Abstract
The purpose of this work was to validate the use of Pluronic fluorescent nanomicelles for in vivo two-photon imaging of both the normal and the tumor vasculature. The nanomicelles were obtained after encapsulating a hydrophobic two-photon dye: di-stryl benzene derivative, in Pluronic block copolymers. Their performance with respect to imaging depth, blood plasma staining, and diffusion across the tumor vascular endothelium was compared to a classic blood pool dye Rhodamin B dextran (70 kDa) using two-photon microscopy. Pluronic nanomicelles showed, like Rhodamin B dextran, a homogeneous blood plasma staining for at least 1 hour after intravenous injection. Their two-photon imaging depth was similar in normal mouse brain using 10 times less injected mass. In contrast with Rhodamin B dextran, no extravasation is observed in leaky tumor vessels due to their large size: 20–100 nm. In conclusion, Pluronic nanomicelles can be used as a blood pool dye, even in leaky tumor vessels. The use of Pluronic block co-polymers is a valuable approach for encapsulating two-photon fluorescent dyes that are hydrophobic and not suitable for intravenous injection.
Keywords: Animals; Blood Vessels; anatomy & histology; Blood Volume Determination; Brain; blood supply; Cell Line, Tumor; Dextrans; diagnostic use; Diagnostic Imaging; Fluorescent Dyes; diagnostic use; Humans; Mice; Mice, Nude; Micelles; Microscopy, Confocal; methods; Nanostructures; Neoplasms, Experimental; blood supply; Optical Phenomena; Photons; Poloxamer; Rhodamines; diagnostic use; Styrenes; diagnostic use
1. Introduction
Two-photon laser scanning microscopy (TPLSM) allows deep imaging (< 1 mm) of biological tissues in living animals with a micrometric spatial resolution[1]. The observation depth depends among others on: tissue parameters, laser characteristics, optics and finally the physical-chemical properties of the fluorescent dye. Relevant fluorescent dyes for optical imaging are often hydrophobic and can therefore not be injected in animals. In the present study, a new approach of encapsulating hydrophobic two-photon dyes in Pluronic block copolymers was validated for two-photon imaging of the normal and the tumor vasculature in mice. In both cases, especially in the leaky vasculature of tumors, the nanomicelles should not diffuse across the vascular endothelium for proper detection of the local functional blood volume[2,3]. In other words, an enhanced permeability and retention (EPR) effect is not desired during the measurements. This will overestimate the local blood volume and blur the two-photon images. This latter parameter is of crucial interest in biomedical applications such as tumor diagnosis and therapy follow-up[4]. Tumors have most often an abnormal vascular blood volume in comparison to normal adjacent tissue, which determines drug delivery and consequently their therapy response.
The new two-photon dyes should allow imaging at low laser power (< 30 mW) at non-toxic concentrations without modifying the blood viscosity. For TPLSM, main fluorescent dye specifications are: a high two-photon absorption cross section (σTPA) and a fluorescence quantum yield Φ with an emission and a two-photon absorption wavelength located in the spectral region of interest for bio-imaging (680–1040 nm). Actually, regular intravascular dyes consist of branched polysaccharides, e.g.: dextrans, grafted with organic dyes such as fluorescein or rhodamin[5,6]. Numerous other strategies use quantum dots[7], water-soluble dendrimers[8] or fluorescent dye doped silica mesoporous nanoparticles[9]. Most of these particles are of limited use due to their in vivo instability or biotoxicity. Another approach involves nanodispersion of hydrophobic organic preformed compounds in aqueous solutions using special formulation techniques such as micro-emulsions[10].
In this field, we recently reported the elaboration of organic fluorescent nanomicelles based on a micro-emulsion process using a well known polyethylene–polypropylene glycol (Pluronic) surfactant [11]. This process leads to hydrophilic micelles encapsulating hydrophobic fluorescent molecules with dimensions ranging from 20 to 100 nm. This method can be applied to numerous hydrophobic dyes[12]. Here, a di-stryl benzene-modified derivative[13] has been used, exhibiting a 2500 GM (1 GM = 10−50 cm4 s photon−1 molecules−1) two-photon absorption cross section at 800 nm. Its structure is shown in figure 1. To illustrate the potential for vasculature imaging of such probes, imaging of the cerebral vasculature has been performed in the cortex of normal mice. The maximum imaging depth and blood plasma staining have been compared to the regular blood pool dye Rhodamin B Dextran (70 kDa). Quantitative blood volume measurement has been performed for both dyes and compared to values in the literature. Finally, the diffusion properties of the Pluronic nanomicelles has been analyzed, and compared to those of Rhodamin B dextran, in the leaky tumor neo-vasculature of human lung carcinoma growing in dorsal skin fold chambers.
Figure 1.
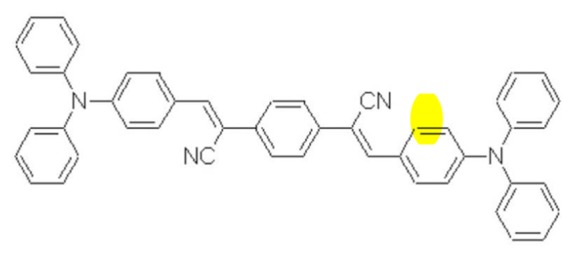
Structure of the di-strylbenzene derivative
2. Materials and Methods
2.1. Dyes preparation
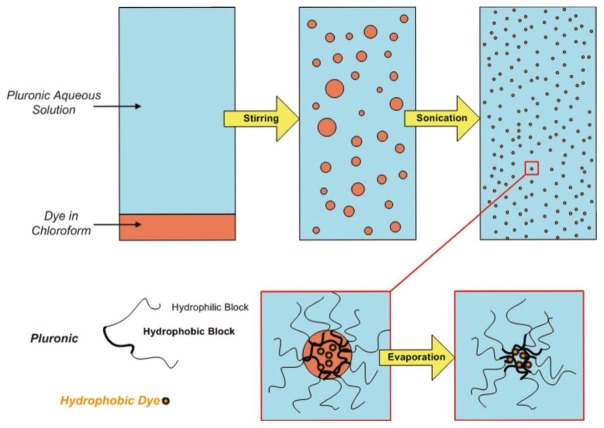
Pluronic F68 was purchased from Sigma-Aldrich (France). Synthesis of the di-stryl benzene derivative (figure 1) has already been reported[13]. For the Pluronic nanomicelles (figure 2), 2 mg of di-stryl benzene-modified derivative was dissolved in 100 μl chloroform and added to 5 ml of Pluronic F68 (2 wt. %) aqueous solution containing 0,9% NaCl. After 15 min stirring for pre-emulsification, the micro-emulsion was prepared by ultrasonicating for 15 minutes (bath, 150 W). After sonication, the mixture was stirred in a regulated bath at 50° C for 30 min to evaporate the chloroform. The colloidal particle size (20–100nm) was fixed by mechanical filtration (Nylon-Cameo, Fisher, France) of the solution. Typically dye concentrations, ranging from 0.1 to 0.15 g.L−1 (0.15 mmol.L−1 to 0.23 mmol.L−1), can be obtained in a 10 g.L−1 Pluronic aqueous solution[11]. As a reference, a 100 g.L−1 Rhodamin B dextran 70-kDa solution (Sigma-Aldrich, France) in 0.9% NaCl was prepared. Depending on the degree of Rhodamin B labeling of the dextran, the Rhodamin B dye concentrations varied between 4 mmol.L−1 and 11 mmol.L−1.
Figure 2.
Schematic representation of the Pluronic micelles synthesis (see text for details).
2.2. Animal models
Healthy Swiss white mice (n = 6, 6 – 8 weeks old) and tumor bearing Swiss nude mice (n = 3, 6 months old, Charles River Laboratories, Lyon, France) were used. All experiments were performed in accordance with the French Government guidelines (license A3851610008). During surgery and imaging, mice were anesthetized by a continuous inhalation of 2% isoflurane in a gas mixture of 30% O2 and 70% N2O, and placed on a modified stereotaxic frame or on a homemade frame for proper fixation of mice with dorsal skin fold chambers. Core temperature was maintained at approximately 37°C using a warm water blanket. For the cerebral micro-vessel imaging experiments, a craniotomy (ø = 3 mm) was carried out above the left parietal cortex. A rubber ring was placed on the top of the skull to trap saline for continuous objective immersion.
For tumor vasculature imaging, 3 Swiss nude mice were implanted with small dorsal skin fold chambers (small dorsal kit SM100, APJ Trading Co., Ventura, CA, USA) as described in a previous protocol[4]. Briefly, two symmetrical titanium frames with central holes (ø = 1 cm) flanked the dorsal skin-fold of animals sandwich the extended double layer of skin and create the dorsal skin-fold chamber, which consisted of one layer of striated muscle, s.c. tissue, and epidermis. An observation window, covered with a glass coverslip, allowed for intravital microscopic observations. One day after surgery, mice were checked on the presence of infection. At the absence of infection, 2.10[6] human lung carcinoma cells (NCI-H460, ATCC # HTB-177) in 200 μL vehicule (50 % RPMI medium, 50 % matrigel (BD Matrigel TM Matrix #354234, Bedford, MA)) were injected in the dorsal skin fold chamber. Two-photon imaging of the tumor vasculature was performed at 14 days after tumor cell injections.
For each experiment, 100 μl of Pluronic microemulsion or Rhodamin B dextran solution in saline was injected in the tail vein of the mice approximately 1 min before imaging.
2.3. Microscopy set up
In vivo two-photon laser scanning microscopy was performed with a confocal microscope consisting of a Biorad (MRC 1024) scanhead and an Olympus BX50WI microscope. A 800 nm excitation beam originating from a femtosecond Ti:sapphire laser (5 W pump; Spectra-Physics, Millenia V) was focused into the cortex using a 20× water-immersion objective (0.95 numerical aperture, Xlum Plan FI Olympus). The laser beam was then scanned in the x–y plane to acquire a 512×512 image (0.9 s/image) with laser powers varying between 10 and 30 mW. The z scan (variation of the observation depth) was realized by translation of the motorized objective. For these experiments, the confocal configuration was not used and the non-descanned fluorescence was directly collected with an external photomultiplier (PMT) protected by a BG39 filter (Schott Glass–Jena, Germany). Planar scans of the fluorescent intensity were acquired at successive depths in the animal with a 5 μm z step between scans. Image reconstruction and analysis were performed using free Image J software (http://rsbweb.nih.gov/ij/).
2.4. Relative blood volume measurement
Relative blood volume measurements (= total blood volume/total sample volume) have been performed using a method described by Verant et al.[5]. For each stack, the background (rolling ball algorithm with a radius varying between 30–60 μm) and the saturated pixels due to artifacts are removed using Image J software. Per slice in a z-stack, fluorescence intensities are normalized between 0 and 255 gray values using plunging vessels with maximum intensity (gray value 255). This is necessary to correct for the variation of fluorescence generation and collection as a function of depth. To finish, an average z-projection of the stack is performed. In this z-projection, the corresponding blood volume is proportional to the sum of the pixel intensities.
3. Results and Discussion
3.1. Cerebral vasculature imaging
Imaging of the cerebral vasculature in the cortex of normal Swiss white mice (6–8 weeks old) has been performed with Pluronic nanomicelles and Rhodamin B dextran. As shown in figure 3, vessels as far as 600 μm below the dura were detected with both dyes. It is noticed that two-photon optimized Pluronic nanomicelles exhibit the same efficiency when compared to Rhodamin B Dextran in spite of a ten time less mass. Minor effects on the blood viscosity and hemodynamics are expected after injection of Pluronic nanomicelles (100μL containing 1 mg Pluronic) in comparison with Dextran dyes (100μL containing 10mg Dextrans) without modifying the observation depth.
Figure 3.
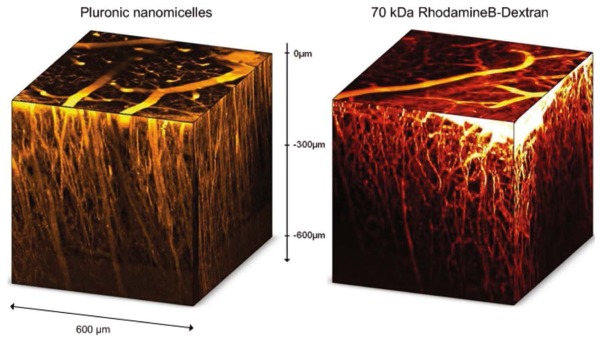
Reconstructed 3D data sets of z-stacks (600*600*600 μm) acquired below the dura in 120 steps of 5μm in the left parietal cortex of healthy Swiss white mice. Mice were injected with 100 μL of 100 g.L−1 Rhodamin B dextran or 100 μL of 10 g.L−1 Pluronic nanomicelles.
Ideally, blood dye concentration must be constant during the intravital microscopic studies which take approximately 1 hour. Therefore, stacks were acquired every ten minutes during 1 hour after injection of both dyes in different mice and the fluorescence intensity of specific vessels was quantified using ImageJ software (figure 4). For both dyes, it was noticed that the fluorescence intensity is rather stable: 90% of the initial signal remained after 1 hour. Thus no important dye accumulation or clearance occurred within 1 hour.
Figure 4.
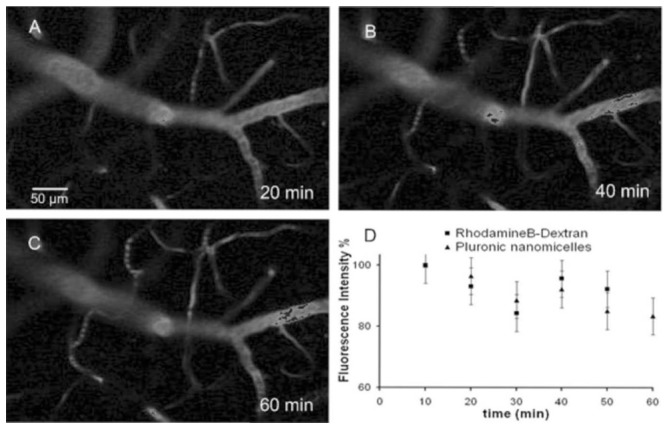
Images acquired at 150 μm bellow the dura at 20 (A), 40 (B) and 60 min (C) after 100 μL Pluronic nanomicelles (10 g.L−1) injection. Figure 4D, shows the decrease of the mean fluorescence signal intensity in all vessels (bars are SD) after injection of Pluronic nanomicelles or 70 kDa Rhodamin B dextran (100 g.L−1).
The stability of the Pluronic nanomicelles in blood was not investigated in details[14]. The stability of the Pluronic nanomicelles depends on many physico-chemical parameters that are unique for each encapsulated hydrophobic dye and Pluronic unimers. In the current study, our di-stryl benzene chromophore is known to aggregate in a hydrophobic environment. This favored π-π stacking and increased the two-photon absorption cross section[12]. This stacking of di-stryl benzene inside the hydrophobic core of the Pluronic nanomicelles might further increase their stability: a larger surface for hydrophobic interactions with the hydrophobic poly (propylene oxide) blocks. Binding of the aggregated di-stryl benzenes to hydrophobic components of the blood could not be excluded[14], but might be retarded several hours.
3.2. Relative cortical blood volume measurements
Relative cortical blood volume (rCBV) measurements have been performed with previous stacks after Pluronic nanomicelles or Rhodamin B dextran injection using for both dyes 3 mice. For the relative blood volume calculations, the slices were taken at 50 μm below the dura to suppress large superficial vessels of the subarachnoid space. An example of average Z-projection of a specific area is shown in figure 5.
Figure 5.
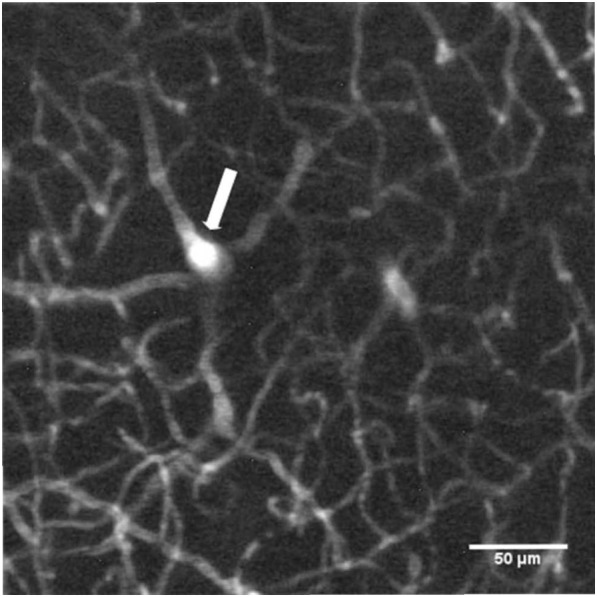
Z-projection of the average fluorescence intensity of 41 images acquired from 100 to 300 μm bellow the dura in the left parietal cortex of a Swiss white mouse after injection of 100 μL of Pluronic nanomicelles (10 g.L−1). The plunging vessel (white arrow) is crucial for the normalization of the light intensity per slice.
Using Pluronic nanomicelles, a rCBV of 2,4 ± 0,5 (standard deviation) % was found, which was in good agreement with Rhodamin B dextran results: rCBV = 2,3 ± 0,3 %. Those results, close to the rCBV literature values[15], demonstrate that our Pluronic nanomicelles are strictly plasmatic dyes.
3.3. Two photon imaging of the tumor vasculature
In most high grade tumors, the endothelium of new neo-vessels is often leaky for small molecules[16], which leads to an higher interstitial fluid pressure[17,18]. For proper local tumor blood volume measurements, the dye should stay in the vasculature and not diffuse across the leaky endothelium. In the present study, we tested if Pluronic nanomicelles could be used as blood pool dyes in tumors and compared their performance to the Rhodamin B dextran 70 kDa dye. In figure 6, both dyes were injected with a 30 minutes interval in a mouse bearing a dorsal skin fold chamber with human lung carcinoma. The largest Pluronic nanomicelles were injected first and during 30 minutes, no dye diffusion across the vascular endothelium into the extravascular space was observed: the background between the vessels remained black and showed a good contrast with the functional tumor vessels (figure 6A). In a previous study, Pluronic nanomicelles were found to stay inside the tumor vasculature until 4 hours after i.v. injection (results not shown).
Figure 6.
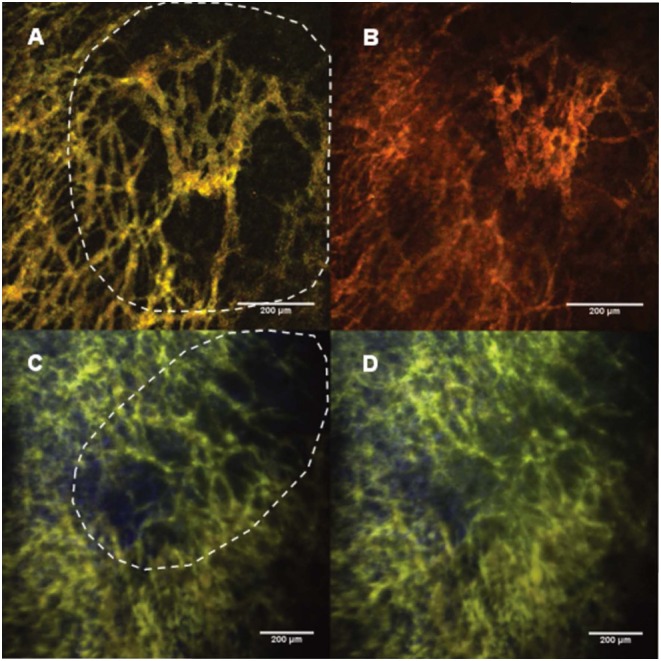
Images (size: 848.53 μm2) of the tumor vasculature in Swiss nude mice bearing human lung carcinoma in a dorsal skin-fold chamber. The tumor areas are indicated by the dashed lines and the length of the scale bares represent 200 μm. 6A: 30 min after 100 μL of Pluronic nanomicelles (10 g.L−1) injection. 6B: 30 min after a second injection of 100 μL of 70 kDa Rhodamin B dextrans (100 g.L−1). 6C: Z-projection of average intensities in a stack of 40 images (thickness 195 μm, image size: 1214.56 μm2) of the tumor vasculature in another human lung carcinoma, after 100 μL of Pluronic nanomicelles (10 g.L−1) injection at 1 minute after I.P. injection of a vascular disrupting agent (VDA) and 30 min after VDA treatment (6D).
Thirty minutes after the second injection of Rhodamin B dextran 70 kDa, the contrast between the vessels and the background got lost due to the diffusion of the rhodamin B dextran dyes into the extravascular space (figure 6B). This difference can be related to the important size of Pluronic nanomicelles (20 to 100 nm) in comparison to 70 kDa Rhodamin B dextran with a hydrodynamic radius of 6 nm. Even, the largest dextrans (200 kDa) with a hydrodynamic radius of 27 nm are known to diffuse across the leaky tumor blood vessels[16],[17]. Note that at this molecular size, the solubility of the dextran decreases (maximum concentration 10 mg/ml) in comparison to the 70 kDa dextran (maximum concentration 100 mg/ml), which results in approximately 10 times less dyes in the blood plasma.
The fast uptake of Pluronic nanomicelles could only be observed after treatment with a vascular disrupting agent that was known to drastically increase the vascular permeability (figure 6C–D)
4. Conclusions
In the present study, we have demonstrated that Pluronic nanomicelles can be used as blood pool dyes in intravital two-photon imaging of both the normal and the tumor vasculature. In the normal vasculature, the blood plasma staining is homogeneous and similar to the results obtained with the Rhodamin-B isothicyanate–Dextran at ten times less injected mass (1 mg Pluronic versus 10 mg 70 kDa grafted Dextran). In comparison to Dextran dyes, minor effects on the blood viscosity and hemodynamics are expected with Pluronic nanomicelles. In a human lung carcinoma, Pluronic nanomicelles stayed in the vasculature, whereas Rhodamin B dextran diffused across the leaky tumor vascular endothelium. Therefore, Pluronic nanomicelles can be used for local tumor blood volume measurements during tumor growth and follow up of anti-angiogenic therapies.
In general, the use of Pluronic block co-polymers is a valuable approach for encapsulating two-photon fluorescent dyes that are hydrophobic and not suitable for intravenous injection.
Acknowledgments
This work was supported by a grant from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche. The authors thank Patrice Baldeck for helpful discussion, Cyril Zenga and Aurelie Juhem for biotechnical assistance in two-photon imaging of the tumor vasculature.
References
- 1.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BD, Chen X, Lee TY. Serial changes in CT cerebral blood volume and flow after 4 hours of middle cerebral occlusion in an animal model of embolic cerebral ischemia. AJNR Am J Neuroradiol. 2007;28(4):743–749. [PMC free article] [PubMed] [Google Scholar]
- 3.Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32(2):332–348. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Tozer GM, Ameer-Beg SM, Baker J, Barber PR, Hill SA, Hodgkiss RJ, Locke R, Prise VE, Wilson I, Vojnovic B. Intravital imaging of tumour vascular networks using multi-photon fluorescence microscopy. Advanced Drug Delivery Reviews. 2005;57(1):135–152. doi: 10.1016/j.addr.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Verant P, Serduc R, Van Der Sanden B, Remy C, Vial JC. A direct method for measuring mouse capillary cortical blood volume using multiphoton laser scanning microscopy. J Cereb Blood Flow Metab. 2007;27(5):1072–1081. doi: 10.1038/sj.jcbfm.9600415. [DOI] [PubMed] [Google Scholar]
- 6.Andrieux K, Lesieur P, Lesieur S, Ollivon M, Grabielle-Madelmont C. Characterization of fluorescein isothiocyanate-dextrans used in vesicle permeability studies. Anal Chem. 2002;74(20):5217–5226. doi: 10.1021/ac020119l. [DOI] [PubMed] [Google Scholar]
- 7.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300(5624):1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 8.Krishna TR, Parent M, Werts MHV, Moreaux L, Gmouh S, Charpak S, Caminade AM, Majoral JP, Blanchard-Desce M. Water-soluble dendrimeric two-photon tracers for in vivo imaging. Angewandte Chemie-International Edition. 2006;45(28):4645–4648. doi: 10.1002/anie.200601246. [DOI] [PubMed] [Google Scholar]
- 9.Lebret V, Raehm L, Durand JO, Smaihi M, Gerardin C, Nerambourg N, Werts MHV, Blanchard-Desce M. Synthesis and characterization of fluorescently doped mesoporous nanoparticles for two-photon excitation. Chemistry of Materials. 2008;20(6):2174–2183. [Google Scholar]
- 10.Horn D, Rieger J. Organic nanoparticles in the aqueous phase - theory, experiment, and use. Angewandte Chemie-International Edition. 2001;40(23):4331–4361. doi: 10.1002/1521-3773(20011203)40:23<4330::aid-anie4330>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Maurin M, Vurth L, Vial JC, Baldeck P, Marder SR, Van der Sanden B, Stephan O. Fluorescent Pluronic nanodots for in vivo two-photon imaging. Nanotechnology. 2009;20(23):235102. doi: 10.1088/0957-4484/20/23/235102. [DOI] [PubMed] [Google Scholar]
- 12.Maurin M, Vurth L, Vial JC, Baldeck P, Marder SR, Van der Sanden B, Stéphan O. Pluronic Organic Fluorescent Probes for Two-photon Vascularisation Imaging. Nonlinear Optics, Quantum Optics: Concepts in Modern Optics. 2010;40(1–4):175–181. [Google Scholar]
- 13.Pond SJK, Rumi M, Levin MD, Parker TC, Beljonne D, Day MW, Bredas J-L, Marder SR, Perry JW. One- and two-photon spectroscopy of donor-acceptor-donor distyrylbenzene derivatives: effect of cyano substitution and distortion from planarity. J Phys Chem A. 2002;106(47):11470–11480. [Google Scholar]
- 14.Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo forster resonance energy transfer imaging. Langmuir. 2008;24(10):5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- 15.Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29(46):14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133(1):95–109. [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 18.Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52(23):6553–6560. [PubMed] [Google Scholar]