Abstract
Dyslexic children are known to be slower than normal readers in rapid automatized naming (RAN). This suggests that dyslexics encounter local processing difficulties, which presumably induce a narrower perceptual span. Consequently, dyslexics should suffer less than normal readers from removing parafoveal preview. Here we used a gaze-contingent moving-window paradigm in a RAN task to experimentally test this prediction. Results indicate that dyslexics extract less parafoveal information than control children. We propose that more attentional resources are recruited to the foveal processing because of dyslexics’ less automatized translation of visual symbols into phonological output, thereby causing a reduction of the perceptual span. This in turn leads to less efficient pre-activation of parafoveal information and hence more difficult in processing the next foveal item.
Keywords: dyslexia, eye movement, perceptual span
Both normal readers and readers with reading disability extract foveal and parafoveal information during reading, but the perceptual span (i.e., the area of text from which information is processed during a fixation; McConkie & Rayner, 1975; see below) may differ between the groups. Here we test whether dyslexic readers operate with a smaller perceptual span than normal readers when they perform Rapid Automatized Naming (RAN; naming as fast as possible a number of single digits arranged in a matrix). This task is known for its diagnostic value for reading disability. The main goal of our experiment was to determine whether normal readers obtain a larger amount of parafoveal information than dyslexic readers in this non-reading task with oculomotor and saccade-programming demands similar to normal reading.
Perceptual Span during Reading
During a fixation the spatial extent of visual processing is quite narrow. As demonstrated in the gaze-contingent moving-window paradigm, normal skilled readers of English need visual information only from up to 4 letters to the left and at most 14–15 letters to the right of fixation in order to maintain a normal reading rate (McConkie & Rayner, 1975). The area from which useful visual information is extracted during a single fixation is termed the perceptual span. The perceptual span in reading normal English text is asymmetric to the right because readers need to move their eyes in this direction, and the act of programming an eye-movement causes an obligatory shift of covert attention to the saccade target (Deubel & Schneider, 1996).
The asymmetry and the extent of the perceptual span are not constant but depend on various factors such as reading skill: for example, Rayner (1986) reported that beginning readers have a reduced perceptual span, extending about 11 letters to the right of fixation. Presumably because of lower cognitive processing efficiency, beginning readers need to devote more of their attentional resources to the foveal word than skilled readers do (see also Häikiö, Bertram, Hyönä, & Niemi, 2009). It has also been demonstrated that the perceptual span varies as a function of reading material. Inhoff, Pollatsek, Posner, and Rayner (1989) found that the asymmetry of the perceptual span of English readers shifted to the left when they were forced to read from right to left. Similarly, the perceptual span of Israeli readers is also asymmetric to the left because the reading direction of Hebrew text is leftwards (Pollatsek, Bolozky, Well, & Rayner, 1981). The width of the span is limited by cognitive rather than perceptual factors: While approximately constant if measured using the amount of information in bits, the size of the span measured in characters is smaller in writing systems with more densely packed information. For example, the perceptual span in Chinese extends 1 character to the left and 2–3 characters to the right of fixation (Inhoff & Liu, 1998).
The size of the perceptual span is also relevant in the gaze-contingent boundary paradigm (Rayner, 1975). In this paradigm the visibility of a parafoveal word N+1 during fixations on pretarget words N is under experimental control, that is, identical or masked previews are replaced by the target words only after readers’ eyes cross an invisible boundary located between words N and N+1. Preview benefit (PB) is indicated by a positive difference between fixation durations on target word N+1 when preview has been denied compared to when preview has been available during the previous fixation.
Importantly for the present context, readers dynamically adjust the size of their perceptual spans to the processing difficulty of texts. Inhoff et al. (1989) demonstrated that readers were able to extract more parafoveal information when the order of letters in a word was normal than when it was transformed. Henderson and Ferreira (1990) reported that parafoveal processing efficiency was reduced when pretarget foveal words were more difficult, suggesting that the attentional focus on the difficult foveal word narrowed the perceptual span, and thus less parafoveal information was acquired. For Chinese reading Yan, Kliegl, Shu, Pan, and Zhou (2010) demonstrated that the size of the perceptual span is modulated not only by foveal but also by parafoveal processing load.
Dyslexia and Eye-Movement Control
It is generally accepted that decisions about when and where to move the eyes are driven by two largely independent processes in eye-movement control (Rayner & McConkie, 1976; Findlay & Walker, 1999). How do dyslexic readers compare to normal readers in this respect? Eye-movement control deficit, especially about where to fixate, was once considered as a possibly causal factor for dyslexia. For example, Pavlidis (1981) reported group differences in eye-movements between dyslexic and normal readers in a simple saccadic task. However, Pavlidis’s results (1981) were not replicated in later studies using similar tasks (e.g., Olson, Kliegl, & Davidson, 1983; Stanley, Smith, & Howell, 1984). Several other studies also failed to establish differences between normal and dyslexic readers in eye-movements during non-reading tasks (e.g., Hutzler, Kronbichler, Jacobs, & Wimmer, 2006), but there is some evidence that eye-movements measured in visual-attention tasks can be used to discriminate between the groups—at least when naming or storage of letters in visual-short memory is required (e.g., Hawelka & Wimmer, 2005).
On the other hand, as there is a general agreement that eye-movements reflect cognitive processing and understanding of the text, even if dyslexic readers’ oculomotor control is not a cause of reading problems, their eye-movements may well reflect cognitive processing difficulties (e.g., Hawelka, Gagl, & Wimmer, 2010). There is already some evidence that reading deficiency may translate into a smaller perceptual span. Rayner, Murphy, Henderson, and Pollatsek (1989) reported for two dyslexic readers that their reading rate peaked for a two-word gaze-contingent window condition whereas the rate of normal readers was usually at a maximum for a three-word window.1
Rapid Automatized Naming
We propose to follow up on this research but rather than using normal reading, we will test whether there are group differences in the perceptual span during Rapid Automatized Naming (RAN; Denckla & Rudel, 1976). In the RAN task, readers are required to name a series of familiar stimuli, such as letters, digits, colors, or objects. The RAN task is one significant indicator of development status in reading skill (Kirby, Parrila, & Pfeiffer, 2003; Logan, Schatschneider, & Wagner, 2011). Dyslexic children on average perform poorer than normal children in this task (see Norton & Wolf, 2012, for a review). RAN measures the speed of accessing phonological codes from visual stimuli, however, it is not clear how RAN relates to reading, and the reason for the RAN difference between normal and dyslexic readers is an active area of research. In particular, the group differences appear to be largest for rapid naming of digits and letters (termed alphanumeric below) as compared with naming of objects and colors (Bowey, McGuigan, & Ruschena, 2005; Cardoso-Martins & Pennington, 2004).
This interaction might be explained by the multicompoential view of the RAN task (Wolf & Bowers, 1999; Norton & Wolf, 2012), which suggests that, aside from dyslexics’ phonological-processing deficit, normal readers’ alphanumeric naming is more automatized and closer to reading than object naming. We propose that if automaticity of the independent components plays a key role, then dyslexic readers are predicted to spend more attentional resources on the task of translating symbols to phonology. Given the documented attentional modulation of the perceptual span, it follows that their perceptual span should be restricted, because relatively fewer resources are available for parafoveal preprocessing.
The best way to test this prediction is to experimentally restrict the amount of preview. Surprisingly, this prediction has rarely been put to experimental test. One exception is a study by Jones, Branigan, and Kelly (2009), who used a discrete format with voice-activated presentation of the next item and found somewhat reduced group differences with the discrete format. However, by the time an item is voiced, most of the visual processing is presumably finished. Using a gaze-contingent paradigm, Logan (2009) manipulated the amount of parafoveal information (full preview, one-letter preview, and no preview) in the RAN task among normal first-grade readers and found preview benefit from the one-letter preview condition. Jones, Ashby, and Branigan (in press) manipulated the orthographic and phonological similarity between the foveal and the parafoveal letters. They found that adult dyslexics were more affected by parafoveal orthographic confusability. However, little is known about how dyslexic children differ from normal children in parafoveal processing efficiency in the RAN task from all these previous studies. In the present study we restricted the available preview using gaze-contingent presentation to more directly study influences of automaticity on the perceptual span in the RAN task.
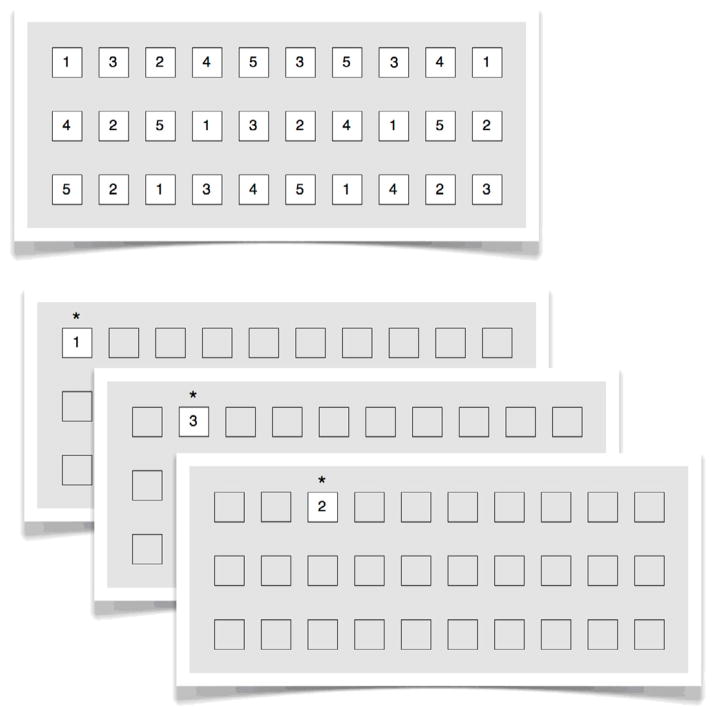
Given the high predictive power of digit-RAN in classifying dyslexic readers especially in the Chinese population (Shu, McBride-Chang, Wu, & Liu, 2006), we presented RAN digits in either the original format (termed continuous-RAN below) or in a gaze-contingent discrete-RAN format, in which items were presented serially in positions analogous to the continuous-RAN (i.e., an item-based version of the gaze-contingent moving-window paradigm in which the boundary of the window always coincided with the boundaries of the items). Only the currently fixated item was legible in this condition, and thus no preview was available (see Figure 1). Therefore, comparison of performance in the discrete- and continuous-RAN directly shows the amount of parafoveal processing used in RAN performance. The continuous- versus discrete-RAN design in our experiment is similar to the full preview and no preview conditions in Logan (2009). However, different from Logan (2009) and other previous studies, in addition to fixation duration measures, we were also interested in whether landing position would be affected by the presentation type, as a test of saccade-target selection difference between normal and dyslexic readers. Marking the locations of the to-be-fixated items allowed us to investigate whether the “where” decision remains intact among dyslexic children when there is no influence from higher cognitive level (i.e., in the discrete-RAN).
Figure 1.
An illustration of continuous-RAN (A, top panel) and discrete-RAN (B, bottom panel) tasks. In discrete-RAN, all previews were parafoveally masked and were replaced by the target items as soon as reader’s eyes (as indexed by the asterisks in the figure) crossed the invisible vertical boundary located between every two items.
Method
Subjects
A total of 35 native Chinese dyslexic children from Grade 5 (20 boys and 15 girls) with normal or corrected-to-normal vision participated in the eye-tracking experiment. Twenty-eight fifth-graders (13 boys and 15 girls) also participated in the experiment serving as a control group. Parents of the participating children gave their consent on the test by signing a form. Eye-movement data from all subjects were analyzed, but naming data from 7 subjects were removed from the naming speed analysis due to a sound recording error (5 boys from the dyslexic group and 2 boys from the control group).
As shown in Table 1, the two groups were matched in age and a nonverbal IQ test (Picture Completion in Wechsler Intelligence Scale for Children, Chinese revision, C-WISC, Gong & Cai, 1993). Children in the dyslexic sample scored 85 or higher in WISC with two exceptions of 83 and 84. Dyslexia was diagnosed through a standard test based on a character recognition task, which has proven to be diagnostic in previous studies in mainland China (Shu, et al., 2006; Zhang, Zhang, Shu, Xi, Wu, Zhang, & Li, 2012). None of our participants were diagnosed with attentional deficits.
Table 1.
Means (and standard deviations) of subjects’ age and test scores.
| Control (N=28) | Dyslexic (N=35) | t-value | |
|---|---|---|---|
| Age (year) | 10.65 (.35) | 10.75 (.38) | −1.07 |
| Character recognition (max: 150) | 127.82 (9.29) | 81.37 (17.89) | 13.05*** |
| Nonverbal IQ | 10.39 (2.78) | 9.80 (2.43) | .90 |
Note: Children classified as dyslexics scored at least 1.5 standard deviations below their respective grade mean in the character recognition task.
p<.001.
Material
Five digits (1, 2, 3, 4, and 5) were used. Each of them extended 66*70 pixels on the screen and was repeated 6 times in a 10-column by 3-row matrix in a trial. The digits were listed in random orders with the constraint that adjacent items were not the same. Digits were displayed in black and were centered on white rectangles marking item locations, which were presented on a gray background. The horizontal inter-item distance was 116 pixels, and the distance between rows of items was 256 pixels.
Apparatus
Eye-movements were recorded using an EyeLink1000 Desktop system. The items were presented on a 19-inch Viewsonic G90f monitor (resolution, 1280 by 1024 pixels; frame rate, 85 Hz). Therefore, it took at most 15 ms to complete the display change. Subjects were seated comfortably with a forehead rest at a distance of 57cm from the monitor. At this distance, the width of each item was approximately 2 degrees and the empty space between neighboring item locations was about 1.5 degrees of visual angle. All recordings and calibrations were done monocularly based on the right eyes; viewing was binocular. Voice responses were recorded using an ASIO compatible Sound Blaster Audigy sound card, guaranteeing a fixed audio latency of 10 ms.
Procedure
Subjects were calibrated with a nine-point grid. They were instructed to read all items on the screen aloud as quickly and as accurately as possible, then to fixate a dot in the lower right corner of the monitor to signal completion of the trial. Subjects’ gaze on a fixation point that occupied the position of the first item initiated presentation of the next screen. An extra calibration was scheduled if the tracker did not detect subjects’ eyes within a pre-defined window around the initial fixation point.
As shown in Figure 1, in continuous-RAN, all digits were presented on the screen simultaneously. In contrast, in discrete-RAN, crossing an invisible vertical boundary located between the to-be-fixated item and the previous one triggered the appearance of the next digit, and simultaneously also eliminated the previous one so that only a single item was displayed at a time during reading (see Logan, 2009, for a similar paradigm but using # as masks). All other item positions were marked by empty rectangular black frames. Each subject read 20 screens in total. Trials with the same condition were blocked and the order of the blocks was counterbalanced across subjects.
Data Analysis
For eye movement duration measures, we distinguish among first-fixation durations (FFDs; the first fixation on a word, irrespective of the number of fixations), single-fixation durations (SFDs; cases in which a word was inspected with exactly one fixation), and gaze durations (GDs; the sum of fixations during the first-pass reading of the word). In reading, FFD and SFD are often considered as an index for early lexical processing whereas GD is particularly sensitive to post-lexical processing (Inhoff, 1984). In addition, we also analyzed the fixation landing-position and the saccade amplitude. In terms of parafoveal processing, landing position further into a region and longer saccades are interpreted as evidence for more information obtained parafoveally during previous fixations (Inhoff, 1989; Hirotani, Frazier, & Rayner, 2006).
Although each item in the RAN tasks occupied only 66*70 pixels, the AOI (i.e., area of interest) for each item was defined as a region of 116*256 pixels centered on the item so that most fixations in a trial could be assigned to an AOI. Data were selected for analyses in three steps: First and last items in a row were deleted. Further, if a blink happened during first pass reading of a certain item, this item was excluded (i.e., 4.5% of all items). Finally, items with FFDs shorter than 60 ms or longer than 800 ms, or GDs longer than 1000 ms, were also removed from the analyses (i.e., 4.3% of all items without blinks). Totally, 26400, 16608 and 26400 observations contributed to the following FFD, SFD and GD analyses, respectively.
Three fixed effects were specified in linear mixed models (LMMs) for data analyses. The first fixed effect, as a test of the main effect for the group difference, compared normal against dyslexic readers. The second fixed effect, as a test of the main effect of condition, compared continuous-RAN against discrete-RAN. The third fixed effect tested the interaction between these two factors. Estimates are based on LMMs with crossed random factors for subjects and items using the lmer program of the lme4 package (Bates & Maechler, 2011) in the R environment for statistical computing and graphics. Estimates larger than 1.96 time of their standard errors (t>1.96) were interpreted as significant at the 5% level. This is justified because, given the number of subjects and the large number of observations for each subject, the t-statistic in LMMs (i.e., M/SE) effectively corresponds to the z-statistic. Analyses for untransformed and log-transformed durations yielded the same pattern of significance.
Results
Naming Speed Analysis
The first row of Table 2 shows the total naming time. The main effects of group (b=−3.14, SE=0.58, t=−5.38) and condition (b=−5.83, SE=0.13, t=−46.56) and their interaction (b=−0.60, SE=0.11, t=−5.37) were all significant. Further simple analyses showed that both groups were slower in the discrete-RAN than in the continuous-RAN condition (control: b=6.43, SE=0.15, t=44.21; dyslexic: b=5.24, SE=0.16, t=31.98). The larger difference between the two conditions for the control group indicated by the significant interaction suggests that they process parafoveal information more efficiently; they are more impaired by denial of preview. We address this topic in a more detailed way in the following eye-movement analyses.
Table 2.
Group comparisons of RAN measures.
| Control | Dyslexic | |||
|---|---|---|---|---|
|
| ||||
| Continuous | Discrete | Continuous | Discrete | |
| Total naming time (sec) | 9.6 (1.4) | 16.0 (2.6) | 13.3 (2.3) | 18.6 (3.1) |
| Single Fixation Duration (ms) | 274 (28) | 379 (58) | 322 (40) | 417 (51) |
| First Fixation Duration (ms) | 265 (27) | 320 (50) | 297 (35) | 324 (47) |
| Gaze Duration (ms) | 307 (34) | 444 (59) | 377 (51) | 504 (49) |
| Single Fixation Probability (%) | 83 (8) | 57 (17) | 70 (15) | 45 (17) |
| Landing Position (AOI) | .55 (.08) | .43 (.10) | .49 (.08) | .41 (.08) |
| Saccade Amplitude (°) | 3.30 (.23) | 2.92 (.32) | 3.19 (.30) | 2.89 (.28) |
Note: Means and standard deviations are based on subject means.
Duration Analyses
The means and standard deviations of duration measures are presented in Table 2. There were significant main effects of group in SFDs (b=−0.107, SE=0.029, t=−3.7) and GDs (b=−0.161, SE=0.027, t=−6.0) with a similar numerical trend in FFDs (b=−0.042, SE=0.031, t=−1.3), indicating overall slower speed for dyslexics. The main effect of condition was significant for all three measures (b=−0.307, SE=0.006, t=−51.7; b=−0.125, SE=0.005, t=−25.2 and b=−0.359, SE=0.005, t=−75.1; for SFD, FFD and GD analyses, respectively), indicating a higher processing difficulty for discrete-RAN over continuous-RAN due to lack of parafoveal information.
Critically, we also observed significant interactions between group and condition for all three measures (b=−0.028, SE=0.006, t=−4.7; b=−0.049, SE=0.005, t=−9.9 and b=−0.032, SE=0.005, t=−6.8; for SFD, FFD and GD analyses, respectively). As shown in Table 1 and Figure 2, task strongly modulated the group differences. The two groups differed in all three duration measures in continuous-RAN (b=0.140, SE=0.028, t=4.93; b=0.090, SE=0.027, t=3.28 and b=0.195, SE=0.029, t=6.65; for SFD, FFD and GD analyses, respectively), but the group difference was greatly reduced in discrete-RAN for SFDs and GDs (b=0.080, SE=0.039, t=2.04 and b=0.126, SE=0.029, t=4.3) and was not significant for FFDs (b=−0.008, SE=0.039, t=−0.2).
Figure 2.
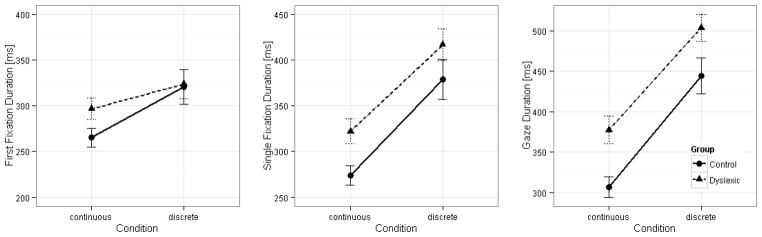
Mean first-fixation (left panel), single-fixation (middle panel) and gaze duration (right panel) by condition and group. Error bars indicate twice standard errors of the mean. The absolute group difference is smaller in the discrete-RAN than in the continuous-RAN task in virtually all measures.
Landing-Position Analyses
Table 2 also shows the descriptive measure of landing-position and saccade amplitude. There was a significant main effect of group in single-fixation (b=0.041, SE=0.018, t=2.27) and first-fixation landing-positions (b=0.038, SE=0.019, t=1.96). The main effects of condition were significant (b=0.056, SE=0.003, t=21.04 and b=0.097, SE=0.002, t=40.47 for SF and FF analyses, respectively). Finally, interactions were significant for SF (b=0.024, SE=0.003, t=9.18) and FF (b=0.021, SE=0.002, t=8.66) landing-positions. Simple effect analyses indicated that normal readers landed further into the AOIs as compared with the dyslexics in continuous-RAN (b=−0.069, SE=0.021, t=−3.3 and b=−0.060, SE=0.021, t=−2.93 for SF and FF landing-positions, respectively), but the two groups did not differ from each other in discrete-RAN (b=−0.010, SE=0.020, t=−0.49 and b=−0.017, SE=0.022, t=−0.794 for SF and FF landing-positions, respectively). The difference in landing-position was a consequence of inter-item progressive saccade amplitude: there was a significant main effect of condition (b=0.341, SE=0.011, t=32.07) and a significant interaction (b=0.042, SE=0.011, t=3.92) although the main effect of group was not significant (b=0.075, SE=0.065, t=1.15).
Discussion
There has been suggestive evidence for a reduction of the perceptual span associated with dyslexics in text reading, however, such an assumption has never been statistically confirmed nor extended to non-reading tasks. We tested the difference in parafoveal processing efficiency between normal and dyslexic readers in RAN tasks, and compared a standard format with a gaze-contingent discrete version of the RAN. The three main results were, first, a general slowdown in discrete-RAN, presumably due to the increase of processing difficulty given the lack of any preview. Second, eye-movement measures revealed that this slowdown arose from prolongations of fixation durations and a reduction of saccade amplitude. Most importantly, third, the interactions between RAN condition and subject group consistently indicated that dyslexics extract less parafoveal information. In other words, they do not look as far ahead and, therefore, are less impaired by denial of parafoveal preview.
RAN and Eye-Movement Control
The mechanism underlying the statistical relationship of RAN and reading has long been discussed. Subjects’ performance in RAN has been shown to be a strong predictor of their later reading ability, presumably because the components of RAN share essential features with text reading (Norton & Wolf, 2012). Wolf and Bowers (1999) list seven subprocesses involved in RAN performance, including attentional, visual, and phonological processes. However, most of these subprocesses are shared with discrete-RAN and with a rapid naming task (of individual items at the center of screen (Logan et al., 2011), both of which are weaker predictors of reading than continuous-RAN.
Thus we would like to point to the importance of processes that are unique to continuous-RAN, such as attentional processes to the stimulus. In particular, just like reading, continuous-RAN involves both foveal and parafoveal information extraction as well as saccade-target selection. Studies by Jones and colleagues (Jones et al., in press; Jones, Obregón, Kelly, & Branigan, 2008) argued similarly that adult dyslexic readers are sensitive to parafoveal information in RAN. These studies jointly suggest the important role that eye-movements play in RAN. The present study provides more conclusive evidence towards this point.
The Reduction of Perceptual Span for Dyslexics
The key finding of the present study is that normal and dyslexic readers differ in the size of the perceptual span. Our results are in agreement with Rayner et al. (1989), who tested two dyslexic readers with a general language-related deficit and suggested that the difficulty in processing foveal words narrowed the perceptual spans for dyslexic readers. The result warranted a replication with a larger sample. Also, Rayner et al.’s conclusion referred mainly to overall reading rate (in words per minute; see also Footnote 1).
Results from the present study statistically consolidate Rayner et al.’s (1989) assumption through the significant interactions between group and condition: normal readers acquired more parafoveal information and exhibited stronger preview benefits whereas dyslexics needed to spend more attentional resources presumably due to their difficulty in translating from visual symbols to their phonological representations (Manis, Seidenberg, & Doi, 1999). Logan (2009) reported a similar result that children with better performance at RAN were more impaired by the lack of parafoveal information, although her conclusion was made by comparing good and poor (but not dyslexic) beginning readers. Our results extended previous findings to parafoveal processing efficiency between normal and dyslexic children and to an arguably more basic non-reading situation.
Since Jones et al. (in press) did not manipulate the amount of parafoveal information, it is difficult to judge from their experiment whether parafoveal information facilitates or interferes with later processing. In the present study, the positive preview benefit for dyslexics indicates that they were able to effectively use parafoveal information like normal readers (although with reduced efficiency). This interpretation is also supported by another recent study. Pan, Yan, Laubrock, Shu, and Kliegl (2012) reported results largely in agreement with the predicted pattern of alphanumeric (digits) and symbolic (dice surface) RAN for Chinese dyslexic and normal children. In that study, larger group differences for digit-RAN than dice-RAN were observed with respect to various eye-movement measures. Interestingly, after statistical control of group differences, condition effects, and gaze durations, only normal children’s digit-RAN eye-voice span was predictive of psychometric digit-RAN. These results suggest that only when naming is highly automatized (requiring both normal reading skill and digit stimuli) can we unveil the link between eye-voice span and RAN speed. However, neither Jones et al. (in press) nor the present study has tested how dyslexics make use of parafoveal information during natural sentence reading. Further studies would need to address this issue.
The When and Where Decisions of Dyslexics
Obviously, dyslexics’ decisions about when to move the eyes occur at a slower pace than those of normal readers due to the deficiency in retrieving phonological representations or in achieving a comparable degree of automaticity in this process. This causes prolonged fixation durations. Indeed, the group difference in durations was much larger for the continuous-RAN than for the discrete-RAN condition. Thus, the group difference is mainly due to control readers’ better parafoveal processing. However, we think that this larger perceptual span is a consequence of control readers’ more automatic foveal processes, which allows attention to spread more widely, again consistent with the Pan et al., (2012) study described above. Development of automaticity acts like a positive feedback loop in this conception: the more automatized the conversion of symbolic input to phonological output, the more attentional resources can be devoted to parafoveal preprocessing, and more pre-processing of symbolic input leads to a head-start in converting it into phonological output.
There are also results that inform about where decision of dyslexics: Neither inter-word saccade amplitude nor landing-positions differed significantly between groups in discrete-RAN, indicating that in this condition dyslexics’ performance in saccade-target selection was not influenced by their foveal processing deficiency. The results are nicely in agreement with findings that beginning readers can select word centers as the optimal viewing position as early as first-grade in primary school (Aghababian & Nazir, 2000; McConkie, Zola, Grimes, Kerr, Bryant, & Wolff, 1991). If readers obtain information parafoveally, their eyes are expected to target a position further away from word beginnings. The group difference in saccade amplitude and landing-position in continuous-RAN, again, supports an interpretation in terms of differences in perceptual span and extraction of parafoveal information.
Conclusion
Our results clearly indicate that dyslexics make less use of parafoveal information than normal readers. However, we do not think that the lack of use of parafoveal information is the cause for dyslexia. Rather, our results are compatible with the view that “the smaller perceptual span and erratic eye movements are symptoms, rather than causes, of developmental dyslexia” (Morris & Rayner, 1991, p.238). The deficit in translating print into phonological output is likely caused by dyslexics’ less well-automatized translation routines between digits and associated phonological representations (Pan et al., 2012), leading to a lack of availability of attentional resources for parafoveal preprocessing.
We examined perceptual spans in rapid naming among Chinese dyslexic and normal children
Both dyslexic and normal children can use parafoveal information.
Dyslexic readers are less efficient in utilizing parafoveal information.
Basic eye movement control in saccade target selection of dyslexic readers was intact.
The perceptual span difference of the two groups comes from automaticity in naming.
Acknowledgments
This research was funded by Chinesisch-Deutsches Forschungsprojekt (GZ 633) to Hua Shu and Reinhold Kliegl, by Deutsche Forschungsgemeinschaft Grant KL 955/18 to Reinhold Kliegl, by Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning of P.R. China to Ming Yan and by Academic Award for Doctoral Candidates to Jinger Pan.
Footnotes
They also reported data from one adult dyslexic reader with a selective-attention deficit who actually read faster in the one-word than the two-word condition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghababian V, Nazir TA. Developing normal reading skills: Aspects of the visual processes underlying word recognition. Journal of Experimental Child Psychology. 2000;76(2):123–150. doi: 10.1006/jecp.1999.2540. [DOI] [PubMed] [Google Scholar]
- Bates DM, Maechler M. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42. 2011 [Computer software]. Available at http://cran.r-project.org/web/packages/lme4.
- Bowey JA, McGuigan M, Ruschena A. On the association between serial naming speed for letters and digits and word-reading skill: Towards a developmental account. Journal of Research in Reading. 2005;28:400–422. [Google Scholar]
- Cardoso-Martins C, Pennington BF. The relationship between phoneme awareness and rapid serial naming skills and literacy acquisition: The role of developmental period and reading ability. Scientific Studies of Reading. 2004;8:27–52. [Google Scholar]
- Denckla MB, Rudel RG. Rapid “automatized” naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences. 1999;22:661–674. doi: 10.1017/s0140525x99002150. [DOI] [PubMed] [Google Scholar]
- Gong Y, Cai T. Scale for Children Chinese revision (C-WISC) Changsha, China: Hunan Map Press; 1993. Wechsler Intelligence. [Google Scholar]
- Hawelka S, Wimmer H. Impaired visual processing of multi-element arrays is associated with increased number of eye-movements in dyslexic reading. Vision Research. 2005;45:855–863. doi: 10.1016/j.visres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Ferreira F. Effects of foveal processing difficulty on the perceptual span in reading: Implications for attention and eye movement control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:417–429. doi: 10.1037//0278-7393.16.3.417. [DOI] [PubMed] [Google Scholar]
- Häikiö T, Bertram R, Hyönä J, Niemi P. Development of the letter identity span in reading: Evidence from the eye movement moving window paradigm. Journal of Experimental Child Psychology. 2009;102:167–181. doi: 10.1016/j.jecp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Hawelka S, Gagl B, Wimmer H. A dual-route perspective on eye movements of dyslexic readers. Cognition. 2010;115:367–379. doi: 10.1016/j.cognition.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani M, Frazier L, Rayner K. Punctuation and intonation effects on clause and sentence wrap-up: Evidence from eye movements. Journal of Memory and Language. 2006;54:425–443. [Google Scholar]
- Hutzler F, Kronbichler M, Jacobs AM, Wimmer H. Perhaps correlational but not causal: no effect of dyslexic readers’ magnocellular system on their eye movements during reading. Neuropsychologia. 2006;44:637–48. doi: 10.1016/j.neuropsychologia.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Inhoff AW. Two stages of word processing during eye fixations in the reading of prose. Journal of Verbal Learning and Verbal Behavior. 1984;23:612–624. [Google Scholar]
- Inhoff AW. Parafoveal processing of words and saccade computation during eye fixations in reading. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:544–555. doi: 10.1037//0096-1523.15.3.544. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Liu W. The perceptual span and oculomotor activity during the reading of Chinese sentences. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:20–34. doi: 10.1037//0096-1523.24.1.20. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Pollatsek A, Posner M, Rayner K. Covert attention and eye movements during reading. Quarterly Journal of Experimental Psychology. 1989;41A:63–89. doi: 10.1080/14640748908402353. [DOI] [PubMed] [Google Scholar]
- Jones MW, Ashby J, Branigan HP. Dyslexia and fluency: Parafoveal and foveal influences on rapid automatized naming. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0029710. (in press) [DOI] [PubMed] [Google Scholar]
- Jones MW, Branigan HP, Kelly ML. Dyslexic and nondyslexic reading fluency: Rapid automatized naming and the importance of continuous lists. Psychonomic Bulletin & Review. 2009;16(3):567–572. doi: 10.3758/PBR.16.3.567. [DOI] [PubMed] [Google Scholar]
- Jones MW, Obregón M, Kelly ML, Branigan HP. Elucidating the component processes involved in dyslexic and non-dyslexic reading fluency: An eye-tracking study. Cognition. 2008;109(3):389–407. doi: 10.1016/j.cognition.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Kirby JR, Parrila RK, Pfeiffer SL. Naming speed and phonological awareness as predictors of reading development. Journal of Educational Psychology. 2003;95(3):453–464. [Google Scholar]
- Logan JAR. Unpublished doctoral thesis. The Florida State University; 2009. The role of parafoveal information in rapid letter naming. [Google Scholar]
- Logan JAR, Schatschneider C, Wagner RK. Rapid serial naming and reading ability: The role of lexical access. Reading and Writing. 2011;24:1–25. doi: 10.1007/s11145-009-9199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis FR, Seidenberg MS, Doi LM. See Dick RAN: Rapid naming and the longitudinal prediction of reading subskills in first and second graders. Scientific Studies of Reading. 1999;3:129–157. [Google Scholar]
- McConkie GW, Rayner K. The span of the effective stimulus during a fixation in reading. Perception & Psychophysics. 1975;17:578–586. [Google Scholar]
- McConkie GW, Zola D, Grimes J, Kerr PW, Bryant NR, Wolff PM. Children’s eye movements during reading. In: Stein JF, editor. Vision and visual dyslexia. London: MacMillan Press; 1991. pp. 251–262. [Google Scholar]
- Morris RK, Rayner K. Eye movements in skilled reading: Implications for developmental dyslexia. In: Stein JF, editor. Vision and visual dyslexia. London: MacMillan Press; 1991. pp. 233–242. [Google Scholar]
- Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annal Review of Psychology. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Olson RK, Kliegl R, Davidson BJ. Dyslexics’ and normal readers’ eye movements. Journal of Experimental Psychology: Human Perception and Performance. 1983;9:816–825. doi: 10.1037//0096-1523.9.5.816. [DOI] [PubMed] [Google Scholar]
- Pan J, Yan M, Laubrock J, Shu H, Kliegl R. Eye-voice span during rapid automatized naming of digits and dice in Chinese normal and dyslexic children. 2012. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Pavlidis GTh. Do eye movements hold the key to dyslexia? Neuropsychologia. 1981;19:57–64. doi: 10.1016/0028-3932(81)90044-0. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Bolozky S, Well AD, Rayner K. Asymmetries in the perceptual span for Israeli readers. Brain and Language. 1981;14:174–180. doi: 10.1016/0093-934x(81)90073-0. [DOI] [PubMed] [Google Scholar]
- Rayner K. The perceptual span and peripheral cues during reading. Cognitive Psychology. 1975;7:65–81. [Google Scholar]
- Rayner K. Eye movements and the perceptual span in beginning and skilled readers. Journal of Experimental Child Psychology. 1986;41:211–236. doi: 10.1016/0022-0965(86)90037-8. [DOI] [PubMed] [Google Scholar]
- Rayner K, McConkie GW. What guides a reader’s eye movements. Vision Research. 1976;16:829–837. doi: 10.1016/0042-6989(76)90143-7. [DOI] [PubMed] [Google Scholar]
- Rayner K, Murphy L, Henderson JM, Pollatsek A. Selective attentional dyslexia. Cognitive Neuropsychology. 1989;6:357–378. [Google Scholar]
- Shu H, McBride-Chang C, Wu S, Liu H. Understanding Chinese developmental dyslexia: Morphological awareness as a core cognitive construct. Journal of Educational Psychology. 2006;98:122–133. [Google Scholar]
- Stanley G, Smith GA, Howell EA. Eye movements and sequential tracking in dyslexic and control children. British Journal of Psychology. 1983;74:181–187. doi: 10.1111/j.2044-8295.1983.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for developmental dyslexia. Journal of Educational Psychology. 1999;91:425–438. [Google Scholar]
- Yan M, Kliegl R, Shu H, Pan J, Zhou X. Parafoveal load of word n+1 modulates preprocessing effectiveness of word n+2 in Chinese reading. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1669–1676. doi: 10.1037/a0019329. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang L, Shu H, Xi J, Wu H, Zhang Y, Li P. Universality of categorical perception deficit in developmental dyslexia: An investigation of Mandarin Chinese tones. Journal of Child Psychology and Psychiatry. 2012;53:874–882. doi: 10.1111/j.1469-7610.2012.02528.x. [DOI] [PubMed] [Google Scholar]