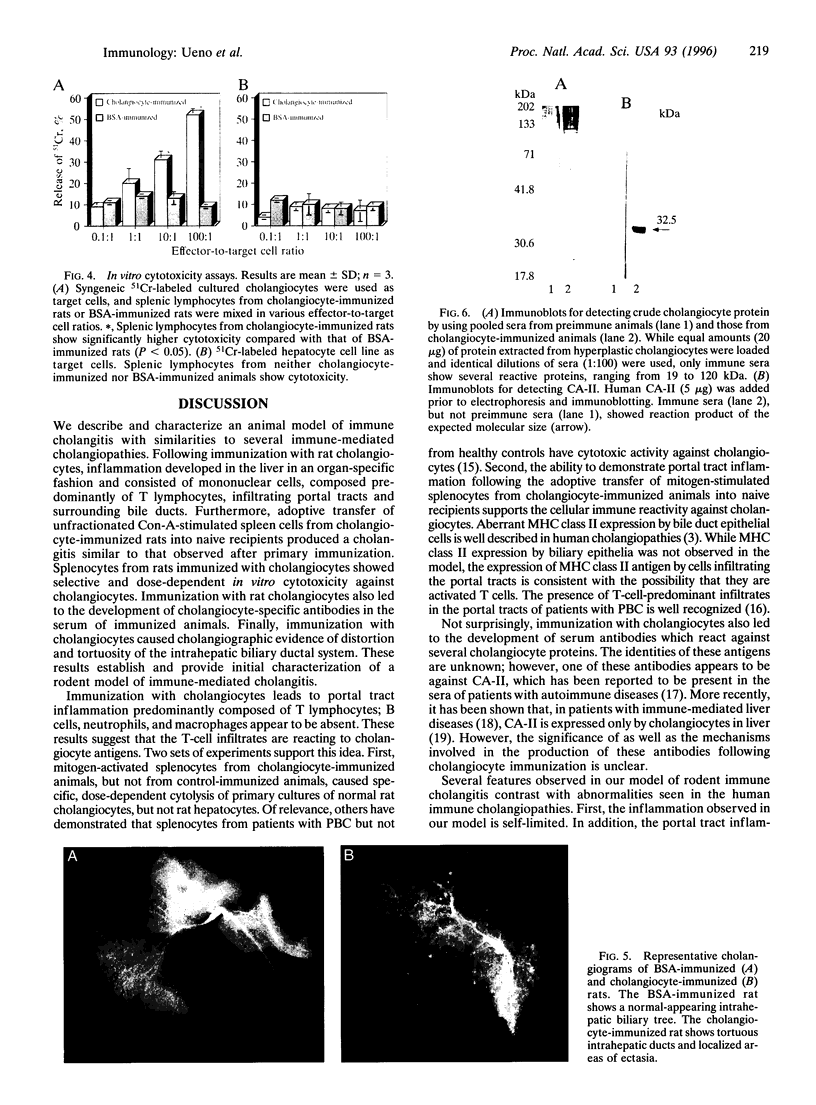
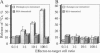Abstract
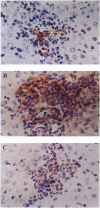
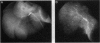
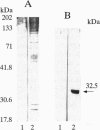
The cholangiopathies are a group of hepatobiliary diseases in which intrahepatic bile duct epithelial cells, or cholangiocytes, are the target for a variety of destructive processes, including immune-mediated damage. We tested the hypothesis that cholangitis could be induced in rodents by immunization with highly purified cholangiocytes. Inbred Wistar rats were immunized with purified hyperplastic cholangiocytes isolated after bile duct ligation from either syngeneic Wistar or allogeneic Fischer 344 rats; control rats were immunized with bovine serum albumin (BSA) or hepatocytes. After immunization with cholangiocytes, recipient animals developed histologic evidence of nonsuppurative cholangitis without inflammation in other organs; groups immunized with BSA or hepatocytes showed no cholangitis. Immunohistochemical studies revealed that portal tract infiltrates around bile ducts consisted of CD3-positive lymphocytes, some of which expressed major histocompatibility complex class II antigen; B cells and exogenous monocytes/macrophages were essentially absent. Transfer of unfractionated ConA-stimulated spleen cells from cholangiocyte-immunized (but not BSA-immunized) rats into recipients also caused nonsuppurative cholangitis. Moreover, these splenocytes from cholangiocyte-immunized (but not BSA-immunized) rats were cytotoxic in vitro for cultured rodent cholangiocytes; no cytotoxicity was observed against a rat hepatocyte cell line. Also, a specific antibody response in sera of cholangiocyte-immunized rats was demonstrated by immunoblots against cholangiocyte proteins. Finally, cholangiograms in cholangiocyte-immunized rats showed distortion and tortuosity of the entire intrahepatic biliary ductal system. This unique rodent model of experimental cholangitis demonstrates the importance of immune mechanisms in the pathogenesis of cholangitis and will prove useful in exploring the mechanisms by which the immune system targets and damages cholangiocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres R. C., Shaw J., Mills C. O., Coleman R., Neuberger J. M. A 51Cr release cytotoxicity assay for use with human intrahepatic biliary epithelial cells. J Immunol Methods. 1991 Jul 26;141(1):117–122. doi: 10.1016/0022-1759(91)90217-4. [DOI] [PubMed] [Google Scholar]
- Brown A. M., McFarlin D. E. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981 Sep;45(3):278–284. [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Howell C. D., Yoder T. D., Vierling J. M. Suppressor function of hepatic mononuclear inflammatory cells during murine chronic graft-vs-host disease. I. Macrophage-enriched cells mediate suppression in the liver. Cell Immunol. 1991 Jan;132(1):256–268. doi: 10.1016/0008-8749(91)90024-6. [DOI] [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Reichlin M. Antibodies to carbonic anhydrase in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 1992 Jan;35(1):73–82. doi: 10.1002/art.1780350112. [DOI] [PubMed] [Google Scholar]
- Kawai K., Kitagawa H., Higashimori T., Matsui T., Fujiyama S., Monna T., Yamamoto S., Morisawa S. Experimental chronic non-suppurative destructive cholangitis in rabbits following immunization with bile duct antigen. Gastroenterol Jpn. 1980;15(4):337–345. doi: 10.1007/BF02774304. [DOI] [PubMed] [Google Scholar]
- Kobashi H., Yamamoto K., Yoshioka T., Tomita M., Tsuji T. Nonsuppurative cholangitis is induced in neonatally thymectomized mice: a possible animal model for primary biliary cirrhosis. Hepatology. 1994 Jun;19(6):1424–1430. [PubMed] [Google Scholar]
- Kochar D. K., Gupta K. D., Jatkar P. R., Vyas U. K. Primary biliary cirrhosis like lesions following experimental autoimmunization. Indian J Pathol Microbiol. 1982 Jul;25(3):173–174. [PubMed] [Google Scholar]
- LaRusso N. F., Wiesner R. H., Ludwig J., MacCarty R. L. Current concepts. Primary sclerosing cholangitis. N Engl J Med. 1984 Apr 5;310(14):899–903. doi: 10.1056/NEJM198404053101407. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtman S. N., Keku J., Clark R. L., Schwab J. H., Sartor R. B. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991 Apr;13(4):766–772. [PubMed] [Google Scholar]
- Lichtman S. N., Okoruwa E. E., Keku J., Schwab J. H., Sartor R. B. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. J Clin Invest. 1992 Oct;90(4):1313–1322. doi: 10.1172/JCI115996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Saibara T., Nakata S., Maeda T., Iwasaki S., Iwamura S., Miyazaki M., Yamamoto Y., Enzan H. Cytotoxic activity of spleen-derived T lymphocytes against autologous biliary epithelial cells in autopsy patients with primary biliary cirrhosis. Liver. 1993 Aug;13(4):188–192. doi: 10.1111/j.1600-0676.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., McFarlin D. E. Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol. 1977 Sep;119(3):1134–1137. [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sherlock S., Scheuer P. J. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med. 1973 Sep 27;289(13):674–678. doi: 10.1056/NEJM197309272891306. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Sens M. A., Tashian R. E. Immunocytochemical demonstration of carbonic anhydrase in human epithelial cells. J Histochem Cytochem. 1982 Sep;30(9):864–873. doi: 10.1177/30.9.6813372. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv Immunol. 1980;30:159–273. doi: 10.1016/s0065-2776(08)60196-0. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Lasky S., Si L., Van Thiel D. H. Immunologic analysis of mononuclear cells in liver tissues and blood of patients with primary sclerosing cholangitis. Hepatology. 1985 May-Jun;5(3):468–474. doi: 10.1002/hep.1840050321. [DOI] [PubMed] [Google Scholar]
- Yang L., Faris R. A., Hixson D. C. Long-term culture and characteristics of normal rat liver bile duct epithelial cells. Gastroenterology. 1993 Mar;104(3):840–852. doi: 10.1016/0016-5085(93)91021-9. [DOI] [PubMed] [Google Scholar]