Abstract
Rationale
Apocynin suppresses the generation of reactive oxygen species (ROS) that are implicated in ventilator-induced lung injury (VILI). We thus hypothesized that apocynin attenuates VILI.
Methods
VILI was induced by mechanical ventilation with tidal volume (Vt) of 15 ml/kg in isolated and perfused rat lung. Apocynin was administered in the perfusate at onset of mechanical ventilation. A group ventilated with low Vt of 5 ml/kg served as control. Hemodynamics, lung injury indices, inflammatory responses, and activation of apoptotic pathways were determined upon completion of mechanical ventilation.
Results
There was an increase in lung permeability and lung weight gain after mechanical ventilation with high Vt, compared with low Vt. Levels of inflammatory cytokines including interleukin-1b (IL-1b), tumor necrosis factor-alpha (TNF-a), and macrophage inflammatory protein-2 (MIP-2) increased in lung lavage fluids; concentrations of carbonyl, thiobarbituric acid reactive substances, and H2O2 were higher in perfusates and lung lavage fluids, and expression of myeloperoxidase, JNK, p38, and caspase-3 in lung tissue was greater in the high-Vt than in the low-Vt group. Administration of apocynin attenuated these inflammatory responses and lung permeability associated with decreased activation of nuclear factor-κB.
Conclusions
VILI is associated with inflammatory responses including generation of ROS, cytokines, and activation of mitogen-activated protein kinase cascades. Administration of apocynin at onset of mechanical ventilation attenuates inflammatory responses and VILI in the isolated, perfused rat lung model.
Keywords: ARDS, Inflammation, NADPH, MAPK, NF-κB
Introduction
Inappropriate mechanical ventilation can initiate or exacerbate lung injury leading to ventilator-induced lung injury (VILI), as a result of volutrauma [1] and biotrauma [2]. Animal studies demonstrated that mechanical ventilation with high tidal volume (Vt) led to increased neutrophil infiltration [3] and production of proinflammatory cytokines in otherwise healthy lung [4], indicative of a link between volutrauma and biotrauma. In patients with acute respiratory distress syndrome (ARDS), application of low-Vt ventilatory strategies has been shown to decrease mortality [5]. However, low-Vt strategies may not meet the need in all patients, and additional pharmacological therapy may be required to target ongoing inflammatory responses in ARDS.
Lung epithelial cells have been shown to increase nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in response to cyclic mechanical stretch resulting in production of superoxide (O2−) [6]. Reactive oxygen species (ROS) can activate mitogen-activated protein kinase (MAPK) cascades and lead to excessive production of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) [6–8] leading to epithelial drop off or cell death in the context of VILI [9]. In a mouse model [10], early increase in phosphorylation of p38 and extracellular signal regulated kinase (ERK) has been observed in response to mechanical ventilation with no significant change in activation of c-Jun NH2-terminal kinases (JNK). In isolated, nonperfused mouse lung [7], activation of p38, ERK, and JNK has been reported after mechanical ventilation. Taken together, the data suggest that NADPH oxidase may play an important role in the pathogenesis of VILI but may be overlooked as a therapeutic target in the lung research field.
Apocynin (4-hydroxy-3-methoxy-acetophenone), a naturally occurring methoxy-substituted catechol, inhibits NADPH oxidase in activated leukocytes, preventing generation of ROS [11]. Several in vivo studies have shown that apocynin can reduce neutrophil oxidative burst and neutrophil chemotaxis and thus attenuate neutrophil-mediated cell damage [12]. Apocynin has also been shown to decrease monocyte–endothelium interaction in vitro upon stimulation with TNF-α [13]. We hypothesized that apocynin attenuates VILI by inhibition of ROS generation and inflammatory responses.
Methods
Animal preparation
The study protocol was approved by the Institutional Board for Animal Care and Use. The in situ isolated, perfused lung model has been previously described [14]. Briefly, male Sprague–Dawley rats (SD rat) weighing 250–350 g were anesthetized with intraperitoneal injection of sodium pentobarbital. Tracheotomy was performed, and mechanical ventilation was applied (Rodent ventilator model 683; Harvard Apparatus, South Natick, MA, USA) at tidal volume (Vt) of 5 ml/kg and positive end-expiratory pressure (PEEP) of 2 cmH2O. After sternotomy, heparin (1 unit/g) was injected into the right ventricle, through which pulmonary artery was catheterized. The left atrium was cannulated with catheter entering through apex of the heart. The pulmonary venous outflow was diverted into a reservoir. To prevent flow back into the ventricles, an additional ligation was performed above the atrioventricular junction. The lungs were perfused (Minipulse 2; Gilson Medical Electronic, Middleton, WI, USA) at constant flow of 30 μl/min/g body weight. Pulmonary artery pressure (Ppa) and pulmonary venous pressure (Ppv) were monitored. Rat weight was determined to reflect lung weight in the in situ system. Pulmonary arterial resistance (Ra) and venous resistance (Rv) were calculated using the following equations: Ra = (Ppa − Ppc)/Q, and Rv = (Ppc − Ppv)/Q, where Q is perfusate flow, Ppc is pulmonary capillary pressure.
Experimental protocols
The isolated lungs were randomly divided into three groups and ventilated for 2 h with either low Vt of 5 ml/kg (LVt), high Vt of 15 ml/kg (HVt) or high Vt in combination with treatment by apocynin (HVt + Apo). PEEP of 2 cmH2O was applied in all groups. Apocynin (Biomol, USA) was administered at 0.01, 0.1 or 0.2 mM/l in total volume of 40 ml circulating perfusate at onset of mechanical ventilation. Vascular permeability was determined by measuring pulmonary capillary filtration coefficient (Kfc) as previously described [15–17].
Myeloperoxidase assay
Concentration of myeloperoxidase (MPO), an index of neutrophil sequestration in the lungs, was measured as previously described [18].
Carbonyl and thiobarbituric acid reactive substances (TBARS) assay
Protein carbonyl content was measured by protein carbonyl assays (Geneteks Biosciences, Inc., San-Chong City, Taipei). TBARS level in serum was measured using OxiSelectTM TBARS assay kit (Geneteks Biosciences, Inc.).
H2O2 assay
Perfusate was centrifuged at 1,000 9 g within 30 min, the supernatant was collected, and 50 μl H2O2 reaction mix containing 46 μl assay buffer, 2 μl OxiRedTM probe solution, and 2 μl HRP solution (BioVision, USA) was added for incubation for 10 min. Absorbance was read at 570 nm (SpectraMax M5; Molecular Devices, USA). Concentration was calculated based on H2O2 standard curves.
Cytokines assays
Levels of interleukin-1β (IL-1β), TNF-α and macrophage inflammatory protein 2 (MIP-2) in lavage fluids were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Oxon, UK). Absorbance was read at 450 nm (SpectraMax M5; Molecular Devices, USA).
Western blotting analysis
Lung tissues were homogenized using lysis buffer containing protease inhibitor cocktail (Roche, USA) and phosphatase inhibitor cocktail (Roche, USA). Total protein extracts were separated on 10% sodium dodecyl sulfate polyacrylamide gel, and electrotransferred onto polyvinylidine fluoride (PVDF) membrane (Millipore, USA). The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) for 1 h. Antibodies against phosphop44/42 MAPK (ERK1/2), phospho-p44/42 MAPK (ERK1/2), phospho-stress-activated protein kinases (SAPK)/JNK, phospho-p38 MAPK, anti-p44/42 MAPK (ERK1/2), anti-SAPK/JNK, and anti-p38 MAP kinase (1:1,000; Cell Signaling Technology, Beverly, MA, USA) were used. Antibodies against glyceraldehyde 3-phosphate dehydrogenase (GADPH, 1:10,000; Lab Frontier), JNK1 (1:1,000; Santa Cruz Biotechnology), and caspase- 3 (1:2,000; Cell Signaling Technology) were used. The appropriate secondary antibodies were used (1:10,000 horseradish peroxidase antirabbit; Jackson Immuno Research Laboratories). Visualization was performed by enhanced chemiluminescence (Visual Protein Biotechnology Corp., Taiwan). Protein bands were quantified using Kodak 1D image analysis (Eastman Kodak Company, Rochester, NY, USA).
Lung histopathology
Upon completion of experiments, the lungs were dissected and fixed immediately in 10% neutral buffered formalin. The right middle lobes were dehydrated through a graded series of alcohol, cleared in xylene, embedded in paraffin, and stained with hematoxylin/eosin. Three slides from each animal were evaluated for a total of six rats per group. Lung injury score was scaled from 1 to 4 based on our previous study [19]. The score includes perivascular edema 1, peribronchial edema 2, interstitial edema 2, alveolar edema 3, perivascular cell infiltration 2, interstitial cell infiltration 3, and alveolar cell infiltration 4. The lung injury score was evaluated by two pathologists who were blinded to the experimental conditions.
Immunohistochemistry
Lung slides coated with poly-L-lysine (Sigma, St. Louis, MO, USA) were deparaffinized and rehydrated using xylene and ethanol, and placed in 3% H2O2 for 15 min. The slides were incubated with 1:60 dilution of monoclonal nuclear factor kappa-light-chain-enhancer of activated B cells (NF)-κB (Cell Signaling Technology, Beverly, MA, USA), incubated at 4_C overnight, and stained with diaminobenzidine (DAB) (Dako, USA) and Mayer’s hematoxylin (Dako). Analysis was performed under Eclipse 80i microscope (Nikon, Japan) using Image Pro Plus 5.0 (Media Cybernetics, USA). Cells with positive nuclear NF-κB staining were counted out of a total of 100 cells in each slide from three animals in each group of LVt, HVt or HVt + Apo, respectively. Cell counting was performed by a pathologist who was blinded to the experimental conditions.
Statistical analysis
Systat10.0 (Systat Software Inc., San Jose, CA, USA) was used for statistical analysis. Comparisons among all groups were conducted using two-way analysis of variance (ANOVA) for repeated measurements. Comparison between baseline and post-VILI values within group was conducted using Student’s paired t test. Values are expressed as mean ± standard deviation (SD). P<0.05 was considered statistically significant.
Results
VILI model
There was no significant difference in hemodynamics among the groups at baseline and at the end of study (Supplementary Table 1).
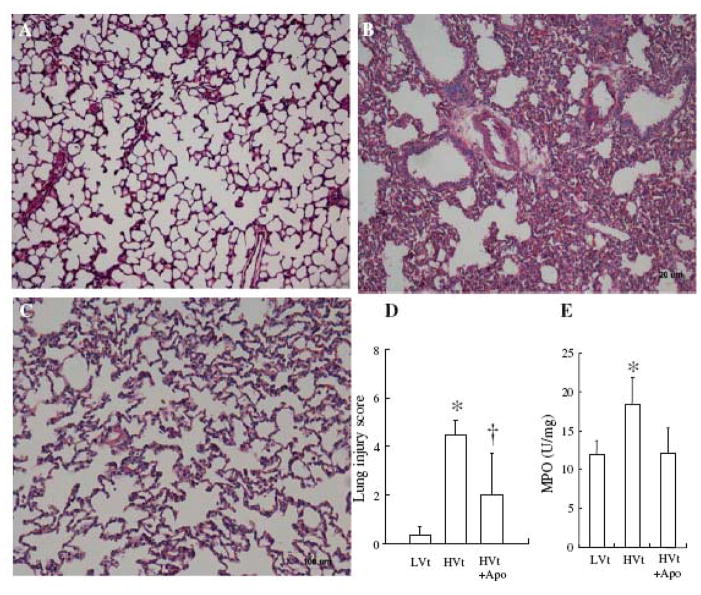
Lung weight gain was higher in the HVt than in the LVt group, which was decreased in the presence of apocynin (Table 1). Lung weight gain was in agreement with increased lung Kfc in the HVt group (Table 1). The increased permeability was further confirmed by the histological analysis showing perivascular edema and intra-alveolar hemorrhage in the HVt group compared with the LVt group. The apocynin-treated group showed decreased histological alterations (Fig. 1a–c) and lung injury score (Fig. 1d).
Table 1.
LWG and Kfc.
| Group | N | LWG (g) | Kfc (cmH2O/min/mL) | |
|---|---|---|---|---|
| Baseline | After injury | |||
| Control | 6 | 0.09 ± 0.06 | 0.17 ± 0.07 | 0.19 ± 0.07 |
| VILI | 6 | 1.37 ± 0.59* | 0.20 ± 0.05 | 0.37 ± 0.04* |
| VILI + apocynin | 6 | 0.79 ± 0.46*# | 0.20 ± 0.04 | 0.24 ± 0.05# |
Values are mean ± SD. LWG lung weigh gain, Kfc pulmonary capillary filtration coefficient.
p < 0.05 compared with control;
p< 0.05 compared with VILI.
Figure 1.
Apocynin improved lung histology. A. Animals received LVt B. Animals were ventilated with HVt. Lung histology was characterized by perivascular edema, interstitial and intra-alveolar leukocyte infiltration, and marked heterogeneity in alveolar inflation. C. Treatment with apocynin improved lung histology. D. Treatment with apocynin decreased lung injury score. E. Treatment with apocynin decreased MPO concentration in lung lavage fluids under mechanical ventilation with HVt. *p< 0.05 for HVt versus LVt; +p < 0.05 for HVt versus HVt + Apo, at indentical condition, respectively.
Inflammatory responses
Concentration of MPO was measured to reflect pulmonary neutrophil infiltration. The level of MPO in lung lavage fluids increased in the HVt group compared with the LVt group (Fig. 1e). This is in agreement with the lung histology analysis showing interstitial and intraalveolar leukocytic infiltrates and proteinaceous exudates in the HVt group (Fig. 1b). Treatment with apocynin decreased concentration of MPO (Fig. 1e) and improved lung histology (Fig. 1c).
A maximal level of carbonyl, TBARS, and H2O2 was noted in perfusates (Supplementary Fig. 1A–C) and lung lavage fluids (Fig. 2a–c) 30 min after onset of mechanical ventilation, and their values were higher in the HVt group than in the LVt group. The levels of MIP-2, IL-1β, and TNF-α in lavage fluids were higher in the HVt group than in the LVt group (Supplementary Fig. 1D–F). Administration of apocynin largely attenuated the oxidative stress and cytokine responses in dose-dependent manner at doses of 0.01 and 0.1 mM/l (Fig. 2 and Supplementary Fig. 1). A higher dose of apocynin at 0.2 mM/l did not produce any further effects as compared with the dose used at 0.1 mM/l (Fig. 2 and Supplementary Fig. 1).
Figure 2.
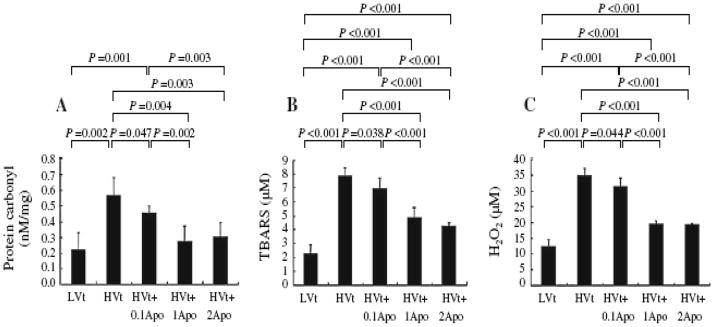
Apocynin dose-dependently decreased oxidative stress. Concentrations of carbonyl, TBARS, and H2O2 were measured in lung lavage fluids (a–c) at the end of study.
MAPK, apoptotic, and NF-κB signaling pathways
Phosphorylation of JNK and P-38 but not ERK (data not shown) increased in lung tissue after high-Vt mechanical ventilation (MV) (Fig. 3a, b). Protein expression of apoptotic caspase-3 in the lung was higher in the HVt than in the LVt group (Fig. 3c). Administration of apocynin attenuated the MAPK and apoptotic signaling pathways (Fig. 3a–c).
Figure 3.
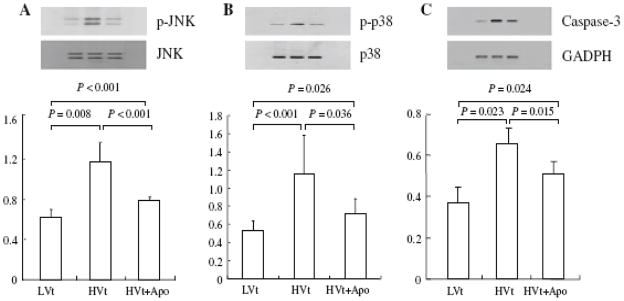
Apocynin attenuated activation of JNK, p-38 and caspase-3 expression in lung. Lung tissue was homogenized for Western blot by using appropriate antibodies.
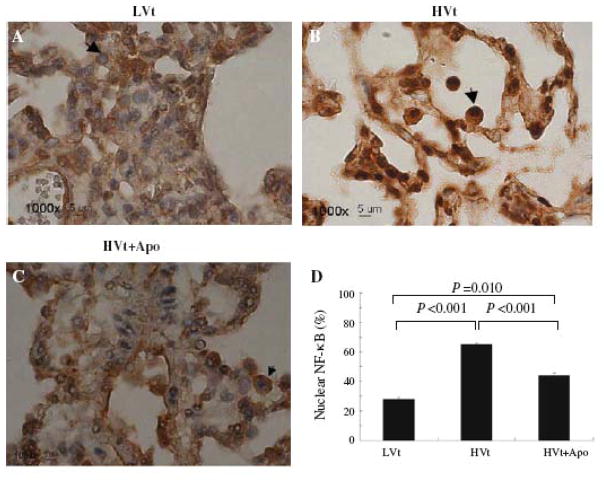
Immune staining of nuclear NF-κB increased in lung tissue after mechanical ventilation with HVt compared with LVt (Fig. 4a, b). Administration of apocynin decreased the NF-κB activation seen in the HVt group (Fig. 4c). The quantitative data of cells with positive nuclear NF-κB staining are reported in Fig. 4d.
Figure 4.
Apocynin decreased NF-κB activation. Nuclear staining of NF-κB in lung tissue after mechanical ventilation with LVt (a) and HVt in the absence (b) and presence of apocynin (c), Arrows indicate NF-κB staining. High power (x100) in lower panel. d. Percentage of cells with positive nuclear staining for NF-κB.
Discussion
A major finding of the present study is that mechanical ventilation with high Vt increased generation of ROS, cytokine responses, and activation of MAPK and NF-κB associated with lung injury. Administration of apocynin depressed oxidative stress, attenuated inflammatory responses, and reduced VILI.
Our VILI model reproduces many features reported in other studies [1–4, 14], including inflammatory responses and structural lung damage. Our results are in agreement with those previously reported showing an inability of endogenous host defenses to increase antioxidant capacity, leading to failure of lung repair mechanism [20]. It is interesting that treatment with apocynin at onset of mechanical ventilation showed dramatic effects in attenuation of VILI. Our study suggests that the oxidative stress implicated in mechanical ventilation is a significant therapeutic target in the context of VILI.
Apocynin is a strong oxidative inhibitor that has been demonstrated to block NADPH oxidase in neutrophils [21, 22], macrophages [22, 23], and endothelium [24] through inhibition of p47phox translocation [25], without interfering with other immune biological functions of cell systems. This is an important therapeutic mechanism of apocynin, as NADPH plays a crucial role in the complex cell–cell interaction during oxidative status [26]. We demonstrate that treatment with the antioxidant apocynin dramatically attenuated inflammatory responses including cytokines and MAPK activation. Although it is difficult to know the exact timing for the generation of cytokines and ROS in the in vivo model, it has been demonstrated that cytokine stimulation leads to accumulation of ROS, which is essential for prolonged MAPK activation and cell death. In turn, ROS generation can stimulate host cytokine production [27]. Thus, the observed decrease in cytokine responses, and reduced activation of MAPK and NF-κB, could be interrelated after the treatment with apocynin.
Apocynin is not well studied as a therapeutic approach in the context of VILI despite the fact that apocynin has been used to decrease TNF-a production and to reduce pulmonary artery endothelial cell apoptosis in endotoxemic mice [28, 29]. Use of apocynin blocks MAPK signal transduction and attenuates apoptotic cell death by blocking intrinsic and extrinsic signaling pathways during ischemia and reperfusion injury [30–37]. Several studies [21, 38, 39] have shown organ protective effects of apocynin by inhibition of cyclooxygenase metabolites in a variety of animal models. Cyclooxygenase byproducts such as thromboxane B2 play an important role to increase pulmonary vascular permeability and to induce pulmonary hypertension. We believe that this is the first report showing beneficial effects of apocynin in the context of VILI.
There are a couple of limitations to the study. We employed an isolated perfused lung model to minimize any hemodynamic effects on lung injury, but the model does not provide interactions with other organ system. Although our approach is relevant clinically, as apocynin was administered at onset of mechanical ventilation to maximize protective effects, late treatment with apocynin is warranted for future studies, as VILI is long established in some clinical situations.
In summary, our results suggest that ROS play an important role in the context of VILI. Application of apocynin can effectively attenuate VILI by inhibition of inflammatory responses associated with depressed oxidative stress in the isolated, perfused lung model.
Supplementary Material
Acknowledgments
This work was supported by grants from Taipei Veterans General Hospital (VGH 95-C1-020and VGH98C-015) and the National Science Council (NSC 95-2314-B-075-008, NSC96-2314-B-075-006), Taiwan, and Canadian Institutes of Health Research (MOP77818 and MOP69042). This work was assisted in part by the Division of Experimental Surgery of the Department of Surgery, Pathology and Statistic, Taipei Veterans General Hospital, Taiwan
Contributor Information
Chi-Huei Chiang, Institute of Emergency and Critical Care Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan. Division of Pulmonary Immunology and Infectious Diseases, Chest Department, Taipei Veterans General Hospital, No. 201, Section 2, Shih-Pai Road, Taipei, Taiwan, Tel.: +886-2-28757713, Fax: +886-2-28765323.
Chiao-Hui Chuang, Division of Pulmonary Immunology and Infectious Diseases, Chest Department, Taipei Veterans General Hospital, No. 201, Section 2, Shih-Pai Road, Taipei, Taiwan, Tel.: +886-2-28757713, Fax: +886-2-28765323.
Shiou-Ling Liu, Division of Pulmonary Immunology and Infectious Diseases, Chest Department, Taipei Veterans General Hospital, No. 201, Section 2, Shih-Pai Road, Taipei, Taiwan, Tel.: +886-2-28757713, Fax: +886-2-28765323.
Tzong-Shyuan Lee, Department and Institute of Physiology, School of Medicine, National Yang-Ming University, Taipei, Taiwan.
Yu Ru Kou, Department and Institute of Physiology, School of Medicine, National Yang-Ming University, Taipei, Taiwan.
Haibo Zhang, The Keenan Research Centre in the Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Toronto, Canada, Department of Anaesthesia, University of Toronto, Toronto, ON, Canada, Department of Physiology, University of Toronto, Toronto, ON, Canada, Interdepartmental Division of Critical Care Medicine, University of Toronto, Toronto, ON, Canada.
References
- 1.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 2.dos Santos CC, Slutsky AS. The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol. 2006;68:585–618. doi: 10.1146/annurev.physiol.68.072304.113443. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury S, Wilson MR, Goddard ME, O’Dea KP, Takata M. Mechanisms of early pulmonary neutrophil sequestration in ventilator induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L902–L910. doi: 10.1152/ajplung.00187.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- 5.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T. Pulmonary atelectasis during low stretch ventilation: “open lung” versus “lung rest” strategy. Crit Care Med. 2009;37:1046–1053. doi: 10.1097/CCM.0b013e3181968e7e. [DOI] [PubMed] [Google Scholar]
- 8.Haddad JJ, Land SC. Redox/ROS regulation of lipopolysaccharide induced mitogen-activated protein kinase (MAPK) activation and MAPKmediated TNF-alpha biosynthesis. Br J Pharmacol. 2002;135:520–536. doi: 10.1038/sj.bjp.0704467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Caro LJL, Marín-Corral J, Sánchez-Rodríguez C, Sánchez-Ferrer A, Nin N, Ferruelo A, de Paula M, Fernández-Segoviano P, Barreiro E, Esteban A. Role of free radicals in vascular dysfunction induced by high tidal volume ventilation. Intensive Care Med. 2009;35:1110–1119. doi: 10.1007/s00134-009-1469-5. [DOI] [PubMed] [Google Scholar]
- 10.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE, 3rd, Kayyali US, Garcia JG. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol. 2006;291:L345–L353. doi: 10.1152/ajplung.00453.2005. [DOI] [PubMed] [Google Scholar]
- 11.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediat Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Paola RD, Bramanti P, Cuzzocrea S. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 2011;81:636–648. doi: 10.1016/j.bcp.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial proatherogenic and pro-inflammatory genes. J Membr Biol. 2005;206:103–116. doi: 10.1007/s00232-005-0783-2. [DOI] [PubMed] [Google Scholar]
- 14.Chiang CH, Pai HI, Liu SL. Ventilator-induced lung injury (VILI) promotes ischemia/reperfusion lung injury (I/R) and NF-kappaB antibody attenuates both injuries. Resuscitation. 2008;79:147–154. doi: 10.1016/j.resuscitation.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Drake R, Gaar KA, Taylor AE. Estimation of the filtration coefficient of pulmonary exchange vessels. Am J Physiol. 1978;234:H266–H274. doi: 10.1152/ajpheart.1978.234.3.H266. [DOI] [PubMed] [Google Scholar]
- 16.Chiang CH, Hsu K, Yan HC, Harn HJ, Chang DM. PGE1, dexamethasone, U-74389G, or Bt2- cAMP as an additive to promote protection by UW solution in I/R injury. J Appl Physiol. 1997;83:583–590. doi: 10.1152/jappl.1997.83.2.583. [DOI] [PubMed] [Google Scholar]
- 17.Chang DM, Hsu K, Ding YA, Chiang CH. Interleukin-1 in ischemia reperfusion acute lung injury. Am J Respir Crit Care Med. 1997;156:1230–1234. doi: 10.1164/ajrccm.156.4.9702095. [DOI] [PubMed] [Google Scholar]
- 18.Lu HL, Chiang CH. Combined therapy of pentastarch, dexamethasone, and dibutyryl-cAMP or beta 2-agonist attenuates ischaemia/reperfusion injury of rat lung. Injury. 2008;39:1062–1070. doi: 10.1016/j.injury.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Yamanel L, Kaldirim U, Oztas Y, Coskun O, Poyrazoglu Y, Durusu M, Cayci T, Ozturk A, Demirbas S, Yasar M, Cinar O, Tuncer SK, Eyi YE, Uysal B, Topal T, Oter S, Korkmaz A. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011;8:48–55. doi: 10.7150/ijms.8.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozar RA, Weibel CJ, Cipolla J, Klein AJ, Haber MM, Abedin MZ, Trooskin SZ. Antioxidant enzymes are induced during recovery from acute lung injury. Crit Care Med. 2000;28:2486–2491. doi: 10.1097/00003246-200007000-00050. [DOI] [PubMed] [Google Scholar]
- 21.Dodd OJ, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 22.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 23.Muijsers RB, van Den Worm E, Folkerts G, Beukelman CJ, Koster AS, Postma DS, Nijkamp FP. Apocynin inhibits peroxynitrite formation by murine macrophages. Br J Pharmacol. 2000;130:932–936. doi: 10.1038/sj.bjp.0703401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson DK, Schillinger KJ, Kwait DM, Hughes CV, McNamara EJ, Ishmael F, O’Donnell RW, Chang MM, Hogg MG, Dordick JS. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 25.Meyer JW, Holland JA, Ziegler LM, Chang MM, Beebe G, Schmitt ME. Identification of a functional leukocyte-type NADPH oxidase in human endothelial cells: a potential atherogenic source of reactive oxygen species. Endothelium. 1999;7:11–22. doi: 10.3109/10623329909165308. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM, Schiffrin EL. Reactive oxygen species and hypertension: a complex association. Antioxid Redox Signal. 2008;10:1041–1044. doi: 10.1089/ars.2007.2012. [DOI] [PubMed] [Google Scholar]
- 27.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Shan L, Peng T. Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J Mol Cell Cardiol. 2009;47:264–274. doi: 10.1016/j.yjmcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Sampath V, Radish AC, Eis AL, Broniowska K, Hogg N, Konduri GG. Attenuation of lipopolysaccharide-induced oxidative stress and apoptosis in fetal pulmonary artery endothelial cells by hypoxia. Free Radic Biol Med. 2009;46:663–671. doi: 10.1016/j.freeradbiomed.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu C, Bilali A, Georgieva GS, Kurata S, Mitaka C, Imai T. Salvage of nonischemic control lung from injury by unilateral ischemic lung with apocynin, a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, in isolated perfused rat lung. Translational Res. 2008;152:273–282. doi: 10.1016/j.trsl.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Sharma Ak, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14:2832–2837. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Sheibani N. PECAM-1 isoform-specific activation of MAPK/ERKs and small GTPases: implications in inflammation and angiogenesis. J Cell Biochem. 2006;98:451–468. doi: 10.1002/jcb.20827. [DOI] [PubMed] [Google Scholar]
- 34.Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A, Choi AM. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS ONE. 2008;3:e1601. doi: 10.1371/journal.pone.0001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.So KS, Oh JE, Han JH, Jung HK, Lee YS, Kim SH, Chun YJ, Kim MY. Induction of apoptosis by a stilbene analog involves Bax translocation regulated by p38 MAPK and Akt. Arch Pharm Res. 2008;31:438–444. doi: 10.1007/s12272-001-1176-7. [DOI] [PubMed] [Google Scholar]
- 36.Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 37.Mates JM, Segura JA, Alonso FJ, Marquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med. 1994;150:1449–1452. doi: 10.1164/ajrccm.150.5.7952574. [DOI] [PubMed] [Google Scholar]
- 39.Lomnitski L, Nyska A, Ben-Shaul V, Maronpot RR, Haseman JK, Harrus TL, Bergman M, Grossman S. Effects of antioxidants apocynin and the natural water-soluble antioxidant from spinach on cellular damage induced by lipopolysaccharide in the rat. Toxicol Pathol. 2000;28:580–587. doi: 10.1177/019262330002800412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.