Abstract
A substantial body of evidence suggests an etiologic role of inflammation and oxidative/nitrosative stress in asthma pathogenesis. Fractional concentration of nitric oxide in exhaled air (FeNO) may provide a non-invasive marker of oxidative/nitrosative stress and aspects of airway inflammation. We examined whether children with elevated FeNO are at increased risk for new-onset asthma.
We prospectively followed 2206 asthma-free children (age 7–10 years) who participated in the Children’s Health Study. We measured FeNO and followed these children for three years to ascertain incident asthma cases. Cox proportional hazard models were fitted to examine the association between FeNO and new-onset asthma.
We found that FeNO was associated with increased risk of new-onset asthma. Children with the highest quartile of FeNO had more than a two-fold increased risk of new-onset asthma compared to those with the lowest quartile (hazard ratio: 2.1; 95% confidence interval: 1.3–3.5). This effect did not vary by child’s history of respiratory allergic symptoms. However, the effect of elevated FeNO on new-onset asthma was most apparent among those without a parental history of asthma.
Our results indicate that children with elevated FeNO are at increased risk for new-onset asthma, especially if they have no parental history of asthma.
Keywords: Incident Asthma, Exhaled Nitric Oxide, Airway Inflammation
Asthma is the most common childhood chronic disease and studies have documented its rise in prevalence over the past several decades [1]. Although the etiology of asthma has been extensively studied, the pathogenesis and the factors causing the rapid rise in prevalence have yet to be firmly established. To reduce the burden from asthma, more research that focuses on asthma pathogenesis is needed. The current understanding of the pathogenesis of asthma suggests that oxidative and nitrosative stress and dysregulated inflammatory responses play a role in asthma etiology [2–3]. Fractional exhaled nitric oxide concentration (FeNO) provides a non-invasive marker of oxidative and nitrosative stress and aspects of airway inflammation that may have a role in childhood asthma and allergic airway disease pathogenesis [4–5]. The potential usefulness of FeNO in studies of asthma etiology is illustrated by a recent Swedish population-based study of healthy adults without respiratory symptoms that found that elevated FeNO predicted the development of wheeze [6].
The Children’s Health Study (CHS), a longitudinal population-based study of respiratory health among school-age children in 13 Southern California communities, provided an opportunity to investigate whether children with elevated FeNO are at increased risk of newonset asthma. We also hypothesized that the elevated risk of new-onset asthma associated with FeNO differs by child’s allergic status and by parental history of asthma. We examined the association of new-onset asthma with FeNO using data collected annually from 2004–2007 from a cohort of 2206 children whose parents did not report a physician diagnosis of asthma at study entry.
METHODS
Study Subjects
Participants were from a CHS cohort enrolled during 2002–2003 when they were in kindergarten or first grade (average 5–6 years old). Informed consent from a parent/guardian and assent from each child were obtained before FeNO testing. The University of Southern California’s Institutional Review Board approved the protocol. Parents completed an annual self-administered questionnaire that included socio-demographic and child’s health characteristics and a brief exposure history, including exposure to secondhand tobacco smoke and in utero exposure to maternal smoking. During 2004–2005, parental consent was obtained and FeNO testing was performed on 2585 subjects out of 3146 eligible cohort members (82%). We further excluded children whose parent reported a physician diagnosis of asthma during the school year of FeNO testing (n=62); children who were lost to follow-up during the year after FeNO testing (n=241); and children whose breath samples were invalidated due to storage or technical problems with the analyzer (n=76); resulting in 2206 subjects.
New-Onset Asthma Definition
An incident asthma case was defined as a child with no prior parental report of a physician diagnosis of asthma at FeNO testing whose parent reported a physician diagnosis of asthma in an annual follow-up questionnaire during the three-year follow-up period.
Socio-demographic and Medical History Information
Race/ethnicity was defined as non-Hispanic white, Hispanic, African American, Asian/Hawaiian/Pacific Islander, and mixed/other ethnicities, based on parental report. Education was defined as the highest level of education attainment of the parent or guardian who completed the questionnaire. Annual household income was used to assess the role of socioeconomic status. We dichotomized self-reported health insurance coverage to assess the role of access to health care.
At study entry, selected aspects of the child’s and parents’ medical histories were collected, and in each successive school year, an update questionnaire inquiring about the child’s intervening year of health was completed by parents and returned to study staff.. Child’s history of respiratory allergy included any hay fever or allergic rhinitis. Child’s history of ever wheezing and wheezing in the past 12 months were defined as yes/no. Parental history of asthma was defined as an asthma diagnosis in either biological parent. During annual school visits, subjects’ height and weight were measured using standardized protocols.
FeNO Collection and Analysis
Details of breath collection and FeNO analysis used in this study were reported previously [7–8]. In brief, offline breath collection was performed in the morning at schools to avoid traffic-related peaks of ambient NO and possible effects of recent eating on FeNO, according to American Thoracic Society recommendations [9–10]. Each CHS community was visited at least twice in different seasons, to minimize confounding of location and season effects. Health status at testing was evaluated by questionnaire; subjects with symptoms of acute respiratory infection within the past 3 days were excluded or rescheduled. Exhaled breath samples for offline testing were obtained using Bag Collection and Sampling Kits and 1.5-l aluminized Mylar bags (Sievers Division, GE Analytical Instruments, Boulder, CO) by the deadspace-discard method at 100 ml/sec expiratory flow, and were stored in temperature-controlled coolers and transported to and analyzed at a central laboratory (Sievers Model 280i chemiluminescent NO analyzer). Indoor air samples were also collected to estimate subjects’ ambient NO exposure at testing. Lag times between collection and analysis ranged from 2 to 26 hours. When this study began, offline collection was the most feasible method of collection for large field studies. Excellent agreement has been demonstrated between offline and online measurements in laboratory-based studies, using a variety of techniques [11–13]. In this study, offline FeNO values were converted to online FeNO values for all children, as would be measured at 50 ml/sec expiratory flow [10], using a prediction model (model adjusted R2=0.94) determined in a later substudy of 362 children with concurrent online and offline FeNO measurements [7]. The substudy included 1 or 2 testing days at each of 15 schools in 8 communities to cover most of the geographic and seasonal range. Online measurements were performed at 50 ml/sec expiratory flow using EcoMedics CLD-88-SP analyzers, with DeNOx accessories to provide NO-free inhaled air (EcoPhysics Inc., Ann Arbor, MI/Duernten, Switzerland), according to the manufacturer’s instructions based on professional societies' recommendations [4, 9–10]. The prediction model included adjustment for ambient NO concentration and lag time between collection and analysis. We used predicted online FeNO (hereafter, FeNO) in subsequent analyses.
Statistical Methods
In order to investigate the relationship of FeNO with new-onset asthma, we calculated incidence rates and conducted descriptive analyses, and we explored a series of multivariate modeling approaches to account for potential confounders and heterogeneity of effects within subgroups of children. Crude incidence rates for new-onset asthma were calculated by dividing the number of cases by the total person-years at risk. For children who developed new-onset asthma, follow-up was considered complete at the time of reported diagnosis. Incidence rates were calculated for age-specific quartiles of FeNO. We have previously shown that FeNO varies with age [8]. Therefore, age-specific FeNO quartiles were computed for each of three age strata (defined as less than 8yrs, 8 to 9 yrs, greater than 9 yrs) for analyses (see Table S1 in the online supplement).
To further investigate the association between FeNO and new-onset asthma, we fitted Cox proportional hazards models with sex- and age-specific baseline hazards (with age defined as integer age at FeNO testing). All models were adjusted for community of residence and race/ethnicity to account for the study design, and we assessed potential confounders identified a priori including parental education, annual family income, health insurance, parental history of asthma, BMI, household pets or pests, humidifier use, average outdoor air pollution levels on the day of the FeNO measurement (NO2, O3, PM2.5, PM10), lifetime secondhand smoke (SHS) exposure, and in utero exposure to maternal smoking. Covariates were considered confounders if the hazard ratio changed by 10% after addition to the base model. The final model was additionally adjusted for lifetime history of wheezing. Heterogeneity of associations among subgroups was assessed by fitting models with appropriate interaction terms, and statistical significance was tested by partial likelihood ratio tests [14]. Stratified analyses were performed in the presence of significant interaction (p-value< 0.05). The nature of the nonlinearity in FeNO effects was explored using splines, piecewise cubic polynomials that are joined smoothly at a number of breakpoints known as knots [15].
Sensitivity analyses were conducted by limiting the asthma case definition to those (1) reporting a diagnosis greater than one year after FeNO testing; (2) without a history of respiratory allergy; and (3) reporting use of inhaled medication in the diagnosis year follow-up questionnaire. To explore the role of wheezing prior to the onset of asthma, we restricted the analysis to children without a history of ever wheezing and 12 months prior to FeNO testing.
All analyses were conducted using SAS software (SAS Institute, Cary, NC) version 9.1. All hypothesis testing was conducted assuming a 0.05 significance level and a two-sided alternative hypothesis.
RESULTS
Study Population and Cohort Follow-up
Descriptive analyses are presented in Table 1. There were approximately equal numbers of boys and girls, and sex was not associated with new-onset asthma. Nearly 50% of children were 8–9 years old at initial FeNO measurement. The population was ethnically diverse: 55% of the participants were Hispanic white. This was largely a middle-class population: the majority of children lived in households earning more than $50,000 per year, had health insurance, and had parents with at least some college education. None of these characteristics was associated with new-onset asthma.
Table 1.
Subject Characteristics and Associations with New-Onset Asthma
| Subject Characteristic* | N=2206 | % | Hazard Ratio† |
95% CI | |
|---|---|---|---|---|---|
| Female | 1155 | 52% | 1.01 | 0.71–1.44 | |
| Age at FeNO testing | |||||
| <8 years | 699 | 32% | 0.77 | 0.48–1.25 | |
| 8–9 years | 1064 | 48% | 0.74 | 0.48–1.16 | |
| >9 years | 443 | 20% | 1 | ||
| Race/Ethnicity | |||||
| Non-Hispanic White | 788 | 36% | 1 | ||
| Hispanic | 1212 | 55% | 0.92 | 0.60–1.41 | |
| African American | 36 | 2% | 1.82 | 0.62–5.31 | |
| Asian/Hawaiian/Pacific Islander | 67 | 3% | 0.64 | 0.20–2.09 | |
| Other | 101 | 5% | 1.30 | 0.61–2.78 | |
| Allergic Status at Study Entry | |||||
| Never allergy | 1104 | 50% | 1 | ||
| Former hayfever or allergic rhinitis | 543 | 25% | 1.65 | 1.05–2.60 | |
| Current hayfever or allergic rhinitis | 557 | 25% | 2.38 | 1.57–3.60 | |
| History of Wheeze | |||||
| No lifetime history of wheeze | 1598 | 73% | 1 | ||
| Lifetime wheeze | 604 | 27% | 4.85 | 3.38–6.96 | |
| No wheeze 12 months prior to study entry | 1983 | 95% | 1 | ||
| Wheeze 12 months prior to study entry | 110 | 5% | 4.95 | 3.12–7.86 | |
| Any Family History of Asthma | 317 | 16% | 1.99 | 1.32–3.01 | |
| Exposure to Secondhand Smoke (SHS) | 206 | 11% | 0.99 | 0.55–1.80 | |
| In Utero Exposure to Maternal Smoking | 131 | 6% | 0.87 | 0.40–1.90 | |
| Annual Household Income | |||||
| <$15,000 | 268 | 14% | 1.29 | 0.69–2.40 | |
| $15,000–$49,999 | 594 | 32% | 1.04 | 0.64–1.69 | |
| >=$50,000 | 1003 | 54% | 1 | ||
| No Health Insurance | 260 | 13% | 1.14 | 0.64–2.03 | |
| Parent Education Level | |||||
| < 12th grade | 425 | 21% | 0.92 | 0.43–1.99 | |
| 12th grade | 368 | 18% | 0.73 | 0.35–1.53 | |
| Some college | 754 | 36% | 1.04 | 0.58–1.87 | |
| College | 284 | 14% | 0.86 | 0.43–1.74 | |
| Some graduate | 242 | 12% | 1 | ||
| BMI Percentile Category | |||||
| Underweight (<5th percentile) | 54 | 2% | 2.05 | 0.82–5.14 | |
| Normal weight (5th to <85th percentile) | 1371 | 62% | 1 | ||
| Overweight/Obese (≥85th percentile) | 781 | 35% | 1.44 | 0.99–2.08 | |
Numbers may not equal 2206 due to missing values
Adjusted for race/ethnicity and community with baseline strata for age and gender (where appropriate)
We ascertained 129 cases of new-onset asthma over a three-year period of follow-up (69 females, 60 males). The overall crude incidence rate was 22.2 per 1000 person-years (pyrs) (see Table S2 in the online supplement for rates by sex, ethnicity, and other select characteristics). The overall mean and median follow-up times were 2.59 and 2.93 years, respectively, and about 25% of the participants were lost prior to the end of follow-up. The proportion of possible follow-up time did not vary substantially by sex, ethnicity, or quartile of FeNO; however, there were small but significant differences in loss to follow-up rates with respect to parental education, family income, and child’s health insurance coverage (data not shown). Based on telephone interviews conducted in the CHS with the families of subjects who left the study schools, loss to follow-up was primarily due to family moves out of the school catchment area related to a change in employment [16].
Selected Health and Exposure Characteristics and Risk of New-Onset Asthma
Fifty percent of the participants reported having no history of respiratory allergy at study entry; the remaining 50% were split evenly between past history of respiratory allergy (history of hay fever or allergic rhinitis but no current symptoms) and current respiratory allergy (symptoms within the previous 12 months) (Table 1). Children with any history of respiratory allergy showed an increased risk of new-onset asthma relative to the never-allergy group. Any history of wheezing and wheezing in the 12 months prior to FeNO testing were present in 27% and 5% in the participants, respectively, and were associated with a nearly five-fold increased risk of new-onset asthma. Sixteen percent reported a parental history of asthma which was associated with a two-fold increased risk of new-onset asthma in the child.
Sixty-two percent of study participants had normal body mass index (BMI) at baseline. Neither underweight (<5th percentile) nor overweight/obese (≥85th percentile) was significantly associated with increased risk of new-onset asthma. Neither lifetime secondhand smoke exposure nor in utero exposure to maternal smoking was associated with risk of new-onset asthma. Prevalence of SHS exposure and in utero exposure to maternal smoking in this population was lower than in previous CHS cohorts[17] (11% and 6%, respectively).
Distribution of FeNO
As we previously reported [8], FeNO followed an approximately log normal distribution (mean: 13.6 ppb; median: 10.1 ppb; standard deviation: 12.0 ppb; range: 2.3–132.4 ppb) (Figure 1). The median concentrations of FeNO and ranges (at baseline) among subjects who developed new-onset asthma was 10.9 ppb (range: 3.2–132.4) versus 10.1 ppb (range: 2.3–107.2) among subjects who did not develop asthma.
Figure 1.
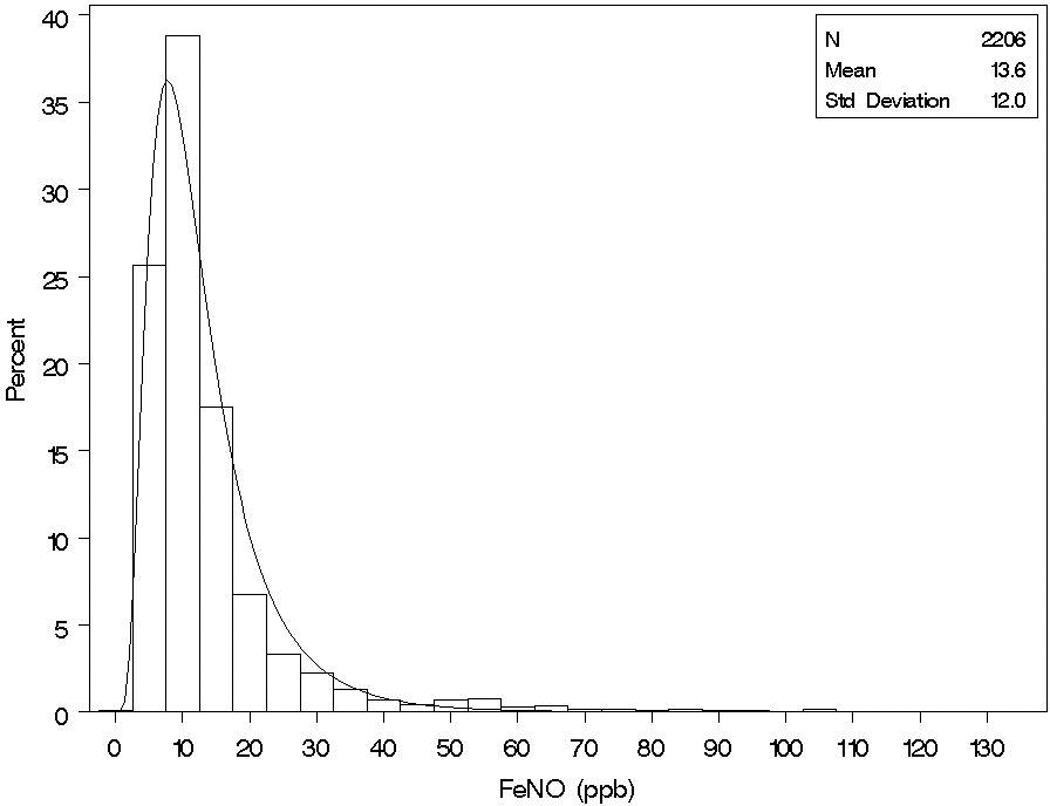
Distribution of Exhaled Nitric Oxide at Baseline.
FeNO and risk of new-onset asthma
Elevated FeNO was associated with an increased risk of new-onset asthma (Table 2 and Figure 2). Children with FeNO in the highest quartile at the start of follow-up had more than a two-fold increased risk of incident asthma compared to those with FeNO in the lowest quartile (HR: 2.11; 95% CI: 1.26–3.51), after adjusting for race/ethnicity, community of residence, and lifetime history of wheeze. We observed an increasing trend of asthma risk with increasing quartiles of FeNO (p trend <0.01). The association of new-onset asthma with FeNO was not substantially affected by adjustment for parental education, family income, health insurance, family history of asthma, household pets or pests, humidifier use, BMI, daily average air pollution levels (on the day of FeNO measurement) secondhand smoke exposure, or in utero exposure to maternal smoking (data not shown).
Table 2.
Association of Exhaled Nitric Oxide (FeNO) with New-Onset Asthma in the Children's Health Study
| Age-Specific Quartiles of FeNO at Baseline |
New-onset Asthma |
No Asthma |
HR1 | 95% CI | ||
|---|---|---|---|---|---|---|
| (N= 129) | % | (N= 2077) | % | |||
| Quartile 1 | 24 | 19% | 528 | 25% | 1 | |
| Quartile 2 | 30 | 23% | 521 | 25% | 1.53 | 0.89–2.63 |
| Quartile 3 | 30 | 23% | 523 | 25% | 1.68 | 0.97–2.90 |
| Quartile 4 | 45 | 35% | 505 | 24% | 2.11 | 1.26–3.51 |
| ptrend<0.01 | ||||||
HR=Hazard ratio, adjusted for race/ethnicity, lifetime wheeze and community with baseline strata for age and gender
Figure 2.
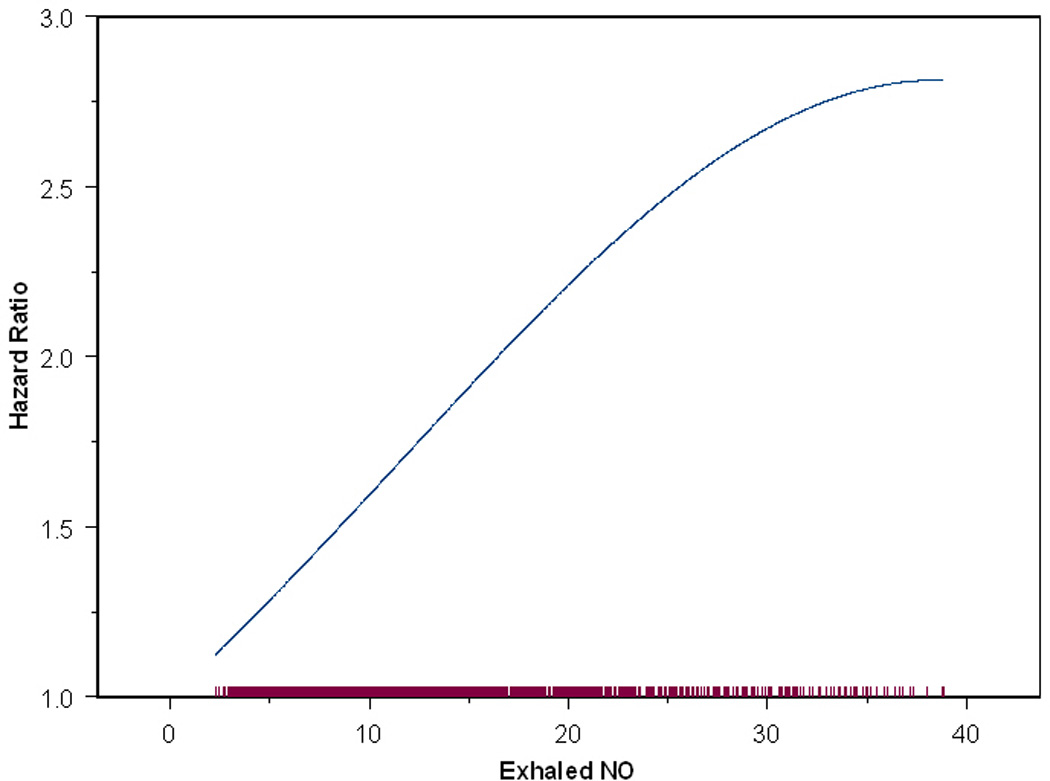
Hazard Ratio Function of the Effect of Exhaled NO on New Onset Asthma. The model was fit for subjects with FeNO <= 40 ppb (approximately 95% of subjects) due to sparseness of data above 40 ppb.
To assess the role of past wheezing, we adjusted the risk estimates for wheezing history and conducted analyses among children without any history of wheezing. Adjustment for any history of wheezing changed the risk estimates by slightly more than 10%. In sensitivity analyses restricting the cohort to children without a history of wheezing (n=1602) or without wheezing in the 12 months prior to FeNO measurement (n=2096), we found a similar increased risk of new-onset asthma for those with the highest quartile of FeNO (Table 3).
Table 3.
Exhaled Nitric Oxide (FeNO) and Risk of New-Onset Asthma, Restricted to Children without Lifetime Wheezing and without Wheezing in the 12 Months Prior to Study Entry
| No lifetime history of wheezing (N=1602) |
No wheezing in 12 months prior to study entry (N=2096) |
|||
|---|---|---|---|---|
| Age-Specific Quartiles of FeNO at Baseline |
HR1 | 95% CI | HR2 | 95% CI |
| Quartile 1 | 1 | 1 | ||
| Quartile 2 | 2.09 | 0.89–4.95 | 1.70 | 0.95–3.06 |
| Quartile 3 | 0.99 | 0.36–2.66 | 1.41 | 0.76–2.63 |
| Quartile 4 | 2.42 | 1.00–5.86 | 2.19 | 1.22–3.90 |
| ptrend=0.19 | ptrend=0.02 | |||
HR=Hazard ratio, adjusted for race/ethnicity, and community and stratified by integer age and sex in children without a history of wheezing (n=48 cases and 1554 noncases)
HR=Hazard ratio, adjusted for race/ethnicity and community and stratified by integer age and sex in children without reported wheezing in the 12 months prior to study entry (n=105 cases and 1991 noncases)
To assess the effect of asthma case definition, we conducted analyses restricting cases to those with recent inhaled medication use. We found that the estimates for the effects of FeNO on new-onset asthma were larger (over four-fold risk comparing the highest to the lowest quartile of FeNO, Tables 4 and S3). To investigate the contribution of delayed asthma diagnosis, we restricted the analysis to follow-up starting in the second year and found that eliminating the first year of follow-up did not substantially alter the relative risk estimates for FeNO (Table 4).
Table 4.
Exhaled Nitric Oxide (FeNO) and Risk of New-Onset Asthma: Restricted Case Definitions
| Restricted to Cases Reporting a Diagnosis After More than One Year of Follow-up |
Restricted to Cases with No History of Allergy |
Restricted to Cases Reporting Recent Inhaled Medication Use† |
Restricted to Cases Categorized as Moderate or Severe# |
|||||
|---|---|---|---|---|---|---|---|---|
| (N=2154; 77 cases) |
(N=2120; 43 cases) |
(N=2133; 56 cases) |
(N=2142; 65 cases) |
|||||
| Age-Specific Quartiles of FeNO at Baseline |
HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | HR* | 95% CI |
| Quartile 1 | 1 | 1 | 1 | 1 | ||||
| Quartile 2 | 1.61 | 0.78–3.31 | 1.59 | 0.63–3.99 | 3.01 | 1.14–7.89 | 1.45 | 0.58–3.60 |
| Quartile 3 | 2.09 | 1.29–4.25 | 1.68 | 0.66–4.27 | 3.57 | 1.35–9.44 | 2.21 | 0.94–5.60 |
| Quartile 4 | 2.17 | 1.09–4.34 | 1.90 | 0.77–4.72 | 4.29 | 1.70–10.80 | 4.22 | 1.97–9.02 |
| ptrend=0.02 | ptrend=0.18 | ptrend<0.005 | ptrend<0.0001 | |||||
HR=Hazard ratio, adjusted for race/ethnicity, community, and lifetime wheeze and stratified by integer age and sex
Recent inhaled medication use is defined as any rescue or controller medication in the previous 12 months, reported on the diagnosis year follow-up questionnaire.
Moderate to severe asthma (65 cases) was defined as at least one attack of wheezing the past 12 months or awakened at night due to wheezing in the past 12 months (in the year of diagnosis on the follow-up questionnaire). Twenty-four of these cases were defined as severe which were classified as four or more wheeze attacks in the previous 12 months or one or more nights per week awakened with wheeze or limited speech due to wheezing in the previous 12 months (in the diagnosis year on the follow-up questionnaire). Due to the small numbers of severe cases, moderate and severe cases were combined in the analysis.
FeNO and new-onset asthma by allergy status and parental history of asthma
In contrast to our hypothesis, we did not find evidence that the effect of FeNO on new-onset asthma depended on respiratory allergy status (Table 5). The pattern of increasing risk of new-onset asthma with increasing FeNO was observed in children with and without a reported history of respiratory allergy.
Table 5.
Association of Exhaled Nitric Oxide (FeNO) with New-Onset Asthma by Respiratory Allergy Status
| History of Respiratory Allergy |
||||||||
|---|---|---|---|---|---|---|---|---|
| Age-Specific Quartiles of FeNO at Baseline |
Never |
Current or Former |
||||||
| Cases | Non- cases |
HR* | 95% CI | Cases | Non-cases | HR* | 95% CI | |
| Quartile 1 | 8 | 259 | 1 | 16 | 269 | 1.20 | 0.51–2.84 | |
| Quartile 2 | 11 | 310 | 1.30 | 0.52–3.23 | 19 | 211 | 2.10 | 0.91–4.85 |
| Quartile 3 | 11 | 280 | 1.67 | 0.66–4.20 | 19 | 243 | 2.01 | 0.87–4.63 |
| Quartile 4 | 13 | 212 | 2.21 | 0.90–5.38 | 32 | 293 | 2.44 | 1.10–5.39 |
| ptrend<0.05† | ptrend<0.05† | |||||||
| P interaction=0.89‡ | ||||||||
HR=Hazard ratio, adjusted for race/ethnicity, lifetime wheeze and community with baseline strata for age and gender
Trend tests conducted in stratified models
P-value based on the chi-square statistic using the likelihood ratio test to compare a model with base terms to a model also containing the multiplicative interaction term
The effect of FeNO on new-onset asthma differed among children with and without a parental history of asthma (Table 6 and online supplement Figure S1), p interaction<0.05. The observed increasing risk of asthma development was most apparent among children without a parental history of asthma. Compared to children with the lowest quartile of FeNO and no parental history of asthma, children with the highest quartile of FeNO and no parental history of asthma had more than a three-fold increased risk of new-onset asthma (HR: 3.18, 95% CI: 1.66, 6.08). This pattern of increasing risk with higher levels of FeNO was observed among children with either maternal or paternal asthma (data not shown). Although parental history of asthma was directly associated with elevated risk of new-onset asthma, we observed little evidence for association of increasing FeNO with increasing risk of new-onset asthma in children with a parental history of asthma (although power was limited by sample size and number of cases in this group).
Table 6.
Association of Exhaled Nitric Oxide (FeNO) with New-Onset Asthma by Parental History of Asthma
| Parental History of Asthma |
||||||||
|---|---|---|---|---|---|---|---|---|
| No |
Yes |
|||||||
| Age-Specific Quartiles of FeNO at Baseline |
Cases | Non- cases |
HR* | 95% CI | Cases | Non- cases |
HR* | 95% CI |
| Quartile 1 | 13 | 393 | 1 | 9 | 81 | 2.55 | 1.08–6.04 | |
| Quartile 2 | 19 | 408 | 1.66 | 0.82–3.39 | 9 | 61 | 3.97 | 1.67–9.41 |
| Quartile 3 | 18 | 395 | 1.80 | 0.87–3.73 | 7 | 65 | 3.77 | 1.48–9.61 |
| Quartile 4 | 36 | 380 | 3.18 | 1.66–6.08 | 8 | 77 | 2.17 | 0.88–5.34 |
| ptrend<0.001† | ptrend=0.33† | |||||||
| P interaction<0.05‡ | ||||||||
HR=Hazard ratio, adjusted for race/ethnicity, lifetime wheeze and community with baseline strata for age and gender
Trend tests conducted in stratified models
P-value based on the chi-square statistic using the likelihood ratio test to compare a model with base terms to a model containing the interaction term
In further analyses, the association of FeNO with new-onset asthma did not differ in boys and girls, children of different ethnicity, those exposed to secondhand smoke, or those exposed in utero to maternal smoking (data not shown).
DISCUSSION
We found that children with higher FeNO had a substantial risk of incident asthma compared to children with low FeNO. The use of FeNO in asthma clinical practice has been extensively investigated [18]. While the role of FeNO in clinical practice remains unclear [19], a number of studies have supported the use of FeNO in monitoring adherence to medication [20], maintaining asthma control and predicting relapse [21–22]. Especially in the presence of symptoms, elevated FeNO (e.g., above 35 ppb in steroid-naïve patients) has been supportive of asthma diagnosis [18, 23]. However, to our knowledge, this is the first investigation to demonstrate the predictive value of FeNO for identifying children at risk for developing asthma, thereby extending the utility of this marker beyond monitoring medication adherence, predicting asthma exacerbations or verifying a diagnosis.
A number of studies have identified a subgroup of individuals with elevated FeNO without asthma or asthma-related symptoms [24–27]. In a cross-sectional study of 13–14 year old schoolchildren, Nordvall et al. [27] suggested that a small subset of participants with elevated levels of FeNO who reported no symptoms of asthma in the ISAAC questionnaire [28] may represent “early asthma.” Sivan et al. [29] compared the use of FeNO, spirometry, and induced sputum for eosinophil count in consecutive school-age children referred for evaluation of possible asthma. The sensitivity, specificity, and positive and negative predictive values for best cutoff points of FeNO (19 parts per billion) were all above 80% and were very similar to those for sputum eosinophil count, suggesting that FeNO testing is about as effective as sputum induction in aiding the diagnosis of childhood asthma.
In a population-based prospective study of adults who were free of asthma or wheeze at study entry, Olin et al. [6] found that baseline FeNO over the 90th percentile predicted new-onset wheeze at four-year follow-up among adults. Because their study was underpowered to investigate new-onset asthma, the authors used new-onset wheeze as a surrogate and early marker of asthma. Regardless of wheeze history, elevated FeNO was predictive of new-onset asthma in our population of schoolchildren.
It is widely accepted that genetic factors account for a significant proportion of allergy and asthma occurrence [30–31]. In this study, we found nearly a two-fold increased risk of new-onset asthma in children with a parental history of asthma; however, the size of that risk did not vary significantly by quartiles of FeNO. The effect of elevated FeNO on new-onset asthma was more marked among children without a parental history of asthma. We previously reported that 23% of the parent cohort indicated a parental history of asthma at cohort establishment [32] (two years before FeNO measurement). The prevalence of parental asthma in the current study was 16%, likely due to the exclusion of prevalent asthma cases and cases diagnosed in the two years before FeNO measurement. Moreover, the proportion of cases with a parental history of asthma was quite similar by child’s history of allergy; 29% of allergic and 24% of non-allergic cases had a parental history of asthma (data not shown).
While our analysis is based on small numbers, the absence of an increased risk in children with higher FeNO and a parental history of asthma [relative to children with lower FeNO and a parental history of asthma] may indicate that the new-onset asthma associated with FeNO is not mediated by the same pathways that account for the asthma in children with a parental history of asthma. Alternatively, our study may demonstrate that beyond the age of 5 to 8 years, the impact of parental history on the development of asthma may be reduced.
Elevated inflammation and oxidative/nitrosative stress could also arise from exposure and/or susceptibility to environmental stressors, such as secondhand smoke or ambient air pollution. We have previously reported that the effects of air pollution on asthma risk may differ in children with and without a parental history of asthma. In a CHS cohort recruited in the 1990s, we showed that children who exercised heavily in high-ozone environments were at increased risk of new-onset asthma especially in the absence of a parental history of asthma [33]. We also demonstrated that traffic-related pollution was associated with a two-fold increased risk of lifetime asthma in children without a parental history of asthma [32]. Taken together, our results and previous findings support an etiologic role for inflammation in asthma pathogenesis. Further research is needed to determine whether pro-inflammatory environmental stressors may help to explain why we see the largest effects of FeNO in children without a parental history of asthma.
A body of evidence indicates that FeNO is elevated in allergic airway disease [4–5] and studies have shown FeNO to be elevated in healthy atopic children [34]. We did not find evidence that the effect of FeNO depended on the child’s allergy status. Children with and without a history of respiratory allergy showed similar patterns of increasing risk of new-onset asthma by increasing quartiles of FeNO.
While our results suggest that the relationship of FeNO with new-onset asthma may be independent of the allergic pathway, it is important to note that we used parent-report of hayfever or allergic rhinitis as a measure of children’s respiratory allergy status, which may result in measurement error of true atopic status. However, measurement error is not likely to explain our entire findings as significant residual confounding by atopic status would likely occur only if the true associations between atopy and FeNO and between atopy and new onset asthma are both very strong (e.g. RRs=10.0) [35]. The strong relationships of self-reported measures of allergy with FeNO and asthma provide evidence that measurement error of atopic status is not likely to explain our findings.
The incidence rate of physician-diagnosed asthma in the present study (22.2 per 1000 pyrs) was higher than rates reported in earlier periods [36]. However, in recent decades, rates are more comparable, likely reflecting the increasing occurrence of childhood asthma [37–38]. The incidence rate in the present study is in line with earlier CHS cohorts of approximately the same age (17.8 per 1000 pyrs) [17]. By restricting our case definition to children without any history of wheezing, the incidence rate remained substantial (11.1 per 1000 pyrs)
A recognized limitation of our study is our reliance on self-report of physician-diagnosed asthma for defining eligibility and for case ascertainment. However, physician diagnosis of asthma has been widely accepted as a valid method of classifying asthma status in large epidemiologic studies [39–40]. In a subset of a previous CHS cohort, we independently verified self-reported physician-diagnosed asthma through a review of medical records and found that more than 95% of the children with a self-reported diagnosis had either a definite or probable asthma diagnosis noted on the medical record [41].
Another potential influence on asthma diagnosis is differential access to care and differences in medical practice. We found that adjustment for various indicators of socioeconomic status did not change our results, and we found that by restricting our analysis to cases using recent inhaled medication or by restricting to cases categorized as moderate or severe resulted in stronger risk estimates at each FeNO quartile; therefore, any bias that might arise from differences in medical practice is likely to attenuate the risk estimate toward the null. Children who reported use of inhaled medication after diagnosis could represent children with more bronchial inflammation before asthma onset and more severe asthma at diagnosis.
While this cohort was initially established when the participants were young (ages 5–6 years on average), we cannot definitively state that a new asthma diagnosis represents a true incident case. Misclassification of asthma status at cohort entry is not likely to explain our findings, as excluding cases reporting a diagnosis in the first year of follow-up did not substantially alter the relative risk estimates. To further limit the inclusion of possible undiagnosed asthma, we restricted our analysis to children without a history of wheeze or without wheeze in the twelve months prior to FeNO measurement. The results remained consistent with the highest risk of new-onset asthma occurring in children with the highest quartile of FeNO.
Conclusions
Our results suggest that FeNO is a marker of risk for the development of asthma especially among children without a parental history of asthma. FeNO may be valuable in developing primary prevention strategies.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the school principals, teachers, students and parents in each of the 13 study communities for their cooperation and especially to the members of the health testing field team for their efforts. This work was supported by the Southern California Environmental Health Sciences Center (grant # 5P30ES007048) funded by the National Institute of Environmental Health Sciences, the Children’s Environmental Health Center (grant #s 5P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency, the National Institute of Environmental Health Sciences (grant # 5P01ES011627) the National Heart, Lung and Blood Institute (grant #s 5R01HL061768 and 5R01HL076647) and the Hastings Foundation.
REFERENCES
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Steerenberg PA, Janssen NA, De Meer G, Fischer PH, Nierkens S, Van Loveren H, Opperhuizen A, Brunekreef B, Van Amsterdam JG. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003;58(3):242–245. doi: 10.1136/thorax.58.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djukanovic R, Lai CK, Wilson JW, Britten KM, Wilson SJ, Roche WR, Howarth PH, Holgate ST. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992;5(5):538–544. [PubMed] [Google Scholar]
- 4.Baraldi E, de Jongste JC. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20(1):223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers. 2002;7(1):1–32. doi: 10.1080/13547500110104233. [DOI] [PubMed] [Google Scholar]
- 6.Olin AC, Rosengren A, Thelle DS, Lissner L, Toren K. Increased Fraction of Exhaled Nitric Oxide Predicts New-onset Wheeze in a General Population. Am J Respir Crit Care Med. 2010;181:324–327. doi: 10.1164/rccm.200907-1079OC. [DOI] [PubMed] [Google Scholar]
- 7.Linn WS, Berhane KT, Rappaport EB, Bastain TM, Avol EL, Gilliland FD. Relationships of online exhaled, offline exhaled, and ambient nitric oxide in an epidemiologic survey of schoolchildren. J Expo Sci Environ Epidemiol. 2008;19(7):674–681. doi: 10.1038/jes.2008.64. [DOI] [PubMed] [Google Scholar]
- 8.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res. 2009;10(28) doi: 10.1186/1465-9921-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999;160(6):2104–2117. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 10.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 11.Barreto M, Villa MP, Martella S, Falasca C, Guglielmi F, Pagani J, Darder MT, Ronchetti R. Off-line exhaled nitric oxide measurements in children. Pediatr Pulmonol. 2001;32(2):159–167. doi: 10.1002/ppul.1102. [DOI] [PubMed] [Google Scholar]
- 12.Deykin A, Massaro AF, Drazen JM, Israel E. Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. Am J Respir Crit Care Med. 2002;165(12):1597–1601. doi: 10.1164/rccm.2201081. [DOI] [PubMed] [Google Scholar]
- 13.Linn WS, Avila M, Gong H., Jr Exhaled nitric oxide: sources of error in offline measurement. Arch Environ Health. 2004;59(8):385–391. doi: 10.3200/AEOH.59.8.385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox Regression models and life tables. J Royal Statistical Soc. 1972;B(34):187–202. [Google Scholar]
- 15.Hastie TJ, Tibshirani RJ. Generalized Additive Models. New York: Chapman and Hall; 1990. [Google Scholar]
- 16.Islam T, Gauderman WJ, Berhane K, McConnell R, Avol E, Peters JM, Gilliland FD. Relationship between air pollution, lung function and asthma in adolescents. Thorax. 2007;62(11):957–963. doi: 10.1136/thx.2007.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, Peters JM. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174(10):1094–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim KG, Mottram C. The use of fraction of exhaled nitric oxide in pulmonary practice. Chest. 2008;133(5):1232–1242. doi: 10.1378/chest.07-1712. [DOI] [PubMed] [Google Scholar]
- 19.Petsky HL, Cates CJ, Li AM, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2008;(2):CD006340. doi: 10.1002/14651858.CD006340.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164(5):738–743. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch M, Uxa S, Horak F, Jr, Putschoegl B, Dehlink E, Szepfalusi Z, Frischer T. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol. 2006;41(9):855–862. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 22.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60(3):215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004;169(4):473–478. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 24.Ludviksdottir D, Janson C, Hogman M, Hedenstrom H, Bjornsson E, Boman G. Exhaled nitric oxide and its relationship to airway responsiveness and atopy in asthma. BHR-Study Group. Respir Med. 1999;93(8):552–556. doi: 10.1016/s0954-6111(99)90154-3. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen AH, Lingaas-Holmen T, Sue-Chu M, Bjermer L. Combined use of exhaled nitric oxide and airway hyperresponsiveness in characterizing asthma in a large population survey. Eur Respir J. 2000;15(5):849–855. doi: 10.1034/j.1399-3003.2000.15e07.x. [DOI] [PubMed] [Google Scholar]
- 26.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130(5):1319–1325. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]
- 27.Nordvall SL, Janson C, Kalm-Stephens P, Foucard T, Toren K, Alving K. Exhaled nitric oxide in a population-based study of asthma and allergy in schoolchildren. Allergy. 2005;60(4):469–475. doi: 10.1111/j.1398-9995.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Strachan D, Sibbald B, Weiland S, Ait-Khaled N, Anabwani G, Anderson HR, Asher MI, Beasley R, Bjorksten B, Burr M, Clayton T, Crane J, Ellwood P, Keil U, Lai C, Mallol J, Martinez F, Mitchell E, Montefort S, Pearce N, Robertson C, Shah J, Stewart A, von Mutius E, Williams H. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatr Allergy Immunol. 1997;8(4):161–176. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 29.Sivan Y, Gadish T, Fireman E, Soferman R. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr. 2009;155(2):211–216. doi: 10.1016/j.jpeds.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Moffatt MF, Cookson WO. Gene identification in asthma and allergy. Int Arch Allergy Immunol. 1998;116(4):247–252. doi: 10.1159/000023952. [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Valdez R, Yoon PW, Crocker D, Moonesinghe R, Khoury MJ. The association between family history of asthma and the prevalence of asthma among US adults: National Health and Nutrition Examination Survey, 1999–2004. Genet Med. 2009;11(5):323–328. doi: 10.1097/GIM.0b013e31819d3015. [DOI] [PubMed] [Google Scholar]
- 32.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 34.Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999;159(1):69–73. doi: 10.1164/ajrccm.159.1.9804134. [DOI] [PubMed] [Google Scholar]
- 35.Kelsey JL, Thompson WD, Evans AS. Methods in Observational Epidemiology. New York: Oxford University Press; 1986. [Google Scholar]
- 36.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980;122(4):567–575. doi: 10.1164/arrd.1980.122.4.567. [DOI] [PubMed] [Google Scholar]
- 37.Basagana X, Sunyer J, Zock JP, Kogevinas M, Urrutia I, Maldonado JA, Almar E, Payo F, Anto JM. Incidence of asthma and its determinants among adults in Spain. Am J Respir Crit Care Med. 2001;164(7):1133–1137. doi: 10.1164/ajrccm.164.7.2012143. [DOI] [PubMed] [Google Scholar]
- 38.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312(7040):1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225–1232. [PubMed] [Google Scholar]
- 40.Burr ML. Diagnosing asthma by questionnaire in epidemiological surveys. Clin Exp Allergy. 1992;22(5):509–510. doi: 10.1111/j.1365-2222.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 41.Salam MT, Li YF, Langholz B, Gilliland FD. Early-life environmental risk factors for asthma: findings from the Children's Health Study. Environ Health Perspect. 2004;112(6):760–765. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


