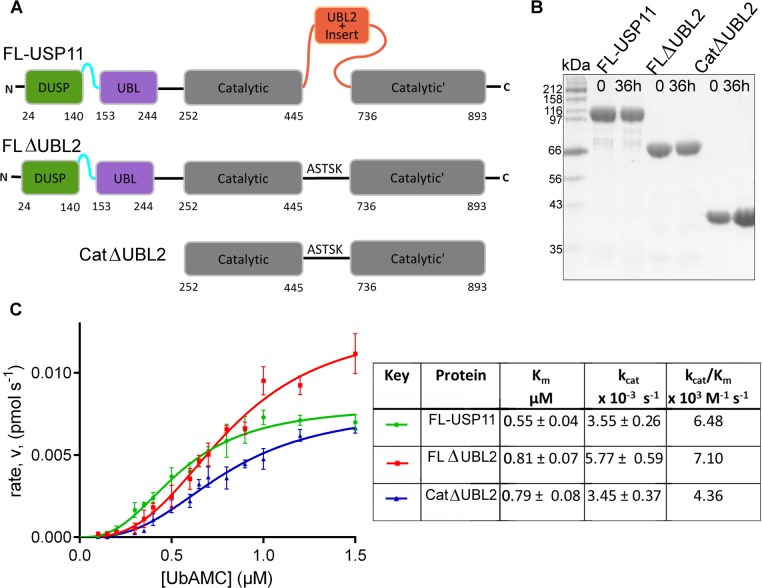
Figure 4.
Kinetic parameters for USP11 and deletion mutants. (A) Schematic representation of the hUSP11 constructs used in the activity assays. FL-USP11: full-length enzyme; FLΔUBL2: missing the UBL2 and insert nested in the catalytic domain; CatΔUBL2: additionally lacking the N-terminal DUSP and UBL domains. Deletion mutants lacking the UBL2 and insert have been replaced with a linker from USP8. (B) SDS-PAGE analysis of samples of FL-USP11 (107 kDa, lanes 2 and 3), FLΔUBL2 (77 kDa; lanes 4 and 5) and CatΔUBL2 (42 kDa; lanes 6 and 7), before the assays (time 0) and after the assay (time 36 h). (C) Graph of rate of reaction against substrate concentration for FL-USP11 (green), FLΔUBL2 (red) and CatΔUBL2 (blue). Each point represents the mean for data points measured in triplicate. Values for Vmax and Km were used to calculate the turnover number, kcat, and catalytic efficiency kcat/Km and are listed in the table. Errors are given as standard error mean.