Abstract
Aims/hypothesis
Human adipose tissue macrophages (ATMs) display an alternatively activated (M2) phenotype, but are still able to produce excessive inflammatory mediators. However, the processes driving this particular ATM phenotype are not understood. Genome-wide association studies associated the CDKN2A locus, encoding the tumour suppressor p16INK4A, with the development of type 2 diabetes. In the present study, p16INK4A levels in human ATMs and the role of p16INK4A in acquiring the ATM phenotype were assessed.
Methods
Gene expression of p16INK4A in ATMs was analysed and compared with that in monocyte-derived macrophages (MDMs) from obese patients or with macrophages from human atherosclerotic plaques (AMs). Additionally, p16INK4A levels were studied during macrophage differentiation and polarisation of monocytes isolated from healthy donors. The role of p16INK4A in MDMs from healthy donors was investigated by small interfering (si)RNA-mediated silencing or adenovirus-mediated overproduction of p16INK4A.
Results
Compared with MDMs and AMs, ATMs from obese patients expressed lower levels of p16INK4A. In vitro, IL-4-induced M2 polarisation resulted in lower p16INK4A protein levels after differentiation of monocytes from healthy donors in macrophages. Silencing of p16INK4A in MDMs mediated by siRNA increased the expression of M2 marker genes and enhanced the response to lipopolysaccharide (LPS), to give a phenotype resembling that of ATM. By contrast, adenovirus-mediated overproduction of p16INK4A in MDMs diminished M2 marker gene expression and the response to LPS. Western blot analysis revealed that p16INK4A overproduction inhibits LPS- and palmitate-induced Toll-like receptor 4 (TLR4)–nuclear factor of κ light polypeptide gene enhancer in B cells (NF-κB) signalling.
Conclusions/interpretation
These results show that p16INK4A inhibits the acquisition of the ATM phenotype. The age-related increase in p16INK4A level may thus influence normal ATM function and contribute to type 2 diabetes risk.
Keywords: Adipose Tissue; metabolism; Cell Polarity; Cyclin-Dependent Kinase Inhibitor p16; biosynthesis; genetics; Diabetes Mellitus, Type 2; metabolism; Down-Regulation; Female; Gene Silencing; Humans; Macrophages; metabolism; Male; NF-kappa B; metabolism; Obesity; metabolism; Plaque, Atherosclerotic; metabolism; RNA, Small Interfering; metabolism; Toll-Like Receptor 4; metabolism
Introduction
Macrophage infiltration into adipose tissue (AT) reflects the low-grade inflammatory state characterising obesity and insulin resistance [1]. Obesity-induced adipocyte hypertrophy causes adipocyte inflammation and cell death triggering macrophage accumulation. These AT macrophages (ATMs) accumulate in AT, surrounding dead adipocytes and forming so-called crown-like structures (CLS) [2]. Macrophages exhibit different activation states allowing them to exert specialised functions: classically activated (M1) macrophages produce high levels of inflammatory mediators such as TNF, IL-6 and cyclo-oxygenase 2 (COX2), while alternatively activated (M2) macrophages produce anti-inflammatory cytokines [3]. In vitro, monocyte differentiation into M2 macrophages is induced by stimulation with T helper 2 (Th2) cytokines, such as IL-4 or IL-13, or into M1 macrophages by stimulation with IFNγ or lipopolysaccharide (LPS) [4]. M2 markers include mannose receptor, C type 1 (CD206), the haptoglobin-haemoglobin scavenger receptor (CD163) and the chemokine chemokine (C-C motif) ligand 18 (pulmonary and activation-regulated) (CCL18 [also known AMAC1]) [4]. In obesity, inflammatory cytokine production by ATMs increases significantly. Based on animal studies it was proposed that obesity promotes the recruitment of M1 macrophages into AT and/or induces a shift of M2 resident macrophages toward the M1 state [5]. Indeed, mouse ATMs form a heterogeneous population including both M1 and M2 subsets [6]. However, in humans, ATMs have a more complex phenotype, possibly depending on their localisation within the AT [7,8]. Notwithstanding, the molecular mechanisms determining this particular polarisation remain elusive.
p16INK4A, encoded by the CDKN2A locus, is a cell cycle inhibitor which blocks cyclin D/cyclin-dependent kinase 4 (CDK4) kinase activity by binding to the catalytic subunit of CDK4, thereby preventing retinoblastoma protein phosphorylation and subsequent liberation of the E2F1 transcription factor. Hence, the transcription of genes required for progression to the S phase is restrained [9]. Historically, p16INK4A has been studied in relation to cancer and many human tumours display altered p16INK4A levels [10]. Because of its role in cell cycle exit, p16INK4A plays a role in senescence. For instance, mammary epithelial cells, fibroblasts and T lymphocytes accumulate p16INK4A during ageing [11]. More recently, p16INK4A was shown to be absent in induced pluripotent stem cells, but to be re-induced during cellular reprogramming, highlighting a role of p16INK4A in cell fate determination [12]. However, little is known about whether p16INK4A exerts functions unrelated to cell cycle control. Interestingly, a region near the CDKN2A locus has been associated with type 2 diabetes development [13]. As macrophage infiltration and inflammation are key events of type 2 diabetes and obesity, the present study aimed to investigate the connection between p16INK4A levels in human ATMs and their particular phenotype.
Methods
Human ATM purification
Human ATMs were purified from visceral AT biopsies as described by Mayi et al. [14] from non-diabetic morbidly obese patients (BMI 55.7 + 3 kg/m2) undergoing bariatric surgery with informed consent. The study was approved by the ethics committee of the Centre Hospitalier Régional Universitaire de Lille, France, under the ABOS (Atlas Biologique de l’Obesite Sevère) and OMENTOB (OMENTectomie au cours de la gastrectomie en manchon chez l’OBèse sévère: étude pilote contrôlée et randomisée) frameworks. None of the patients had clinical symptoms of systemic inflammation or malignancies.
Immunohistochemical analysis and laser-capture microdissection of human atherosclerotic plaques
Human atherosclerotic plaques were removed from patients eligible for surgical carotid endarterectomy recruited at the Cardiovascular Surgery Department, Hospital of Lille, France. Informed consent was obtained from all patients. For immunohistochemical analysis and macrophage detection, endogenous peroxidase activity was quenched and serial 8 μm cryosections were stained with monoclonal anti-human CD68 antibodies (Dako Corporation, Zone d’Activites du Buisson de la Couldre, France) followed by detection with biotinylated secondary antibodies and streptavidin–horseradish peroxidase. Immunostaining was visualised using the 3-amino-9-ethylcarbazole (AEC) substrate–chromogen system.
Adjacent cryosections of atherosclerotic plaques containing CD68+ macrophage-rich areas were dehydrated in a series of graded alcohols, cleared in xylene and prepared for laser-capture microdissection, performed on an ArcturusXT Microdissection Instrument with CapSure Macro LCM Caps (MDS Analytical Technologies, Saint Gregoire, France). Macrophage-rich areas were captured from four adjacent 8 μm sections and pooled for RNA extraction. RNA extraction from isolated samples was performed using the Picopure RNA extraction kit (MDS Analytical Technologies). RNA quality was assessed using the Agilent 2100 Bioanalyser (Agilent Technologies, Massy, France) and amplified in two rounds using the ExpressArt TRinucleotide mRNA amplification Nano kit (AmpTec, Vicq, France).
Human monocyte differentiation into macrophages
Mononuclear cells isolated from blood of healthy donors (Ficoll gradient) were cultured in RPMI 1640 medium with 10% (vol./vol.) pooled human serum for 7–12 days. During this time, monocytes were differentiated into mature monocyte-derived macrophages (MDMs) or into M2 macrophages by supplementing the medium with IL-4 (15 ng/ml) starting from day 0. Macrophages were treated with either LPS (100 ng/ml) for 1 h or 2 h or with palmitate (500 μmol/l) for 1 h.
Adenovirus-mediated transfer of p16INK4A and p16INK4A inhibition by siRNA transfection
To ectopically overproduce p16INK4A in MDMs, cells were infected with p16INK4A adenovirus (kind gift of Dr. A. Mazo, University of Barcelona, Spain) or lacZ adenovirus (infection control) for 16 h on the seventh day of culture. For p16INK4A inhibition studies, small interfering (si)RNA targeting CDKN2A (M-011007-00; Dharmacon, Courtaboeuf, France) was added to MDMs on the seventh day of differentiation for 12 h. At 24 h after adenoviral infection or siRNA transfection, cells were stimulated or used directly to harvest total RNA, cellular protein and culture media for further analysis.
Gene expression and determination of protein levels
RNA was isolated from healthy MDMs by the TRIzol method (Invitrogen Life Technologies, Cergy Pontoise, France) or from ATMs and MDMs from obese patients using the RNeasy micro kit (Qiagen, Courtaboeuf, France) following the manufacturer’s instructions. Gene expression was examined by quantitative RT-PCR using specific primers (Eurogentec, Angers, France) (see electronic supplementary material [ESM] Table 1). RNA levels were normalised to cyclophilin A mRNA.
Proteins were determined by western blot analysis using a monoclonal antibody against human p16INK4A (Beckton Dickinson), signal transducer and activator of transcription 6, interleukin-4 induced (STAT6) and phosphorylated STAT6 (9362 and 9361), phospho-IKKα,β (2681), IκBα (9242), phospho-P38 (9211) (Cell Signaling), Toll-like receptor 4 (TLR4) (sc-10741), and β-actin (sc-1616) (Santa Cruz). Levels of TNF protein in culture media were measured using an ELISA (R&D Systems, Lille, France).
Statistical analysis
Data are from representative experiments. Each set of experiments was repeated at least three times with similar results. Statistical significance was determined by the Student’s t test. Values are reported as means ± SD. Values of p<0.05 were considered significant and different levels of significance are represented by asterisks: *p<0.05, **p<0.01 and ***p<0.001.
Results
Human p16INK4A expression is lower in ATMs than in MDMs or AMs
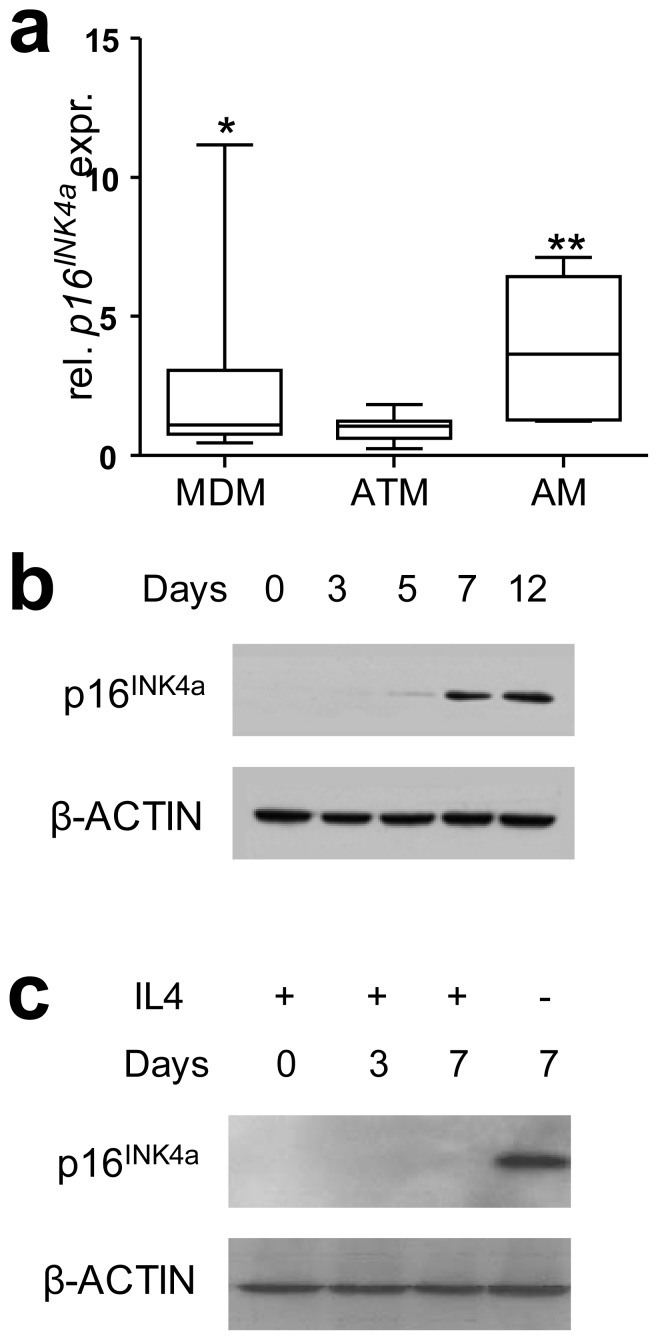
Expression of p16INK4A in human ATMs was compared with MDMs differentiated from monocytes from the same obese donors. Confirming previous reports on the human ATM phenotype [8], mRNA expression of typical M2 markers (AMAC1 [also known as CCL18] and IL10) and of the inflammatory cytokine TNF was higher in ATMs compared with MDMs (data not shown). ATMs expressed significantly lower p16INK4A mRNA levels than MDMs as well as CD68-positive atherosclerotic plaque macrophages (AMs) (Fig. 1a). This observation suggests that p16INK4A expression is specifically decreased in ATMs. Interestingly, in vitro macrophage differentiation led to the induction of p16INK4A protein levels (Fig. 1b), whereas this induction was inhibited in macrophages differentiated in the presence of IL-4, leading to M2 polarisation (Fig. 1c). All observed changes in p16INK4A levels occurred without alterations in cell cycle progression (data not shown).
Fig. 1.
p16INK4A levels are low in ATMs and M2 macrophages. a Human ATMs were purified from visceral AT biopsies; monocytes purified from whole blood of the same obese donors (n=9) were differentiated for 7 days into MDMs. CD68+ AMs were isolated from human carotid plaques (n=6) by laser-capture microdissection. mRNA was isolated and quantitative RT-PCR was performed to measure mRNA expression levels of p16INK4A in the cell types. Statistically significant differences are indicated (t test; *p<0.05, **p<0.01 compared with ATM). b p16INK4A protein was detected by western blot at different time points during macrophage differentiation from blood monocytes of healthy donors. c p16INK4A protein was detected by western blot at different time points during alternative macrophage differentiation of blood monocytes from healthy donors in the presence of IL-4
As peroxisome proliferator-activated receptor (PPAR)α activation induces p16INK4A expression in smooth muscle cells [15] and as PPARγ activation enhances M2 polarisation [16], we investigated whether PPARγ activation with GW929 affects p16INK4A expression. GW929 treatment of mature MDM macrophages resulted in a very mild inhibition of p16INK4A expression while GW929 treatment had no effect on p16INK4A levels in mature M2 macrophages (ESM Fig. 1a). Thus, p16INK4A is not induced by PPARγ activation in human macrophages. Moreover, enhancing M2 polarisation by adding GW929 to monocytes at the start of differentiation [16] did not further decrease p16INK4A expression (ESM Fig. 1b), suggesting that downregulation of p16INK4A is mainly IL-4 dependent.
These data show that p16INK4A expression is low in human ATMs and that in vitro differentiation of primary monocytes into M2 macrophages mimics this effect.
Silencing of p16INK4A in MDMs induces a phenotype resembling human ATMs
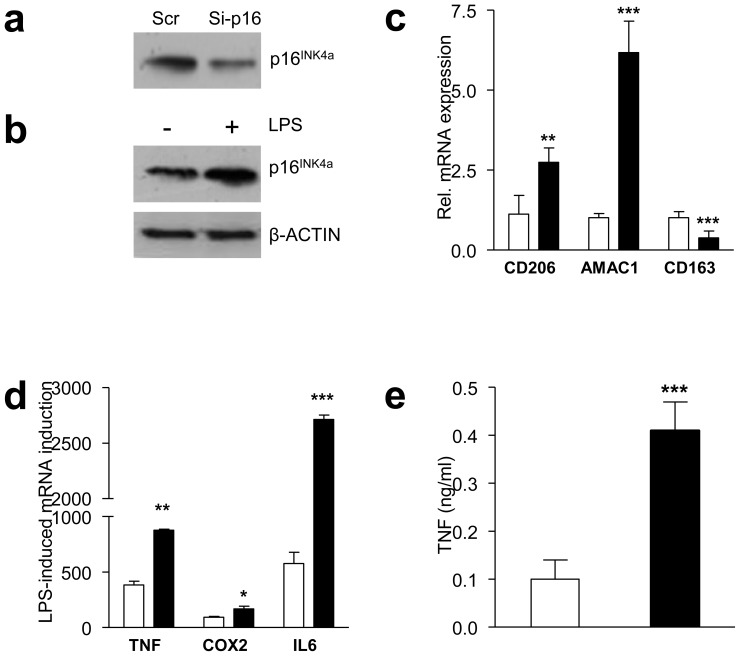
As ATMs display low p16INK4A expression compared with MDMs and as M2 polarisation lowers p16INK4A levels, we determined whether a direct link exists between p16INK4A levels and macrophage polarisation. Silencing p16INK4A in MDMs by approximately 60% using siRNA (Fig. 2a) increased mRNA expression of the M2 markers CD206 (also known as MRC1) and AMAC1, while the expression of CD163, an M2 marker typically downregulated when macrophages are differentiated in vitro with IL-4 [4], decreased (Fig. 2c).
Fig. 2.
Inhibition of p16INK4A levels in MDMs induces a phenotype resembling that of human ATMs. Western blot of p16INK4A protein in MDMs transfected with either control scrambled or siRNA targeting CDKN2A (a) and with or without LPS treatment (b). c After siRNA transfection, mRNA was isolated and expression of CD206, AMAC1, CD163 was quantified by quantitative RT-PCR. d MDMs were treated with LPS for 2 h, RNA was isolated and expression of TNF, COX2, and IL6 was quantified by quantitative RT-PCR. e TNF protein was measured by ELISA in the supernatant fraction 24 h after LPS treatment. Statistically significant differences are indicated (t test; ***p<0.001, **p<0.01 and *p<0.05). Scr, control scrambled, white bars; Si-p16, siRNA targeting CDKN2A, black bars
Activation of MDMs with LPS increased p16INK4A protein levels (Fig. 2b), suggesting a role for p16INK4A in the inflammatory response. To determine whether p16INK4A knockdown also modulates the inflammatory response of macrophages, MDMs from healthy donors were treated with LPS after p16INK4A silencing. Although p16INK4A knockdown induced an M2-phenotype (Fig. 2c), the expression of the inflammatory response genes TNF, COX2 and IL6 was more strongly induced by LPS (Fig. 2d). Furthermore, LPS-induced TNF secretion was higher in cells transfected with p16INK4A siRNA (Fig. 2e). The production of high levels of inflammatory cytokines upon activation, despite being M2 polarised, resembles the human ATM phenotype [8].
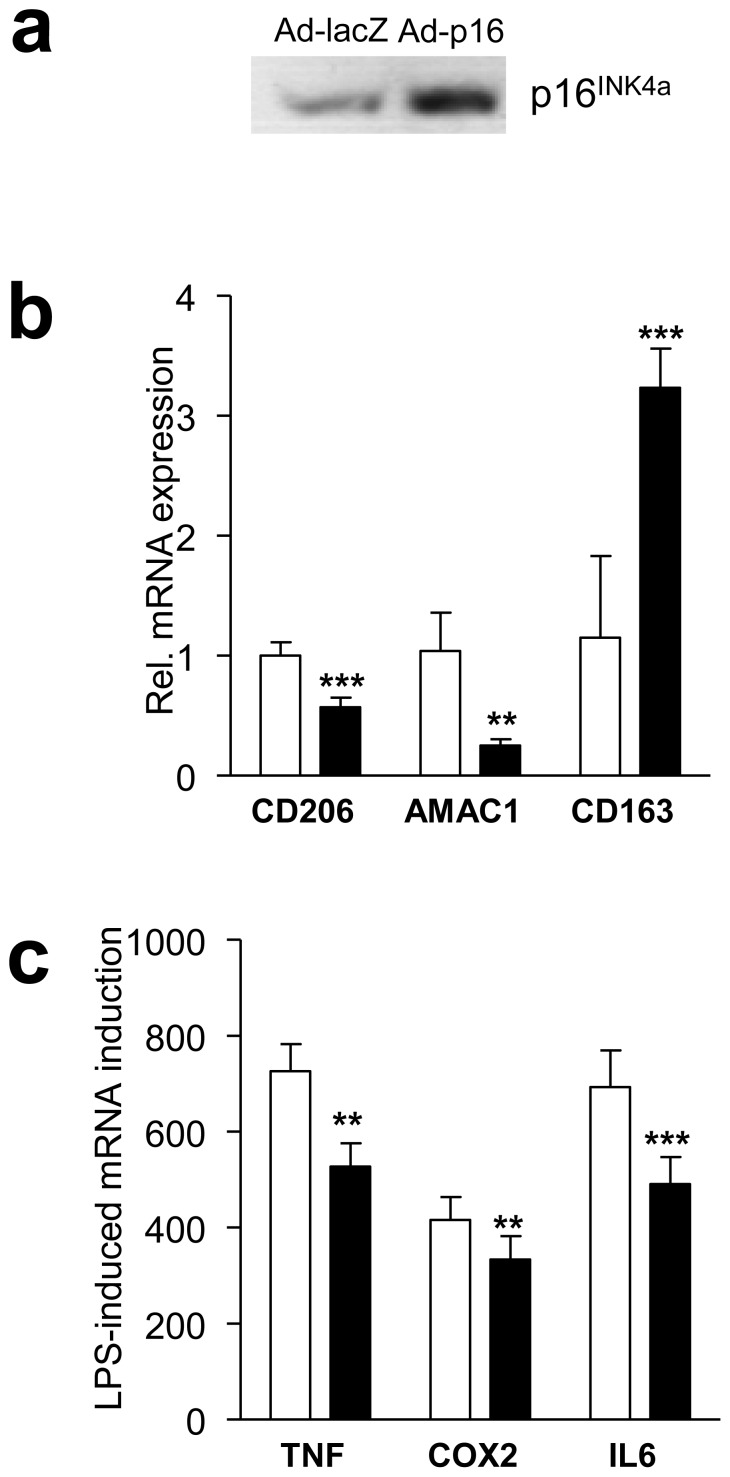
Importantly, p16INK4A overproduction by adenovirus-mediated gene transfer (Fig. 3a) showed an expression pattern opposite to the one observed with p16INK4A silencing, i.e. downregulation of CD206 and AMAC1, upregulation of CD163 (Fig. 3b) and a decreased response to LPS (Fig. 3c). Together, these data indicate that downregulation of p16INK4A levels results in an M2-like phenotype associated with an exacerbated response to inflammation, reflecting the phenotype of ATMs, while p16INK4A overproduction inhibits the ATM phenotype.
Fig. 3.
Overproduction of p16INK4A in MDMs diminishes M2 polarisation and the LPS-induced inflammatory response. MDMs were infected with either control LacZ or p16INK4A. a p16INK4A protein was detected by western blot. b,c After infection, mRNA was isolated and expression of CD206, AMAC1 and CD163 (b) or LPS-induced TNF, COX2 and IL6 (c) was quantified by quantitative RT-PCR. Statistically significant differences are indicated (t test; ***p<0.001 and **p<0.01). Ad-LacZ, MDMs infected with control lacZ, white bars; Ad-p16, MDMs infected with p16INK4A adenovirus, black bars
p16INK4A interferes with the TLR4 response upstream of NFκB without affecting TLR4 levels
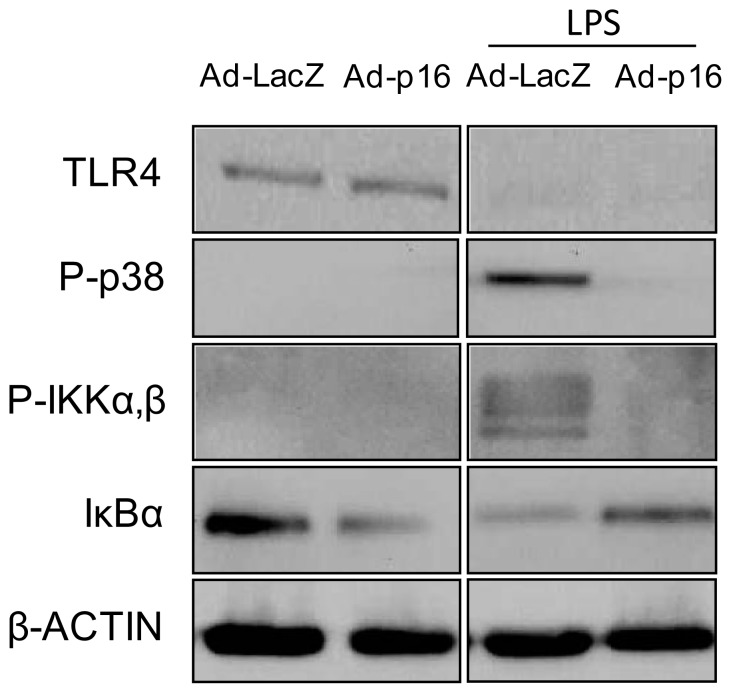
A major pathway involved in regulating M2 genes involves STAT6 signalling [3]. However, modulation of p16INK4A levels did not affect phosphorylation of STAT6 under basal conditions or after IL-4 treatment (data not shown). LPS signalling involves TLR4-mediated phosphorylation of p38 and inhibitor of nuclear factor kappa-B kinase (IKK) α,β [17]. The latter phosphorylates nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), leading to IκBα degradation and subsequent release and nuclear translocation of nuclear factor of κ light polypeptide gene enhancer in B cells (NFκB), thereby initiating the transcriptional activation of inflammatory genes [17]. Subsequent to its activation, TLR4 protein is degraded [18]. Although p16INK4A overproduction decreased the response to LPS (Fig. 3c), this effect was unrelated to TLR4 protein levels in basal or in LPS-stimulated conditions, the latter resulting in the complete degradation of TLR4 (Fig. 4), pointing to an effect downstream in this pathway. Indeed, LPS-induced phosphorylation of p38 and of IKKα,β was less pronounced in cells overproducing p16INK4A (Fig. 4). In line, IκBα, which inhibits the nuclear translocation of NFκB [17], was less degraded on LPS treatment (Fig. 4). Notably, under unstimulated conditions, p16INK4A overexpression induced lower levels of anti-inflammatory IκBα (Fig. 4). Obesity is associated with increased levels of NEFAs, such as palmitate [19], which are known to act as TLR4 ligands [20]. In line with the observations on LPS-treatment, MDM overproducing p16INK4A displayed decreased phosphorylation of p38 and IKKα,β after stimulation with palmitate (ESM Fig. 2). These results suggest that p16INK4A directly interferes with TLR4–NFκB signalling, resulting in an altered inflammatory profile.
Fig. 4.
p16INK4A interferes with TLR4 signalling upstream of NFκB. MDMs were infected with either control lacZ or p16INK4A adenovirus and treated with LPS for 1 h. Signalling pathways were investigated by western blot, using the antibodies indicated. Ad-LacZ, MDMs infected with control lacZ; Ad-p16, MDMs infected with p16INK4A adenovirus. P-, phospho
Discussion
We show that ATMs from obese patients as well as IL4-polarised human M2 macrophages in vitro are characterised by low p16INK4A expression compared with, respectively, MDMs or AMs and to LPS-induced M1 macrophages in vitro. Moreover, siRNA-mediated downmodulation of p16INK4A in MDMs induced an M2 phenotype while increasing the inflammatory response to LPS, thus inducing an ATM-like phenotype. Overproduction of p16INK4A resulted in the opposite phenotype, providing proof that the effects are p16INK4A specific.
Although the dominant phenotype of human ATMs is a mixed M1/M2 phenotype [8,21], recent reports describe the existence of different macrophage subpopulations in obese AT [7,22]. Compared with resident ATMs, which are negative for the M1 marker CD11c and positive for the M2 marker MR, crown-like structure (CLS)-ATMs display a mixed profile of polarisation markers (i.e. they are positive for both CD11c and MR) and produce more pro-inflammatory mediators than resident parenchymal ATMs [22], thus resembling the phenotype observed on downmodulation of p16INK4A levels by siRNA. Interestingly, these CLS macrophages express lower levels of CD163 than resident ATMs [22]. Combined with our results that inhibition of p16INK4A levels leads to a mixed phenotype characterised by low CD163 expression, these observations suggest that p16INK4A may be particularly involved in the differentiation of blood monocytes to CLS-ATMs during obesity. Therefore, it would be interesting to determine the ATM subpopulation in which p16INK4A is silenced and whether its expression levels correlate with CLS formation, AT inflammation or metabolic disturbances.
Our data show that p16INK4A in human macrophages interferes with TLR4–NFκB signalling, identifying a mechanism via which p16INK4A regulates the inflammatory status of these cells. In line, it has been previously shown that local transfer of p16INK4A into bone joints suppresses pro-inflammatory cytokines in a mouse model of rheumatoid arthritis [23] and that the E2F1 transcription factor, a target of p16INK4A, is required for the transcriptional activation of certain NFκB target genes [24]. Moreover, the NFκB pathway has been shown, at least in mice, to play a role in ATM phenotype and function. Mice with a luciferase construct driven by a NFκB-responsive promoter show signal enrichment in ATM clusters during diet-induced obesity [25] and modulation of haematopoietic levels of Ikkβ affects the development of obesity and insulin resistance [26,27]. In parallel, the pro-inflammatory phenotype found in human ATMs involves many NFκB target genes, such as TNF, IL6, MCP1 (also known as CCL2), IL8 and COX2 [21].
Overproduction of p16INK4A resulted in decreased IKKα,β phosphorylation. Moreover, phosphorylation of p38, another component of the LPS-induced signalling pathway converging with NFκB signalling at the level of TNF receptor-associated factor 6 (TRAF-6) [17], was diminished. Although p16INK4A has been shown to induce anti-proliferative responses via negative cross-talk with the p65 subunit of NFκB [28], our results thus indicate that p16INK4A interferes upstream of the p65 subunit of NFκB, possibly at the level, or upstream, of TRAF6. Notably, TLR4 levels were unaffected by p16INKa overproduction.
Interestingly, the main pathway controlling M2 polarisation, i.e. STAT6 phosphorylation, was unchanged on modulation of p16INK4A levels (data not shown). However, under basal conditions, overproduction of p16INK4A also exerted effects on the NFκB pathway, as shown by lower IκBα protein levels. IκBα is known to be anti-inflammatory [17] and inhibition of IκBα phosphorylation and subsequent degradation by adiponectin promotes M2 polarisation [29], suggesting that the anti-inflammatory effects of IκBα can enhance alternative polarisation. The lower IκBα levels in basal conditions after p16INK4A overexpression may thus, at least in part, contribute to the decreased expression of M2 genes. Alternatively, NFκB has been shown to maintain the M2 polarisation status in tumour-associated macrophages [30], suggesting that a decrease in NFκB signalling itself could also contribute to the decreased M2 profile of macrophages overexpressing p16INK4A.
Silencing of the p16INK4A locus is associated with an increased risk of cancer development. For example, in certain haematological malignancies p16INK4A loss of function leads to uncontrolled cell proliferation [31]. However, human MDMs are terminally differentiated cells, which means they are in a cell state tightly linked to cell cycle withdrawal. In line, the mature macrophages were in the G0 phase regardless of their polarisation state (data not shown). Therefore, we propose a direct association between p16INK4A expression and macrophage phenotype, independent of cell cycle control. In parallel with several cancers such as lung cancer and the haematopoietic cancer leukaemia, where the CDKN2A/B genomic methylation status regulating p16INK4A is used as an early diagnostic marker [31], p16INK4A levels in ATMs might serve as a diagnostic marker of ATM function.
Although studies in mice suggest that an age-dependent increase of p16INK4A levels hamper beta cell proliferation, resulting in impaired glucose homeostasis [32], it remains obscure whether p16INK4A plays a similar role in human pancreatic function. Our data show a novel role of p16INK4A in another cell type, i.e. human ATMs, which are important during the development of type 2 diabetes [1]. As in pancreatic beta cells, increased p16INK4A levels causes the age-dependent decline in proliferation of haematopoietic stem cells [33], which give rise to immune cells. As our results show that p16INK4A overproduction inhibits the phenotype of non-proliferating ATMs, age-related increases of p16INK4A levels may disturb normal ATM function, possibly leading to disturbed AT homeostasis. As the locus encoding p16INK4A has been associated with the risk of type 2 diabetes [13], our results suggest that p16INK4A levels in ATMs may partially explain the link between type 2 diabetes and the linkage disequilibrium found with the CDKN2A locus.
In conclusion, this study indicates that downmodulation of p16INK4A is an important event during macrophage maturation into ATMs, acting as a modulator of the cellular inflammatory status.
Supplementary Material
Acknowledgments
This work was supported by the EU FP6 grant (EIF 040851 to L. Fuentes), EU FP7 grants (PIEF-GA-2009-23522 to K. Wouters and grant no. 201608 to T. H. Mayi), an EFSD/GlaxoSmithKline Research Grant 2009 (K. Wouters and B. Staels), the Spanish Government MEC (Ministerio de Educación y Ciencia to L. Fuentes) and the Fondation pour la Recherche Médicale (DCV20070409276 to B. Staels). M. Ploton, M. Daoudi and J. Vanhoutte are acknowledged for technical support.
Abbreviations
- AT
Adipose tissue
- AM
Atherosclerotic plaque macrophage
- ATM
Adipose tissue macrophage
- CDK4
Cyclin dependent kinase 4
- CLS
Crown-like structures
- COX2
Cyclo-oxygenase 2
- LPS
Lipopolysaccharide
- MDM
Monocyte-derived macrophage
- NFκB
Nuclear factor of κ light polypeptide gene enhancer in B cells
- STAT6
Signal transducer and activator of transcription 6, interleukin-4
- TLR4
Toll-like receptor 4
Footnotes
Contribution statement
All authors participated in the conception and design, or analysis and interpretation of the data, contributed to drafting and revising the manuscript and the final approval of the version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Reference List
- 1.Fuentes L, Roszer T, Ricote M. Inflammatory mediators and insulin resistance in obesity: role of nuclear receptor signaling in macrophages. Mediators Inflamm. 2010;2010:219583. doi: 10.1155/2010/219583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 4.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieur X, Mok CY, Velagapudi VR, et al. Differential Lipid Partitioning Between Adipocytes and Tissue Macrophages Modulates Macrophage Lipotoxicity and M2/M1 Polarization in Obese Mice. Diabetes. 2011 doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–314. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 9.Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 11.Huschtscha LI, Reddel RR. p16(INK4a) and the control of cellular proliferative life span. Carcinogenesis. 1999;20:921–926. doi: 10.1093/carcin/20.6.921. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Collado M, Villasante A, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 14.Mayi TH, Duhem C, Copin C, et al. Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages. FEBS J. 2010;277:3308–3320. doi: 10.1111/j.1742-4658.2010.07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gizard F, Amant C, Barbier O, et al. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouhlel MA, Derudas B, Rigamonti E, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter S, O’Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J. 2009;422:1–10. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 18.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 19.Garaulet M, Perez-Llamas F, Perez-Ayala M, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 21.Bourlier V, Zakaroff-Girard A, Miranville A, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 22.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasu K, Kohsaka H, Nonomura Y, et al. Adenoviral transfer of cyclin-dependent kinase inhibitor genes suppresses collagen-induced arthritis in mice. J Immunol. 2000;165:7246–7252. doi: 10.4049/jimmunol.165.12.7246. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Capps C, Willerson JT, Zoldhelyi P. E2F-1 regulates nuclear factor-kappaB activity and cell adhesion: potential antiinflammatory activity of the transcription factor E2F-1. Circulation. 2002;106:2707–2713. doi: 10.1161/01.cir.0000038706.30661.86. [DOI] [PubMed] [Google Scholar]
- 25.Chiang SH, Bazuine M, Lumeng CN, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 27.Saberi M, Woods NB, de Luca C, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff B, Naumann M. INK4 cell cycle inhibitors direct transcriptional inactivation of NF-kappaB. Oncogene. 1999;18:2663–2666. doi: 10.1038/sj.onc.1202617. [DOI] [PubMed] [Google Scholar]
- 29.Lovren F, Pan Y, Quan A, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagemann T, Lawrence T, McNeish I, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackanson B, Guo Y, Lubbert M. The silence of the genes: epigenetic disturbances in haematopoietic malignancies. Expert Opin Ther Targets. 2005;9:45–61. doi: 10.1517/14728222.9.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 33.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.