-year was significantly higher in high FoxP3+ T cells infiltration patients (91.2%) than low FoxP3+ T cells infiltration patients (84.5%) with a combined OR of 1.93 (95%CI = 1.51–2.48, P<0.001. Figure 3A). The overall survival rate during follow-up 3-year was significantly higher in high FoxP3+ T cells infiltration patients (76.4%) than low FoxP3+ T cells infiltration patients (67.9%) with a combined OR of 1.56 (95%CI = 1.31–1.87, P<0.001. Figure 3B). The overall survival rate during follow-up 5-year was significantly higher in high FoxP3+ T cells infiltration patients (69.9%) than low FoxP3+ T cells infiltration patients (58.9%) with a combined OR of 1.65 (95%CI = 1.40–1.95, P<0.001. Figure 3C).
Figure 3. Forest plot of HR for survival of CRC patients.
Fixed effect model of odds ratio for survival of follow-up 1 (A), 3 (B), 5-year (C) of CRC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
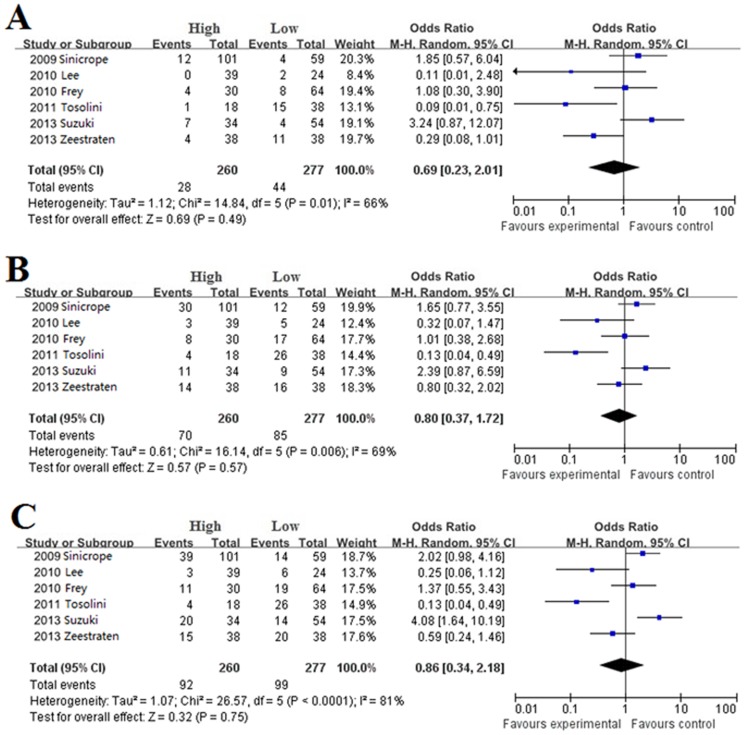
Recurrence during follow-up 1, 3, 5-year after surgical resection: There were no differences in 1(OR = 0.69, 95%CI = 0.23–2.01, P = 0.49. Figure 4A), 3 (OR = 0.80, 95%CI = 0.37–1.72, P = 0.57. Figure 4B) and 5-year (OR = 0.86, 95%CI = 0.34–2.18, P = 0.75. Figure 4C) recurrences between high and low FoxP3+ T cells infiltration patients.
Figure 4. Forest plot of HR for recurrence of CRC patients.
Random effect model of odds ratio for recurrence of follow-up 1 (A), 3 (B), 5-year (C) of CRC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
Meta-analysis for GC
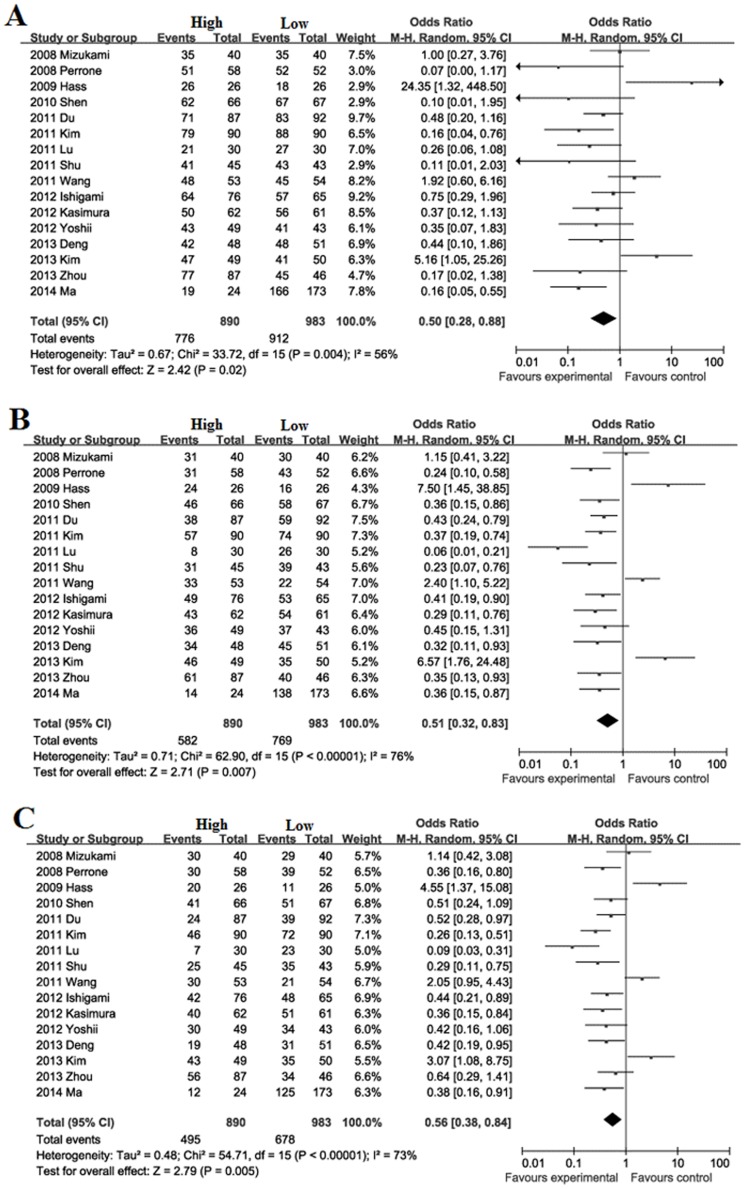
Survival during follow-up 1, 3, 5-year after surgical resection: The overall survival rate during follow-up 1-year was significantly lower in high FoxP3+ T cells infiltration patients (87.2%) than low FoxP3+ T cells infiltration patients (92.8%) with a combined OR of 0.50 (95%CI = 0.28–0.88, P = 0.02. Figure 5A). The overall survival rate during follow-up 3-year was significantly lower in high FoxP3+ T cells infiltration patients (65.4%) than low FoxP3+ T cells infiltration patients (78.2%) with a combined OR of 0.51 (95%CI = 0.32–0.83, P = 0.007. Figure 5B). The overall survival rate during follow-up 5-year was significantly lower in high FoxP3+ T cells infiltration patients (55.6%) than low FoxP3+ T cells infiltration patients (69.0%) with a combined OR of 0.56 (95%CI = 0.38–0.84, P = 0.005. Figure 5C).
Figure 5. Forest plot of HR for survival of GC patients.
Random effect model of odds ratio for survival of follow-up 1 (A), 3 (B), 5-year (C) of GC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
Recurrence during follow-up 1, 3, 5-year after surgical resection: The recurrence rate during follow-up 1-year was significantly higher in high FoxP3+ T cells infiltration patients (26.9%) than low FoxP3+ T cells infiltration patients (10.8%) with a combined OR of 3.06 (95%CI = 1.95–4.80, P<0.001. Figure 6A). The recurrence rate during follow-up 3-year was significantly higher in high FoxP3+ T cells infiltration patients (43.4%) than low FoxP3+ T cells infiltration patients (22.4%) with a combined OR of 2.77 (95%CI = 1.92–3.98, P<0.001. Figure 6B). The recurrence rate during follow-up 5-year was significantly higher in high FoxP3+ T cells infiltration patients (52.5%) than low FoxP3+ T cells infiltration patients (33.6%) with a combined OR of 2.52 (95%CI = 1.76–3.62, P<0.001. Figure 6C).
Figure 6. Forest plot of HR for recurrence of GC patients.
Fixed effect model of odds ratio for recurrence of follow-up 1 (A), 3 (B), 5-year (C) of GC patients after surgery: high FoxP3+ T cells infiltration patients vs low FoxP3+ T cells infiltration patients.
Publication bias
Publication bias may exist when no significant findings remain unpublished, thus artificially inflating the apparent magnitude of an effect. Survival and recurrences following high and low FoxP3+ T cells infiltration patients with HCC, CRC and GC were calculated by the fixed-effect model and random-effect model, respectively. The results were similar and the combined results were highly reliable.
The funnel plots on survival and recurrence following high and low FoxP3+ T cells infiltration patients with HCC (Figure S2), CRC (Figure S3) and GC (Figure S4) showed basic symmetry, which suggested no publication bias.
Discussion
Tregs are functionally immunosuppressive subsets of CD4+ T, which were found by Sakaguchi et al [2] in 1995. They control the balance between tolerance and rejection of self and altered self by secreting IL-4, IL-10 and TGF-β and other cytokines [48]. For the identification of Tregs, many markers such as CTLA-4, GITR, OX-4, CD127 and transcription factor FoxP3 can be used [49]. FoxP3 is now considered as the most specific marker for Tregs [50], because it is critical for the development and function of Tregs. And FoxP3 became a popular single marker for Tregs studies in tumor. However, the conclusions from published research regarding its prognostic value for different tumors were controversial in different gastrointestinal cancers. Even in the same kind of tumor, this conclusion was not entirely consistent such as CRC and GC [3]–[5].
Meta-analysis is useful to integrate results from independent studies for a specified outcome. Pooled results from the combining relevant studies are statistical powerful, and make it possible to detecting effects that may be missed by individual studies. To date, no meta-analysis has been undertaken for any studies that evaluate tumor-infiltrating FoxP3+ T cells as a prognostic marker in HCC, CRC or GC. In this meta-analysis, 13 studies involving 1964 patients were analyzed. All the studies consistently shown high density of tumor-infiltrating FoxP3+ T cells have been associated with poor survival and high recurrences in HCC, consistent with the initial hypothesis that FoxP3+ T cells inhibit antitumor immunity. These conclusions were confirmed by our previous reports [18], [20], [51]. In all our data clarified the results of individual studies and to identify patients at high risk for whom specific- or adjuvant-therapy might be necessary since high density of FoxP3+ T cells is a prognostic factor for HCC.
For CRC, 10 studies involving 2756 patients were analyzed. Be different from HCC, studies of the prognostic value of FoxP3+ T cells in CRC have lead to highly discrepant findings. Some studies investigating colorectal cancer concluded that FoxP3+ T cells correlated with a good prognosis, whereas other studies found no prognostic association or even a bad prognostic claim [3]–[5], [22]–[31]. The data were organized according to overall survival and recurrence; then combined results strongly demonstrated that high density of tumor-infiltrating FoxP3+ T cells was a good prognosis for CRC. The result has challenged the conventionally theory that FoxP3+ T cells can suppress tumor immunity. It is regrettable that very few studies in the literature have examined the exact functional properties of FoxP3+ T cells isolated from human CRC. In considering CRC grows in a septic microenvironment, researchers recently hypothesized that the favorable prognostic effect of FoxP3+ T cells may reflect their ability to preferentially suppress tumor-promoting inflammatory responses to gut microbes and Th17-cell-dependent proinflammatory [4].
For GC, the prognostic significance of tumor-infiltrating FoxP3+ T cells for the survival of patients with gastric cancer remains controversial. There are 16 studies involving 1873 patients that compared the survival of HCC according to FoxP3+ T cells expression level of the primary tumor met the enrollment criteria. In the 16 studies, studies looking at gastric cancers show a split among poor (n = 11), neutral (n = 2), and good (n = 3) prognostic claims. Base on those studies, the Meta-Analysis results consistent with HCC, high density of FoxP3+ T cells was associated with poor survival and high recurrences.
However, one should be cautious when interrupting these results due to the limitations of our studies. Further high-quality studies are still needed to confirm these results. There are several important limitations also need to be considered. First of all, patients had received different treatments and postoperative treatment; preoperative TNM category and histologic types were various. Whereas, we were unable to assess these potential confounders present in individual studies. Second, although we tried to identify all relevant data, potential publication bias was unavoidable and some data could still be missing. Third, the antibody, cell-scoring strategy and the cutoff value were defined differently in some studies. Finally, this study was constrained to studies published in English and Chinese language; it was difficult to completely rule out publication bias.
HCC, CRC and GC are gastrointestinal tumors, and come from immune tolerance organs which exposed to high levels exogenous antigens. However, the role and function of FoxP3+ T cells were different completely. Thus, the original view that FoxP3+ T cells invariably suppress tumor immunity is oversimplified for CRC. The discrepancy in different tumors could arise from differences in study methodologies or in the biologic properties of specific tumor types. We require better understanding of the functional subtypes of FoxP3+ T cells and their biologic properties in different tumor microenvironments if we wish to rationally modulate their behavior to enhance tumor immunity. We believe that the interaction between the different components of the tumor microenvironment and the diversity of signals provided by the tumor cells can explain these discrepancies in the prognostic studies relying on the presence of Tregs in tumor infiltrates. Recent findings have shown that a subset of FoxP3+ Tregs could acquire the capacity to produce IL-17 instead of IL-10 and TGF-β [52]. The double-positive T cells exhibit functions of both Th17 and Tregs, or act as a transient population that may eventually generate either Th17 or Tregs, presenting a potential mechanism for the Tregs/Th17 regulation in the progression of tumor.
In summary, some studies fit with the general notion that FoxP3+ T cells suppress adaptive immune responses and led many groups to pursue strategies to deplete FoxP3+ T cells from patients or mouse with cancer as a means to enhance tumor immunity [53]–[55]. However, our findings suggest that the treatment of depletion or attenuation of FoxP3+ T cells can be used for the treatment of HCC and GC but detrimental for CRC.
Supporting Information
Flow diagram of study selection. A: Flow diagram of study selection for HCC; B: Flow diagram of study selection for CRC; C: Flow diagram of study selection for GC.
(DOC)
Funnel plots for HCC. A: 12 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 11 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 12 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 11 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 10 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 11 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
Funnel plots for CRC. A: 8 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 8 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 8 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 6 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 6 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 6 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
Funnel plots for GC. A: 16 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 16 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 16 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 4 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 4 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 4 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
PRISMA Checklist.
(ZIP)
Funding Statement
This work was supported by Tianjin Science and Technology commission funded key project (No. 12ZCZDSY02700, No. 13JCYBJC22500). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various auto-immune diseases. J Immunol 155: 1151–1164. [PubMed] [Google Scholar]
- 3. deLeeuw RJ, Kost SE, Kakal JA, Nelson BH (2012) The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 18: 3022–3029. [DOI] [PubMed] [Google Scholar]
- 4. Ladoire S, Martin F, Ghiringhelli F (2011) Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother 60: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei Z, Liu Y, Liu C, Cui A, Liang Z, et al. (2014) Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed]
- 6. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG (2013) Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS One 8: e71930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han K, Qi W, Gan Z, Shen Z, Yao Y, Min D (2013) Prognostic value of Ezrin in solid tumors: a meta-analysis of the literature. PLoS One 8: e68527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, et al. (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, et al. (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13: 902–911. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, et al. (2008) Prognostic value of tumor-infiltrating FOXP3 regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol 34: 173–179. [DOI] [PubMed] [Google Scholar]
- 12. Li SP, Peng QQ, Ding T, Xu J, Zhang CQ, et al. (2008) Clinical significance of regulatory T cells proportion in the peripheral blood and tumor tissue in primary hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 30: 523–527. [PubMed] [Google Scholar]
- 13. Shen SL, Liang LJ, Peng BG, He Q, Kuang M, et al. (2011) Foxp3+ regulatory T cells and the formation of portal vein tumour thrombus in patients with hepatocellular carcinoma. Can J Surg 54: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou J, Ding T, Pan W, Zhu LY, Li L, et al. (2009) Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 125: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 15. Lin GH, Wang J, Li SH, Wang J, Xu L, et al. (2010) Relationship and clinical significance of TGF-beta1 expression with Treg cell infiltration in hepatocellular carcinoma. Chin J Cancer 29: 403–407. [DOI] [PubMed] [Google Scholar]
- 16. Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, et al. (2011) Selective Recruitment of Regulatory T Cell through CCR6-CCL20 in Hepatocellular Carcinoma Fosters Tumor Progression and Predicts Poor Prognosis. PLoS One 6: e24671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, et al. (2012) Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol 29: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 18. Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, et al. (2012) Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 86: 329–337. [DOI] [PubMed] [Google Scholar]
- 19. Wu H, Chen P, Liao R, Li YW, Yi Y, et al. (2012) Overexpression of Galectin-1 Associates with Poor Prognosis in Human Hepatocellular Carcinoma Following Resection. J Gastroenterol Hepatol 27: 1312–1319. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, et al.. (2013) Intrahepatic IL-17+ T cells and FoxP3+ Tregs cooperate to promote development and affect the prognosis of hepatocellular carcinoma.J Gastroenterol Hepatol. doi: 10.1111/jgh.12418. [DOI] [PubMed]
- 21. Lin SZ, Chen KJ, Xu ZY, Chen H, Zhou L, et al. (2013) Prediction of Recurrence and Survival in Hepatocellular Carcinoma Based on Two Cox Models Mainly Determined by FoxP3 Regulatory T Cells. Cancer Prev Res 6: 594–602. [DOI] [PubMed] [Google Scholar]
- 22. Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, et al. (2009) Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137: 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee WS, Park S, Lee WY, Yun SH, Chun HK (2010) Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 116: 5188–5199. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, et al. (2010) Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 59: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, et al. (2010) High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 126: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 26. Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, et al. (2010) Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 222: 350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, et al. (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 28. Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, et al. (2012) Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One 7: e42274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki H, Onishi H, Morisaki T, Tanaka M, Katano M (2013) Intratumoral FOXP3+VEGFR2+ regulatory T cells are predictive markers for recurrence and survival in patients with colorectal cancer. Clin Immunol 146: 26–33. [DOI] [PubMed] [Google Scholar]
- 30. Zeestraten EC, Van Hoesel AQ, Speetjens FM, Menon AG, Putter H, et al. (2013) FoxP3- and CD8-positive Infiltrating Immune Cells Together Determine Clinical Outcome in Colorectal Cancer. Cancer Microenviron 6: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim M, Grimmig T, Grimm M, Lazariotou M, Meier E, et al. (2013) Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS One 8: e53630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, et al. (2008) Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer 98: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, et al. (2008) Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 44: 1875–1882. [DOI] [PubMed] [Google Scholar]
- 34. Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, et al. (2009) Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen Z, Zhou S, Wang Y, Li RL, Zhong C, et al. (2010) Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 136: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du L, Xiao X, Wang C, Zhang X, Zheng N, et al. (2011) Human leukocyte antigen-G is closely associated with tumor immune escape in gastric cancer by increasing local regulatory T cells. Cancer Sci 102: 1272–1280. [DOI] [PubMed] [Google Scholar]
- 37. Kim HI, Kim H, Cho HW, Kim SY, Song KJ, et al. (2011) The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol 104: 728–733. [DOI] [PubMed] [Google Scholar]
- 38. Lu X, Liu J, Li H, Li W, Wang X, et al. (2011) Conversion of intratumoral regulatory T cells by human gastric cancer cells is dependent on transforming growth factor-β1. J Surg Oncol 104: 571–577. [DOI] [PubMed] [Google Scholar]
- 39. Shu P, Qin J, Qin XY, Sun YH, Shen ZB, et al. (2011) Expression and prognostic value of M2 macrophages and regulatory T cells in gastric carcinoma tissue. Zhonghua Wei Chang Wai Ke Za Zhi 14: 368–371. [PubMed] [Google Scholar]
- 40. Wang B, Xu D, Yu X, Ding T, Rao H, et al. (2011) Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol 18: 2585–2593. [DOI] [PubMed] [Google Scholar]
- 41. Ishigami S, Arigami T, Uenosono Y, Matsumoto M, Okumura H, et al. (2012) Cancerous HLA class I expression and regulatory T cell infiltration in gastric cancer. Cancer Immunol Immunother 61: 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kashimura S, Saze Z, Terashima M, Soeta N, Ohtani S, et al. (2012) CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 15: 144–153. [DOI] [PubMed] [Google Scholar]
- 43. Yoshii M, Tanaka H, Ohira M, Muguruma K, Iwauchi T, et al. (2012) Expression of Forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer 106: 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng B, Zhu JM, Wang Y, Liu TT, Ding YB, et al. (2013) Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-β1 in gastric cancer. PLoS One 8: e63777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim KJ, Lee KS, Cho HJ, Kim YH, Yang HK, et al. (2013) Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol 45: 285–293. [DOI] [PubMed] [Google Scholar]
- 46. Zhou S, Xu S, Tao H, Zhen Z, Chen G, et al. (2013) CCR7 Expression and Intratumoral FOXP3(+) Regulatory T Cells are Correlated with Overall Survival and Lymph Node Metastasis in Gastric Cancer. PLoS One 8: e74430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma GF, Miao Q, Liu YM, Gao H, Lian JJ, et al.. (2014) High FoxP3 expression in tumour cells predicts better survival in gastric cancer and its role in tumour microenvironment. Br J Cancer. doi: 10.1038/bjc.2014.47. [DOI] [PMC free article] [PubMed]
- 48. Chang KM (2005) Regulatory T cells and the liver: a new piece of the puzzle. Hepatology 41: 700–702. [DOI] [PubMed] [Google Scholar]
- 49. Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I (2008) The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp 56: 181–191. [DOI] [PubMed] [Google Scholar]
- 50. Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6: 345–352. [DOI] [PubMed] [Google Scholar]
- 51. Wang F, Jing X, Li G, Wang T, Yang B, Zhu Z, et al. (2012) Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int 32: 644–655. [DOI] [PubMed] [Google Scholar]
- 52. Kryczek I, Wu K, Zhao E, Wei S, Vatan L, et al. (2011) IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 186: 4388–4395. [DOI] [PubMed] [Google Scholar]
- 53. Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, et al. (2008) Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Powell DJ Jr, Attia P, Ghetie V, Schindler J, Vitetta ES, et al. (2008) Partial reduction of human FOXP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration. J Immunother 31: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou S, Tao H, Zhen Z, Chen H, Chen G, et al. (2013) Depletion of CD4+ CD25+ regulatory T cells promotes CCL21-mediated antitumor immunity. PLoS One 8: e73952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of study selection. A: Flow diagram of study selection for HCC; B: Flow diagram of study selection for CRC; C: Flow diagram of study selection for GC.
(DOC)
Funnel plots for HCC. A: 12 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 11 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 12 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 11 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 10 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 11 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
Funnel plots for CRC. A: 8 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 8 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 8 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 6 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 6 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 6 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
Funnel plots for GC. A: 16 articles in the meta-analysis of survival during follow-up 1-year after treatment; B: 16 articles in the meta-analysis of survival during follow-up 3-year after treatment; C: 16 articles in the meta-analysis of survival during follow-up 5-year after treatment; D: 4 articles in the meta-analysis of recurrence during follow-up 1-year after treatment; E: 4 articles in the meta-analysis of recurrence during follow-up 3-year after treatment; F: 4 articles in the meta-analysis of recurrence during follow-up 5-year after treatment.
(TIF)
PRISMA Checklist.
(ZIP)