Abstract
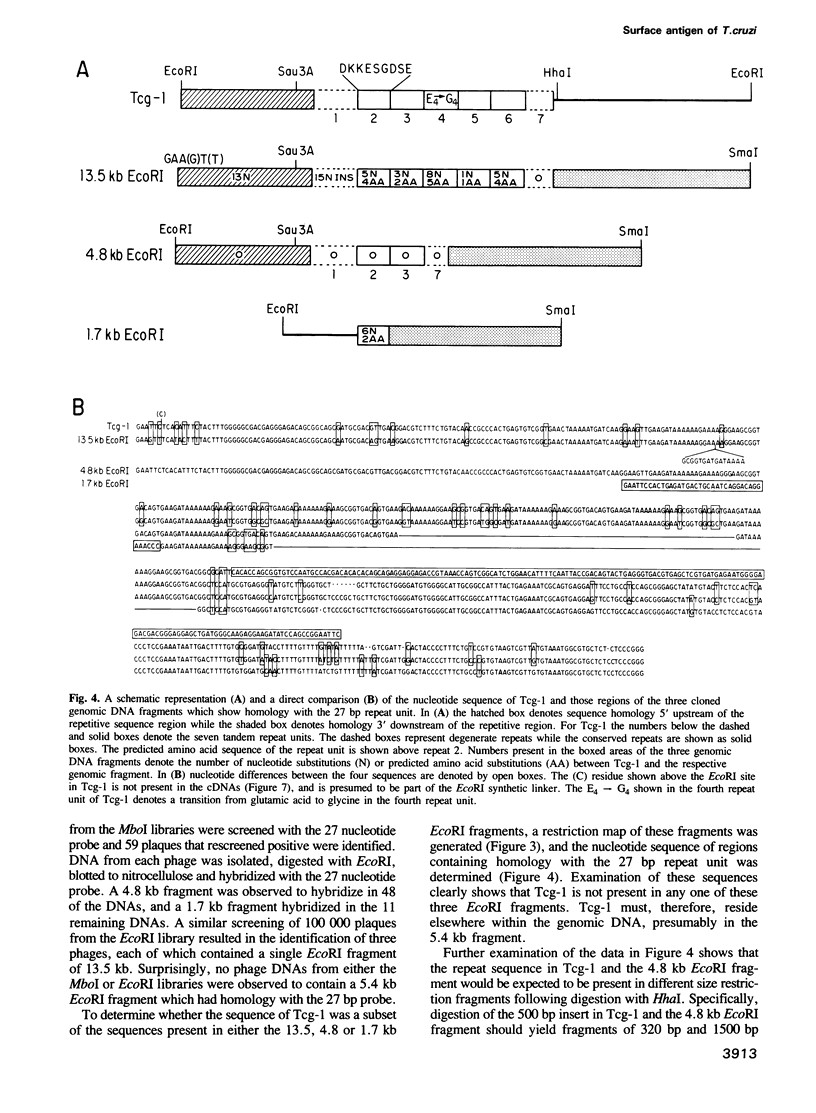
In previous studies we identified a 500 bp DNA fragment from the genome of Trypanosoma cruzi which encoded epitopes present in an 85 kd trypomastigote-specific surface antigen. A unique feature of this DNA insert was the presence of a 27 bp tandem repeat unit within the putative coding region. The findings presented here show that the gene which encodes this particular surface protein is a member of a multigene family, and that the 27 bp repeat unit defines a subset of this family. Only four separate members of the family contain sequences homologous to the 27 bp repeat unit. Of these, three have been cloned and shown by direct nucleotide sequence analysis not to contain the original 500 bp fragment. By restriction enzyme analysis, the 500 bp fragment is inferred to be present in a 5.4 kb EcoRI genomic DNA fragment that is refractory to isolation by standard cloning procedures. Preferential sensitivity of this fragment to digestion with Bal31 nuclease indicates that it is likely to be telomeric, thus explaining the inability to obtain it in several different recombinant DNA libraries. In order to determine which of the four members were transcribed, 26 cDNA recombinants having sequence homology with the 27 bp repeat were examined. Restriction enzyme maps and nucleotide sequence analysis of these cDNAs indicate that transcription occurs almost exclusively from the telomeric member of the family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Robbins E. S., Ley V., Hong K. S., Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988 Feb 1;167(2):300–314. doi: 10.1084/jem.167.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Characterization of stages and strains of Trypanosoma cruzi by analysis of cell membrane components. J Immunol. 1981 Sep;127(3):855–859. [PubMed] [Google Scholar]
- Beard C. A., Wrightsman R. A., Manning J. E. Identification of monoclonal antibodies against the trypomastigote stage of Trypanosoma cruzi by use of iminobiotinylated surface polypeptides. Mol Biochem Parasitol. 1985 Aug;16(2):199–212. doi: 10.1016/0166-6851(85)90087-8. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Fouts D. L., Manning J. E., Fox G. M., Schmid C. W. A complex repeated DNA sequence within the Drosophila transposable element copia. Nucleic Acids Res. 1981 Dec 21;9(24):7053–7064. doi: 10.1093/nar/9.24.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanar D. E., Levy L. S., Manning J. E. Complexity and content of the DNA and RNA in Trypanosoma cruzi. Mol Biochem Parasitol. 1981 Sep;3(5):327–341. doi: 10.1016/0166-6851(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Lanar D. E., Manning J. E. Major surface proteins and antigens on the different in vivo and in vitro forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1984 Apr;11:119–131. doi: 10.1016/0166-6851(84)90059-8. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Tydings J. D., Unkeless J., Cohn Z. Trypanosoma cruzi. Surface antigens of blood and culture forms. J Exp Med. 1981 Mar 1;153(3):629–639. doi: 10.1084/jem.153.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Newport G., Milhausen M., Stuart K., Agabian N. Genomic organization of Trypanosoma brucei variant antigen gene families in sequential parasitemias. Mol Biochem Parasitol. 1983 Nov;9(3):255–269. doi: 10.1016/0166-6851(83)90101-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Wrightsman R. A., Manning J. E. Cloning of a major surface-antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature. 1986 Aug 7;322(6079):566–568. doi: 10.1038/322566a0. [DOI] [PubMed] [Google Scholar]
- Snary D., Hudson L. Trypanosoma cruzi cell surface proteins: identification of one major glycoprotein. FEBS Lett. 1979 Apr 1;100(1):166–170. doi: 10.1016/0014-5793(79)81156-4. [DOI] [PubMed] [Google Scholar]
- Snary D. Trypanosoma cruzi: antigenic invariance of the cell surface glycoprotein. Exp Parasitol. 1980 Feb;49(1):68–77. doi: 10.1016/0014-4894(80)90057-0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Valerio D., De Lange T., Bernards A., Borst P., Grosveld F. G. An analysis of cosmid clones of nuclear DNA from Trypanosoma brucei shows that the genes for variant surface glycoproteins are clustered in the genome. Nucleic Acids Res. 1982 Oct 11;10(19):5905–5923. doi: 10.1093/nar/10.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrightsman R. A., Leon W., Manning J. E. Variation in antigenic determinants specific to the infective stage of Trypanosoma cruzi. Infect Immun. 1986 Aug;53(2):235–239. doi: 10.1128/iai.53.2.235-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Andrews N. W., Kuwajima V. Y., Colli W. Cell surface antigens of Trypanosoma cruzi: possible correlation with the interiorization process in mammalian cells. Mol Biochem Parasitol. 1982 Aug;6(2):111–124. doi: 10.1016/0166-6851(82)90069-x. [DOI] [PubMed] [Google Scholar]