Abstract
Objective
The Carolina Framework for Cervical Cancer Prevention describes 4 main causes of cervical cancer incidence: human papillomavirus (HPV) infection, lack of screening, screening errors, and not receiving follow-up care. We present 2 applications of the Carolina Framework in which we identify high-need counties in North Carolina and generate recommendations for improving prevention efforts.
Methods
We created a cervical cancer prevention need index (CCPNI) that ranked counties on cervical cancer mortality, HPV vaccine initiation and completion, Pap smear screening, and provision of Pap tests to rarely- or never-screened women. In addition, we conducted in-depth interviews with 19 key informants from programs and agencies involved in cervical cancer prevention in North Carolina.
Results
North Carolina’s 100 counties varied widely on individual CCPNI components, including annual cervical cancer mortality (median 2.7/100,000 women; range 0.0–8.0), adolescent girls’ HPV vaccine initiation (median 42%; range 15%–62%), and Pap testing in the previous 3 years among Medicaid-insured adult women (median 59%; range 40%–83%). Counties with the greatest prevention needs formed 2 distinct clusters in the northeast and south-central regions of the state. Interviews generated 9 recommendations to improve cervical cancer prevention in North Carolina, identifying applications to specific programs and policies in the state.
Conclusions
This study found striking geographic disparities in cervical cancer prevention need in North Carolina. Future prevention efforts in the state should prioritize high-need regions as well as recommended strategies and applications in existing programs. Other states can use the Carolina Framework to increase the impact of their cervical cancer prevention efforts.
Keywords: cervical cancer, cancer prevention, Pap test, human papillomavirus (HPV) vaccine, health disparities
Introduction
Cervical cancer mortality in the U.S. has dropped precipitously in the last 60 years, with annual rates falling from 7.9 per 100,000 women in 19501 to 2.4 per 100,000 in 2008,2 largely due to widespread use of Pap screening.3 Despite this remarkable achievement, reductions in mortality have slowed in recent years,2 likely due to a plateauing of screening. Over 4,000 women still die of this preventable cancer each year.4 Disparities in cervical cancer mortality include higher rates among African American, Hispanic, low-income women, and rural-dwelling women, especially in Appalachia and on the U.S.-Mexico border.2,4–11 In addition, states within the U.S. demonstrate wide variation in cervical cancer mortality, ranging from 1.2 per 100,000 women in Utah to 3.8 per 100,000 women in Mississippi.2
Given stalled progress and persistent disparities in mortality, the time is right for reevaluating and refining approaches to addressing cervical cancer. Toward this end, Cervical Cancer-Free North Carolina, a statewide initiative to reduce the burden of cervical cancer, developed the Carolina Framework for Cervical Cancer Prevention (see Box 1). The Carolina Framework guides prevention efforts by identifying and addressing 4 causes of cervical cancer incidence: human papillomavirus (HPV) infection; lack of cervical cancer screening; screening errors; and not receiving follow-up care. In this paper, we discuss 2 applications of the Framework for prioritizing cervical cancer prevention efforts in North Carolina.
Box 1. Carolina Framework for Cervical Cancer Prevention.
Public health programs can better prevent cervical cancer by understanding four factors in the Carolina Framework.59,60 The impact of these factors likely varies by geographic region, but they affect women globally.
1. Human papillomavirus (HPV) infection is responsible for nearly all cases of cervical cancer.59 In the U.S., prevalence of HPV infection among women peaks at more than 40% among 20- to 25-year-olds, with decreasing prevalence with older age.61 Among high-risk populations, including women attending STI clinics or who are HIV-positive, prevalence can be greater than 60%.61 Two strains of HPV, types 16 and 18, cause 70% of cervical cancer cases.59 Estimates of the prevalence of these oncogenic types in U.S. women vary by region, and they range from 1.5% to 17.7% (HPV 16) and from 0.2% to 5.3% (HPV 18).61 The Centers for Disease Control and Prevention (CDC) recommend that all adolescents ages 11–12 receive HPV vaccine to protect against these strains of HPV.62 In addition, females up to age 26 and males up to age 21 are eligible for catch-up vaccination if they have not already received the vaccine.63,64 Unfortunately, rates of vaccination are far below the Healthy People 2020 goal of 80% vaccine completion among adolescent girls ages 13–1765: only 33% of girls and 7% of boys in the U.S. had completed the three-dose vaccine series by 2012.66 Among adolescent girls in the U.S. who initiated HPV vaccine, 67% had completed the series (i.e., received all three doses).66
2. Lack of screening for cervical cancer is responsible for a little over half of new cervical cancers. According to national recommendations, most adult women younger than age 65 should receive a Pap test every three years.67–69 Targeting women without recent Pap tests is a crucial goal in cervical cancer prevention, as detection of precancerous lesions or cervical cancer using a Pap test is most common among women whose previous test was greater than three years earlier or who had never been screened.19,60,70–73 Less than three-fourths of all U.S. women have received a timely Pap test,74 and certain subgroups have even lower rates of adherence to this recommendation.16,74 In North Carolina, 88% of women report receiving a Pap test in the last three years,75 though rates are likely to be much lower given errors in self-report.74 Particularly at risk for cervical cancer are women who have never received a Pap test.70,76
3. Pap screening errors (false-negative tests) are responsible for around a third of new cervical cancers.72 Although a Pap test is a powerful screening tool, 23% to 70% of Pap tests in low-risk women fail to detect cervical abnormalities when present.77 To reduce the number of false negatives, the USPSTF (and other regulatory agencies) recommends co-testing with Pap and HPV DNA tests every 5 years for women ages 30–65.41 Unfortunately, HPV DNA tests have higher rates of false-positives and could lead to overdiagnosis,78 so it is important that clinicians follow guidelines that balance the risks of false-positives and false-negatives, such as the USPSTF co-testing recommendation.
4. Inadequate follow-up care is responsible for around a tenth of new cervical cancer cases.72 Most often, this involves women who have received abnormal results on Pap or HPV DNA tests, but who do not receive confirmatory tests or treatment. The causes of loss to follow-up are likely complex but reflect the deeply fractured health care system in the US.19
We first used the Carolina Framework to characterize counties in terms of prevention need. We next used the Carolina Framework to identify recommendations for improving cervical cancer prevention in North Carolina. In this way, we aim to demonstrate practical applications of the Carolina Framework for guiding prevention efforts that will ultimately reduce the burden of cervical cancer.
Methods
Application 1. Prioritizing Counties by Cervical Cancer Prevention Need
To understand the range of cervical cancer prevention need among the 100 counties in North Carolina, we selected 5 indicators based on the Carolina Framework and availability of data.
Data sources
Cervical cancer mortality
The North Carolina State Center for Health Statistics provided age-adjusted annual cervical cancer mortality rates per 100,000 women for each of the state’s 100 counties for the period from 1998–2007.4 We chose to focus on cervical cancer mortality instead of incidence because incidence is subject to several well-known biases.12,13
HPV vaccination (initiation and completion)
The North Carolina Immunization Registry (NCIR) (http://www.immunize.nc.gov/providers/ncir.htm) is an electronic database that over 90% of the state’s primary care providers use to document vaccination. The North Carolina Immunization Branch provided county-level NCIR data on HPV vaccination among girls, ages 13–17, with active records in the registry. Measures were HPV vaccine initiation (i.e., percentage who received ≥ 1 dose) and completion (i.e., percentage who received 3 doses, among those who initiated). We chose this completion measure, instead of absolute levels of completion, because it does not confound completion with initiation.
Pap test screening among Medicaid-insured women
Community Care of North Carolina (CCNC) provided county-level data on the percentage of Medicaid-insured women, ages 21–64, who received at least 1 Pap test between 2009 and 2011. Previous research has found that Medicaid-insured women are somewhat more likely to receive Pap tests than women with other insurance types.14 Thus, these data likely overestimate the prevalence of Pap screening among other women in the state, comprising a conservative measure of cervical cancer prevention need. Reliable county-level data on screening among privately-insured women were unavailable.
Pap test screening among women without recent tests
The National Breast and Cervical Cancer Early Detection Program (NBCCEDP) provides cervical cancer screening services to low-income and under- and uninsured adult women who do not have Medicaid, a population with low rates of regular Pap testing.15–17 The program targets these higher-risk women by requiring that at least 20% of women newly-enrolled in state programs qualify as rarely- or never-screened (i.e., having had no Pap in the previous 5 years). For this study, the North Carolina Breast and Cervical Cancer Control Program (NC BCCCP) provided county-level data from 2010–2012 on the percentage of newly-enrolled women who qualified as rarely- or never-screened.
Analysis
We created a cervical cancer prevention need index (CCPNI) that reflected each county’s performance on all 5 indicators, with higher scores signifying greater need. For cervical cancer mortality, we assigned scores to counties ranging from 0 to 8, or double the weight of each of the other indicators, as it is the most direct measure of cervical cancer prevention need. For the other 4 indicators, we assigned scores ranging from 0 to 4 (for the percentage of Medicaid-insured women who had a recent Pap test) or 1 to 4 (for the other indicators). Counties received a score of 0 for each indicator if they met relevant national guidelines (e.g., Healthy People 2020 goals). We created the cut-off points for each indicator (available from the last author and online at http://ccfnc.com/etc/Cervical%20Cancer%20Prevention%20in%20NC-Strengthening%20Health%20Programs%20and%20Systems.pdf) by inspecting the distribution of the raw data for each indicator and identifying natural groupings that roughly mapped on to quartiles. For example, we scored counties’ cervical cancer mortality rates with the following rules: counties received a 0 if they had mortality rates < 2.5 per 100,000 women (which meets the Healthy People benchmark relevant to the time these mortality data were collected), 2 for rates between 2.5 and 2.9, 4 for rates between 3.0 and 3.9, 6 for rates between 4.0 and 4.9, and 8 for rates greater than 5.0. We summed each county’s scores on the indicators to obtain an overall score on cervical cancer prevention, with a possible range of 3–24. Sensitivity analyses to test the effect of different weighting schemes on the conclusions of the analysis found similar results.
Application 2. Compiling Recommendations for Improving Cervical Cancer Prevention
To identify strategies for cervical cancer prevention with the most promise for North Carolina, we developed recommendations with applications to specific state programs and policies for 2 key pillars of the Carolina Framework (HPV vaccination and Pap screening).
Data sources
We conducted key informant interviews with 19 leaders of healthcare organizations, non-profits, governmental agencies, and quality improvement organizations from around the state. We generated an original list of informants based on contacts established during CCNFC’s 3.5 years of work in cervical cancer prevention. Using a snowball sampling strategy, we contacted additional interviewees based on original informants’ suggestions of other influential stakeholders. We used a semi-structured interview guide informed by the Carolina Framework to elicit informants’ perspectives on existing programs and policies in North Carolina related to HPV vaccination and cervical cancer screening, as well as suggestions for improving existing efforts.
Informants included representatives from several statewide and local organizations. At the level of state government, the Division of Public Health includes the Immunization Branch, which administers NCIR as well as the state’s Vaccines for Children (VFC) program that provides free vaccines, including HPV vaccine, to eligible children; and the Cancer Prevention and Control Branch that administers NC BCCCP. Local health departments support these efforts through health promotion activities and providing healthcare services, including those funded by VFC and NC BCCCP. In addition, the North Carolina Office of Minority Health and Health Disparities promotes health equity and the reduction of health-related disparities, such as those described above in cervical cancer mortality.
Non-profit organizations include Community Care of North Carolina (CCNC), which is a public-private partnership that aims to improve the efficiency of healthcare services. The North Carolina School Community Health Alliance (NCSCHA) supports alternative healthcare sites, including school-located clinics that may deliver HPV vaccine. Other key stakeholders include professional organizations (e.g., the North Carolina Pediatric Society and the North Carolina Academy of Family Physicians) that offer training to healthcare providers responsible for delivering cervical cancer prevention services.
Analysis
Two coders independently reviewed case notes from each interview for suggestions for improving cervical cancer prevention. We used an iterative process of data collection and analysis to generate a list of suggested activities for key state programs and policies. We grouped these state-specific activities under broader recommendations that rely in part on the national recommendations from the Community Guide to Preventive Services18 and Smith and colleagues’ recommendations.19
The University of North Carolina Institutional Review Board exempted this study from review because it uses publicly-available data and interviews with participants operating in their capacity as public officials.
Results
Application 1. Counties’ Cervical Cancer Prevention Need
North Carolina counties’ median cervical cancer mortality rate was 2.7 per 100,000 women annually in 1998–2007, with counties in the top decile of prevention need ranging from 4.5–8.0 (Table 1). The median rate of adolescent girls receiving at least 1 dose of HPV vaccine was 42%, with counties in the top decile of prevention need ranging from 15%–30%. Among adolescent girls initiating HPV vaccine, the median rate of receiving all 3 doses was 57%; the top decile of need was 40%–45%. The median rate of Medicaid-insured women who received a Pap test in the previous 3 years was 59%, with the top decile of need ranging from 40%–51%. The median rate of women, newly-enrolled in NC BCCCP, who qualified as rarely- or never-screened was 15%; the top decile of need ranged from 0%–5%.
Table 1.
North Carolina counties’ cervical cancer prevention need.
| 0%ile | 50%ile | 70%ile | 80%ile | 90%ile | 100%ile | |
|---|---|---|---|---|---|---|
|
|
||||||
| Cervical cancer mortality, per 100,000 women | 0.0 | 2.7 | 3.2 | 3.7 | 4.5 | 8.0 |
| 1+ doses of HPV vaccine among girls ages 13–17 | 62% | 42% | 38% | 35% | 31% | 15% |
| 3 doses of HPV vaccine among girls ages 13–17 who initiated | 84% | 57% | 54% | 51% | 46% | 40% |
| Percent of Medicaid-insured women with Pap tests, last 3 years | 83% | 59% | 55% | 53% | 51% | 40% |
| Percent of BCCCP’s newly-enrolled women without recent Pap tests | 46% | 15% | 10% | 7% | 5% | 0% |
| CCPNI score | 8 | 12 | 15 | 16 | 18 | 23 |
Note. Percentiles reflect lowest (0%ile) to highest (100%ile) need.
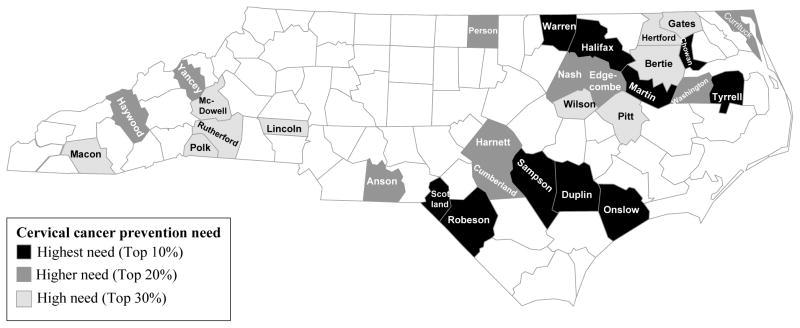
The median CCPNI score based on these 5 measures was 12 (observed range: 8–23; possible range 3–24). Figure 1 highlights the 31 counties with the highest scores (i.e., ≥ 15) on the CCPNI. Counties with high cervical cancer prevention need were primarily in the northeastern and south-central parts of the state, with several counties also in the western part of the state.
Figure 1.
North Carolina counties with high cervical cancer prevention need scores.
Application 2. Recommendations for Improving Cervical Cancer Prevention in North Carolina
Key informants’ suggestions for improving cervical cancer prevention fell into 9 broad recommendations (Table 2).
Table 2.
Recommendations for improving cervical cancer prevention in North Carolina.
| HPV Vaccination |
|
Recommendation 1. Reduce missed opportunities for HPV vaccination among eligible adolescents. Supporting activities:
|
|
Recommendation 2. Encourage pediatricians, family practitioners, nurses, and other healthcare professionals to recommend HPV vaccine. Supporting activities:
|
|
Recommendation 3. Increase provision of adolescent vaccines in alternative settings, including pharmacies and schools. Supporting activities:
|
|
Recommendation 4. Increase funding to establish universal coverage of all CDC-recommended vaccines, including HPV vaccine, to children up to age 18. Supporting activities:
|
| Cervical Cancer Screening |
|
Recommendation 5. Improve recruitment of women rarely- or never-screened for cervical cancer. Supporting activities:
|
|
Recommendation 6. Reduce missed opportunities for cervical cancer screening. Supporting activities:
|
|
Recommendation 7. Encourage adherence to USPSTF guidelines for cervical cancer screening. Supporting activities:
|
|
Recommendation 8. Expand NC BCCCP funding. Supporting activities:
|
| General |
|
Recommendation 9. Increase understanding of the importance of cervical cancer prevention, especially among populations at higher-risk for cervical cancer. Supporting activities:
|
Note. BCCCP = Breast and Cervical Cancer Control Program; CCFNC = Cervical Cancer-Free North Carolina; CCNC = Community Care of North Carolina; CDC = Centers for Disease Control and Prevention; HPV = human papillomavirus; NCIR = North Carolina Immunization Registry; NCSCHA = North Carolina School Community Health Alliance; Pap = Papanicolaou; USPSTF = United States Preventive Services Task Force; WIC = Women, Infants, and Children.
Recommendation 1. Reduce missed opportunities for HPV vaccination among eligible adolescents
Healthcare providers should take every opportunity to address adolescents’ vaccination needs during healthcare visits, including those for acute care and sports physicals,20,21 as adolescents are less likely to attend preventive healthcare visits than younger children. Of the many action steps identified that support this recommendation, immunization quality improvement efforts are particularly important. The state Immunization Branch can train providers using CDC’s Assessment, Feedback, Incentives, and eXchange (AFIX) curriculum, a program that teaches providers techniques they can use to reduce missed opportunities for vaccination. Originally designed to improve early childhood vaccination, AFIX programs for adolescent vaccination are becoming more common. Targeting AFIX visits specifically to HPV vaccination could address the dramatic underuse of this vaccine.22
Recommendation 2. Encourage pediatricians, family practitioners, nurses, and other healthcare professionals to recommend HPV vaccine
Physician or other healthcare provider recommendation is perhaps the most important determinant of HPV vaccine uptake.21,23,24 However, providers often fail to deliver effective recommendations for HPV vaccine.25,26 To support provider recommendation of HPV vaccine, healthcare professional organizations can play a leading role in educating physicians and other healthcare providers about vaccine timetables, contraindications, concomitant vaccination, and strategies for communicating with vaccine hesitant parents. Continuing medical education or maintenance of certification courses could efficiently deliver such education.
Recommendation 3. Increase provision of adolescent vaccines in alternative settings, including pharmacies and schools
Adolescent vaccination in alternative settings is an important complement to vaccination in traditional healthcare settings, especially for adolescents who lack healthcare access. Pharmacies have great potential for HPV vaccine provision, although North Carolina laws currently allow pharmacists to provide vaccines only for adults. In addition, the Community Guide18 recommends school-located programs for adolescent vaccination, which has been tremendously impactful in other countries.27–30 Because school programs to increase HPV vaccination rates in isolation of other CDC-recommended vaccines are less effective,31,32 school-located clinics should offer students the tetanus, diphtheria, and pertussis booster and the meningococcal vaccine in addition to HPV vaccine.
To expand HPV vaccine provision in alternative settings, local health departments can partner with school systems and the North Carolina Institute for Public Health to develop mass vaccination programs. For instance, practitioners in Brunswick County have offered school-located mass vaccination since 2003. In 2010, this program provided HPV vaccine to 137 of 234 (59%) participating adolescents.33
Recommendation 4. Increase funding to establish universal coverage of all CDC-recommended vaccines, including HPV vaccine, to children up to age 18
The cost of HPV vaccine represents a significant barrier to uptake.34–37 One potential solution to this challenge is public funding to support administration to adolescents who may forgo vaccination because of cost. Indeed, studies have demonstrated that HPV vaccination is relatively high in contexts with more generous programs supporting public funding of vaccines.27,38,39 To support this recommendation, healthcare providers, professional organizations, and other individuals could lobby in support of state-level legislation to provide universal coverage of public funding for all CDC-recommended vaccines for children up to age 18. Currently, North Carolina has public funding for HPV vaccine only for children who are Medicaid-eligible, are under- or uninsured, or are American Indian or Alaska Native.40
Recommendation 5. Improve recruitment of women rarely- or never-screened for cervical cancer
As described in Box 1, healthcare providers detect most cases of cervical abnormalities in women who have not received a Pap test in the previous 3 years. Screening programs and healthcare providers should target recruitment efforts at women who have rarely or never received cervical cancer screening, who are Hispanic or African-American, live in rural areas, or who are HIV-positive. As these women may be hard to reach, health departments that are successful in recruiting and screening these women should disseminate effective strategies. To support this recommendation, state and local health departments could identify and adapt existing materials (e.g., from the national Cervical Cancer Resource Directory, http://clearinghouse.cervicalcancerfreecoalition.org/, or from Cancer Control P.L.A.N.E.T., http://cancercontrolplanet.cancer.gov/).
Recommendation 6. Reduce missed opportunities for cervical cancer screening
Systematizing women’s health screenings and modifying intake procedures could simplify preventive health services, thereby reducing missed opportunities. To support this recommendation, CCNC could use its electronic medical records systems (based on Medicaid/Medicare claims data) to alert providers when their patients are overdue for screenings and treatment services.
Recommendation 7. Encourage adherence to USPSTF guidelines for cervical cancer screening
The United States Preventive Services Task Force (USPSTF) adopted new cervical cancer screening guidelines in 2012,41 but many healthcare providers remain unaware of the new guidelines or do not follow them. For instance, USPSTF recommends that most adult women receive Pap tests every 3 years, or co-testing with Pap and HPV DNA tests every 5 years, but many providers screen more often.42–44 To support this recommendation, healthcare professional organizations can educate members about the new USPSTF guidelines through professional conferences and newsletters.
Recommendation 8. Expand NC BCCCP funding
Funding to maintain and improve NC BCCCP’s screening efforts is crucial for continued reductions of the burden of cervical cancer in this state. About 85 of the 360 cervical cancers diagnosed in North Carolina each year happen through this program.6 Thus, NC BCCCP identifies about a quarter of all cases of cervical cancer in the state, though serving less than 1% of adult women. In addition, expanding NC BCCCP (and other BCCCP programs) could allow clinicians to provide follow-up treatment to undocumented immigrant women, who are currently ineligible for treatment funded by BCCCP beyond screening, potentially leading to even more impressive outcomes. Legislators have repeatedly attempted to pass legislation that would allow taxpayers to contribute part of state tax refunds to NC BCCCP. CCFNC coalition partners that do not receive their funding from the state could participate in advocacy activities to demonstrate support for such legislation.
Recommendation 9. Increase understanding of the importance of cervical cancer prevention, especially among populations at higher-risk for cervical cancer
Many parents report that they want more information about HPV vaccine,45–47 have concerns about safety and side effects,25,45 do not understand the recommended ages for routine administration (ages 11–12), or do not know that boys should get the vaccine.48 Similarly, adult women may not understand49,50 or may have misinformation51,52 about cervical cancer screenings, and therefore they may not seek them out. The NC Office of Minority Health and Health Disparities can support this recommendation by expanding the focus of its Community-Focused Eliminating Health Disparities Initiative grants to explicitly include cervical cancer prevention, allowing community organizations to seek their support for campaigns to expand the public’s understanding of cervical cancer screening and HPV vaccination.
Discussion
We applied the Carolina Framework for Cervical Cancer Prevention to identify counties with higher cervical cancer prevention need and to solicit experts’ recommendations for addressing those needs. Cervical cancer prevention need showed striking geographic disparities, with 2 high-need clusters of counties emerging in the northeastern and south-central regions of the state. While the causes of higher need are likely complex, identified counties are notable for being in regions that have lower population density, higher poverty, and higher percentages of African American residents. This pattern suggests that access to care, as well as broader social determinants of health, play a role in cervical cancer prevention need in North Carolina. Whatever the case, state and local program planners must redouble their efforts in these areas in order to counteract the regional challenges that currently undercut the effectiveness of screening and vaccination programs. At the same time, counties with high population density contribute meaningfully to disease burden and should also be a focus of prevention efforts.
Key informants identified a wide array of cervical cancer prevention activities that would work within existing systems, often using current funding. One of the most important overarching recommendations is to reduce missed opportunities for HPV vaccination and cervical cancer screening. Encouraging providers to adopt current national guidelines with regard to screening and vaccination is critical to this goal. The complexity of these problems, together with the large number of identified stakeholders, suggests that recommendations cannot be enacted by any 1 agency alone, but rather require a concerted effort by a coalition of motivated partners. To that end, in September 2013, Cervical Cancer-Free North Carolina convened a statewide conference on optimizing preventive care and reducing cervical cancer mortality for state, county, and community stakeholders. The aim was to foster collaboration for incorporating these recommendations into existing programs and systems. The conference was well attended, with more than 90 participants from over 50 governmental, non-profit, and healthcare organizations across the state. During the conference, participants gave feedback to help refine the recommendations presented here. They prioritized 4 of the 9 recommendations for immediate action (Recommendations 2, 5, 6, and 9, described above).
Strengths of this paper include our pragmatic approach that combined an evidence-based framework with available data and expert opinion to yield actionable, state-specific priorities for cervical cancer prevention. This approach is well-suited for public health practitioners, policymakers, and others who must guide cervical cancer prevention efforts in a timely and resource-efficient manner based on the available evidence. In terms of limitations, we chose to focus our study only on North Carolina, but understanding the national context is also important for improving prevention. County-level estimates of CCPNI indicators are less reliable than state-level estimates,53 so a given county’s performance may be higher or lower than indicated here; however, estimates of health behaviors at smaller geographic areas are becoming increasingly common because of their inherent utility to local decision-makers.54–56 In addition, our use of the Carolina Framework emphasized 2 of the 4 pillars (i.e., HPV infection and lack of screening), but gave less attention to screening errors and follow-up care. While this omission is due largely to the lack of available data through established statewide systems, future work should also address these latter 2 pillars through promising strategies such as co-testing with Pap tests and HPV DNA tests to reduce Pap screening errors57 and patient navigation services to improve follow-up care.58
Future studies should expand on the present application of the Carolina Framework to examine the impact of programs, policies, and approaches we identified in our applications of the Framework. Program planners in North Carolina or other states can use a similar approach to identify high-need areas and generate recommended activities to improve cervical cancer prevention. Evaluation of these applications of the Framework will be important in determining its utility in supporting cervical cancer prevention efforts.
In conclusion, the 4 pillars of the Carolina Framework (HPV infection, lack of screening, screening errors, and lack of follow-up care) identify the main causes of cervical cancer. Addressing these 4 pillars can reduce the incidence of this preventable disease, thereby saving thousands of lives each year. We have demonstrated 2 applications of the Carolina Framework for prioritizing geographic regions and recommended strategies to reduce cervical cancer in North Carolina. In this state, public health practitioners, healthcare providers, non-profit organizations, and other stakeholders can target these areas and activities in order to have the greatest impact on cervical cancer incidence and mortality. Stakeholders in other states can similarly use this approach so as to channel limited funds for cervical cancer prevention to effective programs for the communities that need them most.
Highlights.
We present 2 applications of Carolina Framework for Cervical Cancer Prevention, which outlines 4 causes of cervical cancer incidence.
North Carolina counties varied on cervical health indicators, but 2 high-need regions emerged.
Key informants recommended improvements to existing programs and policies.
Acknowledgments
We wish to thank the key informants who participated in this study.
This research was supported in part by an educational grant from GlaxoSmithKline and the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25 CA57726).
Footnotes
Conflict of Interest Statement
NB has received grants or served on paid advisory boards for GlaxoSmithKline and Merck Sharp & Dohme Corp.
References
- 1.Grauman DJ, Tarone RE, Devesa SS, Fraumeni JF., Jr Alternate ranging methods for cancer mortality maps. J Natl Cancer Inst. 2000;92(7):534–543. doi: 10.1093/jnci/92.7.534. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975–2008. 2011 [Google Scholar]
- 3.U.S. Preventive Services Task Force. Screening for cervical cancer, topic page. [Accessed November 7, 2013.];Updated 2012. [Google Scholar]
- 4.Denslow SA, Knop G, Klaus C, Brewer NT, Rao C, Smith JS. Burden of invasive cervical cancer in North Carolina. Prev Med. 2012;54(3–4):270–276. doi: 10.1016/j.ypmed.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Downs LS, Smith JS, Scarinci I, Flowers L, Parham G. The disparity of cervical cancer in diverse populations. Gynecol Oncol. 2008;109(2 Suppl):S22–30. doi: 10.1016/j.ygyno.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy AM, Dumanovsky T, Visvanathan K, Kahn AR, Schymura MJ. Racial/ethnic and socioeconomic disparities in mortality among women diagnosed with cervical cancer in New York City, 1995–2006. Cancer Causes Control. 2010;21(10):1645–1655. doi: 10.1007/s10552-010-9593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 9.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 10.Pierce Campbell CM, Menezes LJ, Paskett ED, Giuliano AR. Prevention of invasive cervical cancer in the united states: Past, present, and future. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1402–1408. doi: 10.1158/1055-9965.EPI-11-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). . Cancer death rates--Appalachia, 1994–1998. MMWR Morb Mortal Wkly Rep. 2002;51(24):527–529. [PubMed] [Google Scholar]
- 12.Black WC, Ling A. Is earlier diagnosis really better? The misleading effects of lead time and length biases. AJR Am J Roentgenol. 1990;155(3):625–630. doi: 10.2214/ajr.155.3.2117366. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 14.Hsia J, Kemper E, Kiefe C, Zapka J, Sofaer S, Pettinger M, et al. The importance of health insurance as a determinant of cancer screening: Evidence from the Women’s Health Initiative. Prev Med. 2000;31(3):261–270. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- 15.Courtney-Brooks M, Pelkofski EB, Engelhard CL, Duska LR. The Patient Protection and Affordable Care Act: Impact on the care of gynecologic oncology patients in the absence of Medicaid expansion in central Virginia. Gynecol Oncol. 2013;130(2):346–349. doi: 10.1016/j.ygyno.2013.04.468. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). . Cancer screening - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(3):41–45. [PubMed] [Google Scholar]
- 17.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 18.Community Preventive Services Task Force. [Accessed July 29, 2013.];The Guide to Community Preventive Services (The Community Guide) http://www.thecommunityguide.org/index.html. Updated 2013.
- 19.Smith JS, Brewer NT, Saslow D, Alexander K, Chernofsky MR, Crosby R, et al. Recommendations for a national agenda to substantially reduce cervical cancer. Cancer Causes Control. 2013;24(8):1583–1593. doi: 10.1007/s10552-013-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharbanda EO, Stockwell MS, Colgrove J, Natarajan K, Rickert VI. Changes in Tdap and MCV4 vaccine coverage following enactment of a statewide requirement of Tdap vaccination for entry into sixth grade. Am J Public Health. 2010;100(9):1635–1640. doi: 10.2105/AJPH.2009.179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). . Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013 - United States. MMWR Morb Mortal Wkly Rep. 2013;62(29):591–595. [PMC free article] [PubMed] [Google Scholar]
- 23.Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children’s Health. Vaccine. 2012;30(20):3112–3118. doi: 10.1016/j.vaccine.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Reiter PL, McRee AL, Pepper JK, Gilkey MB, Galbraith KV, Brewer NT. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health. 2013;103(8):1419–1427. doi: 10.2105/AJPH.2012.301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimet GD, Rosberger Z, Fisher WA, Perez S, Stupiansky NW. Beliefs, behaviors and HPV vaccine: Correcting the myths and the misinformation. [10.1016/j.ypmed.2013.05.013.];Prev Med. 2013 doi: 10.1016/j.ypmed.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Kepka D, Berkowitz Z, Yabroff KR, Roland K, Saraiya M. Human papillomavirus vaccine practices in the USA: Do primary care providers use sexual history and cervical cancer screening results to make HPV vaccine recommendations? Sex Transm Infect. 2012;88(6):433–435. doi: 10.1136/sextrans-2011-050437. [DOI] [PubMed] [Google Scholar]
- 27.Musto R, Siever JE, Johnston JC, Seidel J, Rose MS, McNeil DA. Social equity in human papillomavirus vaccination: A natural experiment in Calgary Canada. BMC Public Health. 2013;13(1):640–2458-13-640. doi: 10.1186/1471-2458-13-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: National surveillance data. BMJ. 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- 29.Brabin L, Roberts SA, Stretch R, Baxter D, Chambers G, Kitchener H, et al. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: Prospective cohort study. BMJ. 2008;336(7652):1056–1058. doi: 10.1136/bmj.39541.534109.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stretch R. Implementing a school-based HPV vaccination programme. Nurs Times. 2008;104(48):30–33. [PubMed] [Google Scholar]
- 31.Reiter PL, McRee AL, Pepper JK, Brewer NT. Default policies and parents’ consent for school-located HPV vaccination. [10.1007/s10865-012-9397-1.];J Behav Med. 2012 doi: 10.1007/s10865-012-9397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes KA, Entzel P, Berger W, Caskey RN, Shlay JC, Stubbs BS, et al. Early lessons learned from extramural school programs that offer HPV vaccine. J Sch Health. 2013;83(2):119–126. doi: 10.1111/josh.12007. [DOI] [PubMed] [Google Scholar]
- 33.Brunswick County Health Department. Adolescent immunization, 2010. 2011 [Google Scholar]
- 34.Keating KM, Brewer NT, Gottlieb SL, Liddon N, Ludema C, Smith JS. Potential barriers to HPV vaccine provision among medical practices in an area with high rates of cervical cancer. J Adolesc Health. 2008;43(4 Suppl):S61–7. doi: 10.1016/j.jadohealth.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Sudenga SL, Royse KE, Shrestha S. Role and uptake of human papillomavirus vaccine in adolescent health in the United States. Adolesc Health Med Ther. 2011;2011(2):63–74. doi: 10.2147/AHMT.S15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pourat N, Jones JM. Role of insurance, income, and affordability in human papillomavirus vaccination. Am J Manag Care. 2012;18(6):320–330. [PubMed] [Google Scholar]
- 37.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30(24):3534–3540. doi: 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheinfeld Gorin SN, Glenn BA, Perkins RB. The human papillomavirus (HPV) vaccine and cervical cancer: Uptake and next steps. Adv Ther. 2011;28(8):615–639. doi: 10.1007/s12325-011-0045-x. [DOI] [PubMed] [Google Scholar]
- 39.Gowda C, Dempsey AF. Medicaid reimbursement and the uptake of adolescent vaccines. Vaccine. 2012;30(9):1682–1689. doi: 10.1016/j.vaccine.2011.12.097. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) [Accessed November 1, 2013.];VFC childhood vaccine supply policy. 2009 http://www.cdc.gov/vaccines/programs/vfc/about/vac-supply-policy/supply-2009.html. Updated 2010.
- 41.U. S. Preventive Services Task Force. [Accessed July 29, 2013.];Screening for cervical cancer. http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm. Updated 2012.
- 42.Henderson JT, Saraiya M, Martinez G, Harper CC, Sawaya GF. Changes to cervical cancer prevention guidelines: Effects on screening among U.S. women ages 15–29. Prev Med. 2013;56(1):25–29. doi: 10.1016/j.ypmed.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwaiger C, Aruda M, LaCoursiere S, Rubin R. Current guidelines for cervical cancer screening. J Am Acad Nurse Pract. 2012;24(7):417–424. doi: 10.1111/j.1745-7599.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- 44.Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): Symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis. 2010;14(1):73–80. doi: 10.1097/lgt.0b013e3181cec411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilkey MB, Moss JL, McRee AL, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30(41):5928–5934. doi: 10.1016/j.vaccine.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottlieb SL, Brewer NT, Sternberg MR, Smith JS, Ziarnowski K, Liddon N, et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health. 2009;45(5):430–437. doi: 10.1016/j.jadohealth.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med. 2007;45(2–3):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: Results from the National Immunization Survey-Teen, 2010–2011. Vaccine. 2013;31(26):2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler TA. Increasing mammography and cervical cancer knowledge and screening behaviors with an educational program. Oncol Nurs Forum. 2012;39(1):61–68. doi: 10.1188/12.ONF.61-68. [DOI] [PubMed] [Google Scholar]
- 50.Finney Rutten LJ, Nelson DE, Meissner HI. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987–2000) Prev Med. 2004;38(3):258–268. doi: 10.1016/j.ypmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Perry MA. How can the uptake of cervical cytology screening be improved? Nurs Stand. 2001;16(11):33–36. doi: 10.7748/ns2001.11.16.11.33.c3124. [DOI] [PubMed] [Google Scholar]
- 52.Branoff R, Santi K, Campbell JK, Roetzheim R, Oler M. A family practice residency cervical screening project: Perceived screening barriers. Fam Med. 1997;29(2):119–123. [PubMed] [Google Scholar]
- 53.Gowda C, Dong S, Potter RC, Dombkowski KJ, Stokley S, Dempsey AF. A systematic evaluation of different methods for calculating adolescent vaccination levels using immunization information system data. Public Health Rep. 2013;128(6):489–497. doi: 10.1177/003335491312800608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(4):591–599. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider KL, Lapane KL, Clark MA, Rakowski W. Using small-area estimation to describe county-level disparities in mammography. Prev Chronic Dis. 2009;6(4):A125. [PMC free article] [PubMed] [Google Scholar]
- 56.Eberth JM, Hossain MM, Tiro JA, Zhang X, Holt JB, Vernon SW. Human papillomavirus vaccine coverage among females aged 11 to 17 in Texas counties: An application of multilevel, small area estimation. Womens Health Issues. 2013;23(2):e131–41. doi: 10.1016/j.whi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright TC, Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283(1):81–86. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
- 58.Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1691–1700. doi: 10.1158/1055-9965.EPI-12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 60.Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, et al. Cervical cancer in women with comprehensive health care access: Attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 61.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: A global review. J Adolesc Health. 2008;43(4 Suppl):S5–25. S25.e1–41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) [Accessed March 8, 2013.];HPV vaccines. http://www.cdc.gov/hpv/vaccine.html. Updated 2013.
- 63.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention (CDC). . FDA licensure of bivalent human papillomavirus vaccine (HPV2, cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 65.Department of Health and Human Services. Immunization and infectious diseases. http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23. Updated 2012.
- 66.Centers for Disease Control and Prevention (CDC). . National and state vaccination coverage among adolescents aged 13–17 years--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685–693. [PMC free article] [PubMed] [Google Scholar]
- 67.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre H, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention (CDC). . Cervical cancer screening among women aged 18–30 years - United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2013;61(51–52):1038–1042. [PubMed] [Google Scholar]
- 69.Eddy DM. Screening for cervical cancer. Ann Intern Med. 1990;113(3):214–226. doi: 10.7326/0003-4819-113-3-214. [DOI] [PubMed] [Google Scholar]
- 70.Clarke EA, Anderson TW. Does screening by “pap” smears help prevent cervical cancer? A case-control study. Lancet. 1979;2(8132):1–4. doi: 10.1016/s0140-6736(79)90172-7. [DOI] [PubMed] [Google Scholar]
- 71.Kinney W, Sung HY, Kearney KA, Miller M, Sawaya G, Hiatt RA. Missed opportunities for cervical cancer screening of HMO members developing invasive cervical cancer (ICC) Gynecol Oncol. 1998;71(3):428–430. doi: 10.1006/gyno.1998.5135. [DOI] [PubMed] [Google Scholar]
- 72.Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: Systematic review and meta-analysis. Prev Med. 2007;45(2–3):93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 73.van der Graaf Y, Zielhuis GA, Peer PG, Vooijs PG. The effectiveness of cervical screening: A population-based case-control study. J Clin Epidemiol. 1988;41(1):21–26. doi: 10.1016/0895-4356(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 74.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 75.University of North Carolina Lineberger Comprehensive Cancer Center. University cancer research fund: 2007 cancer report card. 2007 [Google Scholar]
- 76.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: A systematic review and meta-analysis. Syst Rev. 2013;2:35–4053-2-35. doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Ann Intern Med. 2000;132(10):810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 78.Malila N, Leinonen M, Kotaniemi-Talonen L, Laurila P, Tarkkanen J, Hakama M. The HPV test has similar sensitivity but more overdiagnosis than the pap test--a randomised health services study on cervical cancer screening in Finland. Int J Cancer. 2013;132(9):2141–2147. doi: 10.1002/ijc.27850. [DOI] [PubMed] [Google Scholar]