Abstract
Low concentrations of endogenous carbon monoxide (CO), generated primarily through degradation of heme from heme-proteins, have been shown to maintain physiological function of organs and to exert cytoprotective effects. However, high concentrations of carboxyhemoglobin (COHb), formed by CO binding to hemoglobin, potentially prevent adequate O2 delivery to tissues by lowering arterial O2 content. Elevated heme-protein concentrations, as found in marine mammals, are likely associated with greater heme degradation, more endogenous CO production and, consequently, elevated COHb concentrations. Therefore, we measured COHb in elephant seals, a species with large blood volumes and elevated hemoglobin and myoglobin concentrations. The levels of COHb were positively related to the total hemoglobin concentration. The maximum COHb value was 10.4% of total hemoglobin concentration. The mean (±s.e.m.) value in adult seals was 8.7±0.3% (N=6), while juveniles and pups (with lower heme-protein contents) had lower mean COHb values of 7.6±0.2% and 7.1±0.3%, respectively (N=9 and N=9, respectively). Serial samples over several hours revealed little to no fluctuation in COHb values. This consistent elevation in COHb suggests that the magnitude and/or rate of heme-protein turnover is much higher than in terrestrial mammals. The maximum COHb values from this study decrease total body O2 stores by 7%, thereby reducing the calculated aerobic dive limit for this species. However, the constant presence of elevated CO in blood may also protect against potential ischemia–reperfusion injury associated with the extreme breath-holds of elephant seals. We suggest the elephant seal represents an ideal model for understanding the potential cytoprotective effects, mechanisms of action and evolutionary adaptation associated with chronically elevated concentrations of endogenously produced CO.
KEY WORDS: Calculated aerobic dive limit, Carbon monoxide, Hemoglobin absorption spectra, Marine mammal, Oxygen stores
INTRODUCTION
Carbon monoxide (CO) is often classified as a strictly toxic gas, depriving the body of oxygen (O2) by binding to heme-proteins such as hemoglobin and forming carboxyhemoglobin (COHb) (Weaver, 2009). Deleterious symptoms (e.g. headache, nausea and shortness of breath) of CO-driven hypoxia are typically seen when COHb values reach ≥20% of total hemoglobin concentration, and death is associated with values of 50–80% (Stewart, 1975; Weaver, 2009). These COHb values drastically reduce blood-O2 transport and O2 storage capacity (decreased arterial O2 content), thus limiting mitochondrial respiration. However, CO is also generated endogenously in low concentrations, and functions in neurotransmission and in protection of tissues and cells against inflammation, apoptosis and ischemia–reperfusion injuries (Snyder et al., 1998; Kevin and Laffey, 2008; Mustafa et al., 2009; Kajimura et al., 2010; Prabhakar, 2012). Therefore, low concentrations of CO can provide beneficial and therapeutic effects up to a specific concentration, at which elevated CO then leads to detrimental effects from reduced O2 delivery. These relatively recent findings give CO a new functional perspective and emphasize the importance of understanding the biological effects of specific CO concentrations in the body which can be viewed as therapeutic.
Ironically, the primary source of endogenous CO comes from the breakdown of heme, which is an essential component of many heme-proteins (e.g. hemoglobin, myoglobin and cytochrome c) that transport O2 or associate closely with aerobic respiration. The breakdown of heme-proteins releases heme, which is then enzymatically degraded by heme-oxygenase, resulting in equimolar production of free iron, CO and biliverdin (Coburn et al., 1963; Tenhunen et al., 1968). Biliverdin is then reduced to bilirubin via biliverdin reductase. These products (biliverdin and bilirubin) and by-products (CO) of heme degradation have been shown to have a multitude of beneficial effects including: vasodilatation, antioxidative properties, attenuation of ischemia/reperfusion injury, inhibition of apoptosis and downregulation of the inflammatory response (Stocker et al., 1987; Barañano et al., 2002; Mustafa et al., 2009). It is these properties that have stimulated investigation of CO therapy and the use of CO-releasing pharmaceuticals for future clinical applications (i.e. sepsis, organ transplants, heart failure, hypertension, inflammation and cancer) (Motterlini and Otterbein, 2010).
Considering that heme-proteins are the primary source of endogenous CO production, marine mammals, which have elevated blood volumes, hemoglobin content and myoglobin concentrations (Ponganis et al., 2011), potentially represent an excellent model for investigating elevated endogenous CO production. For example, in an early study by Pugh, there was an unexpected finding of elevated CO in the blood of Weddell seals (Leptonychotes weddelli) (Pugh, 1959). This study found mean CO levels in Weddell seal blood that were over six times the values seen in the blood of human non-smokers (Pugh, 1959). Bilirubin concentration in Weddell seal plasma was also elevated approximately threefold to fourfold that of human plasma. Similarly, bilirubin and biliverdin have been measured in the blood of adult and juvenile northern elephant seals [Mirounga angustirostris (Gill 1866)], yet the values are in the same range as those seen in healthy humans (Thorson and Le Boeuf, 1994; Dennery et al., 2001; D.E.C., unpublished).
Northern elephant seals have some of the highest known mammalian blood volumes (216 ml kg−1) and hemoglobin concentrations (25 g dl−1) in nature, accounting for over 70% of their total body O2 store (Ponganis, 2011). This elevated blood O2 storage
List of abbreviations
- cADL
calculated aerobic dive limit
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- HHb
deoxyhemoglobin
- O2
oxygen
- O2Hb
oxyhemoglobin
- tHb
total hemoglobin concentration
capacity contributes to their ability to perform repetitive dives of 20 to 25 min duration to depths >500 m with only 2–3 min surface intervals during foraging trips lasting up to 8 months in duration (Robinson et al., 2012). Additionally, these animals are well known for their extended and repeated breath-holds (up to 25 min) during sleep apnea events on land, which are also usually followed by brief eupneic periods (Blackwell and Le Boeuf, 1993). Breath-holds of these seals are accompanied by the dive response [bradycardia and peripheral ischemia (Andrews et al., 1997; Ponganis et al., 2006)] and routine hypoxemia with arterial hemoglobin saturations reaching as low as 10% (Stockard et al., 2007; Meir et al., 2009). The brief periods of eupnea following breath-holds include hyperventilation, tachycardia and vasodilation, which results in reperfusion of tissues with oxygenated blood, thereby increasing the potential for exposure of tissues to reactive oxygen species and damage from oxidative stress. Additionally, a typical elephant seal spends 9–10 months per year at sea with over 90% of its time underwater, and when on land will spend a large portion of time in sleep apnea (Blackwell and Le Boeuf, 1993; Robinson et al., 2012). These life history behaviors drastically limit the amount of time the animals spend in eupnea.
Considering endogenous CO is expelled via respiration, the intermittent breathing patterns (sleep apnea and/or diving) of many air-breathing marine divers introduces a potential limitation for removal of CO. Therefore, increased endogenous CO production and delayed removal of CO could elevate COHb content, leading to a decrease in blood O2 stores and therefore potentially limiting the duration for aerobic metabolism during a dive. On the other hand, elevated endogenous CO may provide protection against potential reperfusion injury associated with these natural breath-holding behaviors.
To address the potential for elevated endogenous CO production in a species with exceptionally large O2 stores, we measured levels of COHb in the blood of northern elephant seals and compared these values against hemoglobin concentrations. Further, because of a natural ontogenetic increase in both hemoglobin and myoglobin (Thorson and Le Boeuf, 1994; Tift et al., 2013), we investigated differences in COHb values associated with age. We hypothesized that (1) the amount of COHb would correlate with the total hemoglobin concentration (tHb), and (2) because of larger blood volumes in adults versus younger seals, the amount of COHb would be highest in adults.
RESULTS
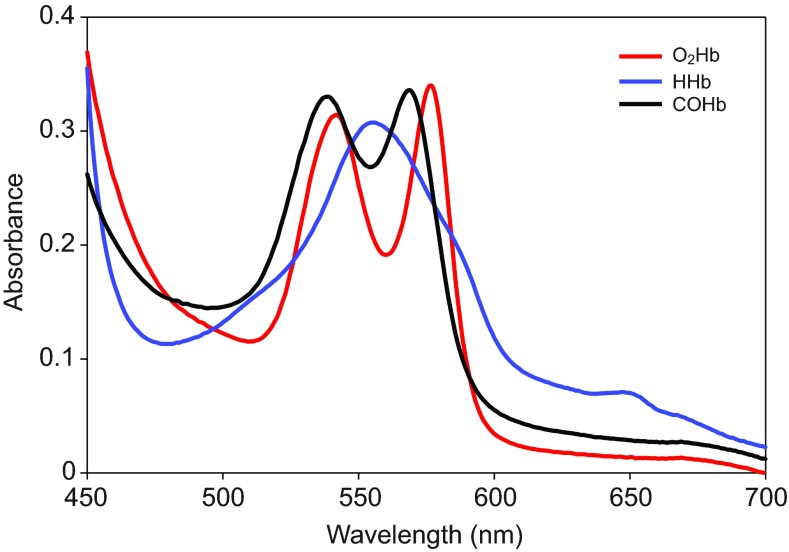
Spectrophotometric absorption peaks of deoxyhemoglobin (HHb), oxyhemoglobin (O2Hb) and COHb from elephant seals, cattle and sheep were measured to validate the use of the co-oximeter for evaluation of the hemoglobin properties in elephant seals. All elephant seal hemoglobin varieties had absorption peaks that were identical to the cattle, sheep and multiple other mammalian species, including humans (Zijlstra et al., 2000) (Fig. 1).
Fig. 1.
Absorption spectra for oxyhemoglobin (O2Hb), deoxyhemoglobin (HHb) and carboxyhemoglobin (COHb) in northern elephant seals. Peaks for O2Hb (542 and 577 nm), HHb (555 nm) and COHb (539 and 569 nm) match those of other mammalian species (human, cow, sheep) (from Zijlstra et al., 2000).
Similar to results found in a previous study (Thorson and Le Boeuf, 1994), adult elephant seal tHb was significantly higher than that of pups, but not juveniles (F2,21=6.5, P=0.0066). Mean tHb in adults was 24.0±1.0 g dl−1, while juveniles and pups had tHb values of 21.7±0.8 and 19.6±0.8 g dl−1, respectively.
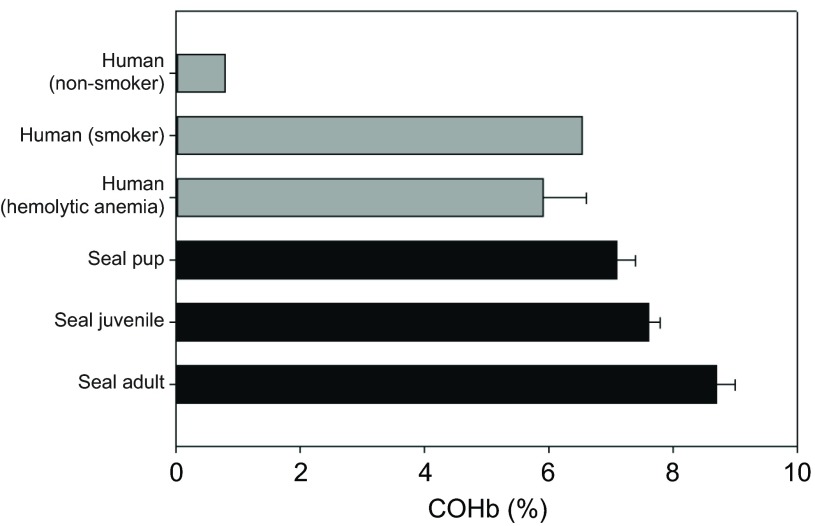
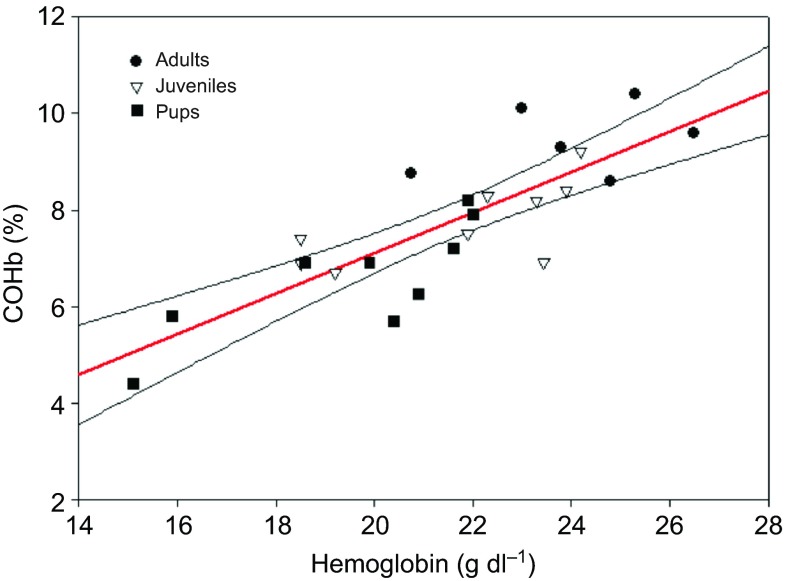
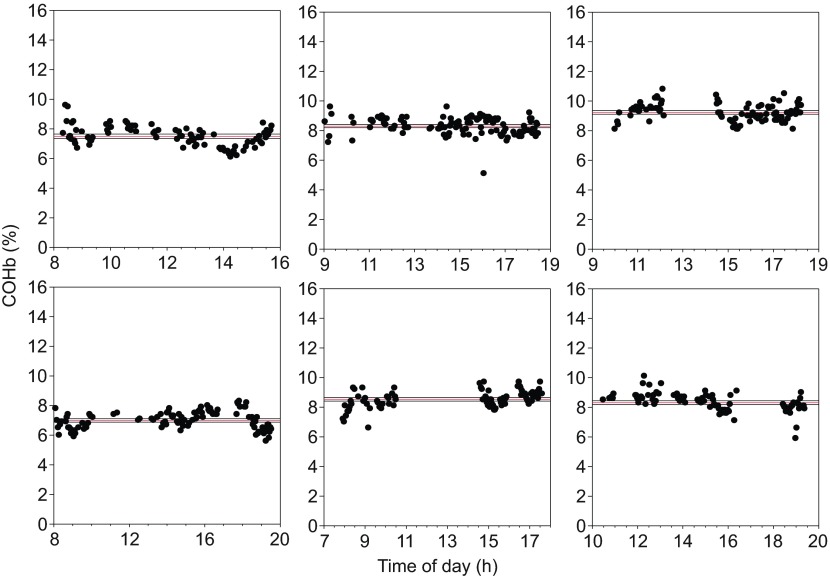
Adults had significantly higher COHb values (8.7±0.3%) than both juveniles (7.6±0.2%) and pups (7.1±0.3%; Fig. 2). Together, age and tHb explained 80% of the variance in COHb values (F3,20=30, P<0.0001; Fig. 3). Over the course of several hours, COHb remained elevated and showed no significant variation from the mean COHb value (F1,801=0.81, P=0.37; Fig. 4).
Fig. 2.
Mean carboxyhemoglobin (COHb) values in the blood of human smokers and non-smokers and elephant seal adults and pups. Human values of COHb for smoking and non-smoking are from Law et al. (Law et al., 1997). Human COHb values of COHb for hemolytic anemia are from Hampson (Hampson, 2007). Values are means + s.e.m.
Fig. 3.
Relationship between the total concentration of hemoglobin and percent carboxyhemoglobin (COHb) in the blood from three different age classes of northern elephant seals. The regression line is in red (COHb=−1.31+0.42tHb), and the 95% confidence intervals are in black (F3,20=30.1, P<0.0001, r2=0.80).
Fig. 4.
Time course of carboxyhemoglobin (COHb) values from six juvenile elephant seals. The red lines represent the best-fit linear regression line for the data and the black lines represent 95% confidence intervals.
DISCUSSION
The values of COHb found in northern elephant seals from this study are higher than values in humans that smoke ≥40 cigarettes per day (Law et al., 1997) and comparable to the highest recorded endogenous values (9.7% COHb) found in a critically ill human patient with hemolytic anemia (Hampson, 2007) (Fig. 2). The maximum COHb value found in an adult elephant seal (10.4% COHb) was also comparable to 12% COHb values measured in one of the first human clinical pharmaceutical investigations using inhaled CO as a therapeutic agent (Motterlini and Otterbein, 2010). These values in healthy humans were well tolerated and showed no adverse effects compared with control levels.
As originally suggested in Weddell seals, we believe that the high endogenous CO values in northern elephant seals from this study can be attributed primarily to the elevated heme stores associated with increased myoglobin content, blood volume, hematocrit and hemoglobin concentrations (Pugh, 1959; Ponganis et al., 1993; Thorson and Le Boeuf, 1994). In elephant seals, the molar equivalents of heme from both hemoglobin and myoglobin stores alone are approximately four and 16 times greater, respectively, than those in adult humans (Fig. 5). The levels of COHb measured in elephant seals suggest: (1) more rapid turnover of heme stores (i.e. shorter half-life of heme-proteins or erythrocytes), as is found in human patients with hemolytic anemia (Coburn et al., 1966; Hampson, 2007), (2) a greater magnitude of heme degradation than has been measured in other mammals, or (3) a combination of both. A greater magnitude of heme degradation could be associated simply with the elevated heme concentrations and/or with an increase in the activity or concentration of heme-oxygenase, the enzyme responsible for heme degradation and endogenous CO production.
Fig. 5.
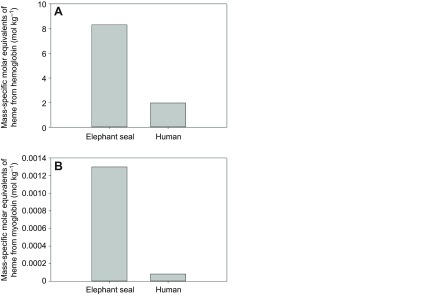
Mass-specific molar equivalents of heme calculated from (A) blood (hemoglobin) and (B) muscle (myoglobin) oxygen stores in northern elephant seals and humans. Molar heme equivalents were calculated using a hemoglobin molecular weight of 65,000 g mol−1 and myoglobin molecular weight of 17,000 g mol−1. Data for species-specific blood volumes, muscle mass, hemoglobin and myoglobin concentrations are from previous studies (Kendrew et al., 1958; Hill et al., 1962; Åkeson et al., 1968; Oscai et al., 1968; Simpson et al., 1970; Bryden, 1972; Möller and Sylven, 1981; Hassrick et al., 2010; Ponganis et al., 2011).
Currently, the life-span of heme-proteins and erythrocytes in marine mammals is unknown, but an increased heme turnover rate could elevate the production rate of endogenous CO. Additionally, the natural and repetitive breath-holds both at sea (diving) and on land (sleep apnea) limit the time elephant seals spend in eupnea, and therefore, decrease or at least delay the exhalation of endogenously produced CO. Between these two breath-hold behaviors, a typical elephant seal will spend a majority of its life in an apneic state (Andrews et al., 2000) and thus, this natural breath-holding behavior may also contribute to the build-up of endogenous CO.
Unlike Weddell seals (Pugh, 1959), elephant seals do not have an elevation in the hemoglobin breakdown product bilirubin (D.E.C., unpublished) to accompany the high blood CO levels seen in these animals. This is especially interesting in elephant seal pups, where neonatal hyperbilirubinemia might be expected because of the common occurrence in human infants, which have much lower heme stores (Dennery et al., 2001). Neonatal hyperbilirubinemia has also been found in some neonate marine mammals (Dierauf et al., 1984), yet there are relatively low levels of bilirubin found in all age classes of elephant seals (Costa and Ortiz, 1982; Champagne et al., 2013; D.E.C., unpublished). One possibility is that the clearance rate or recycling of bilirubin and biliverdin relative to absolute total body heme content and heme turnover in these animals is adequate to maintain lower levels of those metabolites.
Effect of elevated CO on O2 stores and O2 delivery
The concepts of increased O2 storage, a low diving metabolic rate, and aerobic diving underlie current interpretations of diving physiology, dive performance and foraging ecology in apex marine predators (Kooyman, 1989; Butler and Jones, 1997; Ponganis et al., 2003). Total body O2 stores (lung, blood and muscle) are used to calculate the aerobic dive limit (cADL), which is a prediction of the maximum duration of a dive or breath-hold before a post-dive rise in plasma lactate occurs (Kooyman and Kooyman, 1995; Butler and Jones, 1997; Kooyman and Ponganis, 1998; Costa et al., 2001). For this calculation, which is performed by dividing total body O2 stores by the rate of breath-hold O2 consumption, all measured hemoglobin is included in the blood O2 store (which can make up 70% of the total O2 store in some species). However, the present study shows that over 10% of total hemoglobin content can be bound to CO in the premier pinniped diver, effectively reducing its total body O2 stores by ~7%.
When these CO-related reductions in O2 stores are taken into consideration in the calculation of a cADL of adult elephant seals [~30 min (Hassrick et al., 2010)], there is reduction of ~2 min. This reduction might help explain why the majority of dives in female elephant seals are actually 22–23 min (Robinson et al., 2012) instead of the predicted cADL of 30 min. The elevated COHb values seen in elephant seals from this study should heighten awareness of the potential for endogenous CO to reduce the magnitude of total body O2 stores calculated in other species.
Potential therapeutic benefits of elevated CO in marine mammals
The routine hypoxemia, hypercarbia, reduction in peripheral perfusion, and activation of the sympathetic nervous system during both diving and sleep apnea in elephant seals potentially increase the risks of systemic and pulmonary hypertension, ischemia–reperfusion injury, reactive oxygen species formation, and subsequent tissue damage (Kooyman, 1989; Butler and Jones, 1997; Kooyman and Ponganis, 1998; Zenteno-Savín et al., 2002). Adaptations that potentially decrease such risks in marine mammals include hypoxic pulmonary vasodilatation and elevations in both antioxidant concentrations and antioxidant enzyme activities (Zenteno-Savín et al., 2002; Olson et al., 2010; Tift et al., 2011; Vázquez-Medina et al., 2011). We propose that the levels of heme degradation, and associated by-products such as endogenous CO found in elephant seals, may also contribute to the protection against these conditions.
Ischemia–reperfusion injury is commonly seen during a variety of clinical scenarios, including heart attacks, strokes and organ transplantation (Carden and Granger, 2000). Deleterious effects associated with ischemia–reperfusion injury typically include apoptosis, intense inflammation and thrombogenesis. However, exposure to low levels of CO significantly decreases the risk of these injuries during ischemia–reperfusion events, and is currently being applied in clinical investigations (Ozaki et al., 2012). Therefore, the elevated levels of endogenous CO found in elephant seals could provide protection against potential ischemia–reperfusion injury, associated with the marked changes in peripheral perfusion associated with breath-holds (Ponganis et al., 2008). Additionally, low concentrations of CO are known to promote vasodilation and decrease hypertension in several species. For example, in the llama (Lama glama) at high altitude, elevated pulmonary CO production is associated with decreased pulmonary hypertension (Herrera et al., 2008). And lastly, although smoking during pregnancy is associated with numerous adverse outcomes and low birth weights (Cnattingius and Lambe, 2002), there has been shown to be an associated decreased risk of hypertension and pre-eclampsia (Wikström et al., 2010). Thus, elevated CO may also contribute to vascular regulation for coping with hypoxemia and tissue vasoconstriction during breath-holding in seals (Ponganis et al., 2008; Meir et al., 2009).
While the mechanism is still unknown, placental blood flow is maintained during extreme peripheral vasoconstriction associated with the dive response in a phocid seal (Liggins et al., 1980). Recently demonstrated in the pregnant mouse (Venditti et al., 2013), the elevated CO exposure in seals may help optimize uterine blood flow and placental growth during the at-sea pregnancies of continuously diving female elephant seals. However, complicating this suggestion is the negative relationship between smoking (and potentially CO exposure) and endothelial nitric oxide synthase activity, which could reduce vasodilatory capacity in the fetus (Andersen et al., 2009).
Exposure to other heme-protein breakdown products (bilirubin and biliverdin) are also associated with a wide array of potential therapeutic effects in the avoidance of re-perfusion injury and oxidative stress. These include anti-inflammatory, anti-apoptosis and anti-oxidative responses (Wu and Wang, 2005; Ryter et al., 2007; Motterlini and Otterbein, 2010). Thus, although CO decreases O2 storage, it has the potential to offer several other beneficial effects in diving animals and other animals exposed to inflammation, apoptosis and oxidative stress.
We conclude with the suggestion that the elevated endogenous CO and COHb concentrations found in deep-diving phocid seals represent an excellent opportunity to study and understand the fundamentals and extremes of this unique physiological system (Krogh, 1929). With the growing knowledge on the therapeutic potential of carbon monoxide, deep-diving seals may become a valuable model to investigate the potential cytoprotective effects, mechanisms of action and evolutionary adaptation from long-term exposure to elevated concentrations of this endogenously produced gasotransmitter.
MATERIALS AND METHODS
Elephant seal COHb absorption spectrum
To validate the use of a Bayer Rapidlab 845 blood gas analyzer with co-oximeter (Siemens Medical Diagnostics, Bayer, Tarrytown, NY, USA) for the determination of elephant seal hemoglobin values, we confirmed that the hemoglobin absorption spectra of elephant seals were similar to those of humans and other terrestrial animals. Hemoglobin from the blood of healthy juvenile northern elephant seals (n=2, see blood collection technique, below), sheep (n=1) and cattle (n=1) (sheep and cattle blood obtained from Hemostat Laboratories, Dixon, CA, USA) were spectrophotometrically compared with hemoglobin absorption peak values for human, cattle and sheep from Zijlstra et al. (Zijlstra et al., 2000). Isolated hemoglobin absorption peaks for oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb) and COHb were measured and compared using methods similar to that described previously (Kreutzer et al., 1993; Zijlstra et al., 2000). Briefly, blood was collected into chilled vacutainers containing 158 USP units (equivalent to 158 IU) of sodium heparin (BD, Franklin Lakes, NJ, USA). Plasma was discarded and erythrocytes were washed three times with 0.9% NaCl solution. Erythrocytes were then lysed with 1 volume of distilled H2O and 0.4 volumes of toluene and left to sit at 4°C for up to 16 h. This resulted in a top layer of toluene, a middle layer of erythrocyte stromata and a bottom layer of hemoglobin lysate. The bottom layer was collected and centrifuged at 8000 g for 20 min, and the erythrolysate was then filtered through sterile gauze and diluted with deionized water (Milli-Q, Millipore, Billerica, MA, USA). The diluted erythrolysate was then placed inside a tonometer where respective gases were introduced. The different hemoglobin forms (HHb, O2Hb and COHb) were prepared by introducing 100% nitrogen (N2), O2 and CO, respectively, into the tonometer for up to 2 h at a rate of 30–40 ml min−1. For O2Hb and COHb, polystyrene spectrophotometer cuvettes (light-path=1.0 cm) covered with Parafilm® M were filled with 2.5 ml of erythrolysate collected from the tonometer using an airtight blood-gas syringe, thereby minimizing exposure to the ambient environment. For HHb, an addition of 3 mg of sodium hydrosulfite (Na2S2O4) per 2 ml of buffered erythrolysate was included for tonometry to prevent O2 or CO from binding. Once the lysate was transferred, cuvettes were then immediately capped, sealed with Parafilm® M and immediately analyzed.
Absorbance measurements were made at room temperature (20–25°C) with a Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) in the spectral range of 450–700 nm.
Study site and subjects
We sampled 24 northern elephant seals throughout the 2009 winter breeding season (December–April) from Año Nuevo State Reserve, San Mateo County, CA, USA. Every year, a subset of pups at the reserve are marked with flipper-tags (Jumbo Rototag, Dalton, UK) following weaning, which enables reference to the animals' ages. Subjects were divided into three age classes: pups (<3 months old; n=9), juveniles (3 months–3 years old; n=9) and adults (>3 years old; n=6). Adult animals were all female, and were sampled from January to February at the beginning of their breeding season. Pups were sampled in February–March and juveniles were sampled April–May.
Animals were chemically immobilized with an intramuscular injection (1 mg kg−1) of Telazol (tiletamine/zolazepam HCl) and subsequent intravenous ketamine injections (0.5 mg kg−1) as necessary to maintain immobilization for the intravenous catheterization of the extradural vein (all drugs from Fort Dodge Labs, Fort Dodge, IA, USA). Venous blood samples (~3 ml) were collected into lithium heparinized blood tubes (BD Vacutainer®, Fisher Scientific, Franklin Lakes, NJ, USA) and chilled over ice until analysis (<3 h). Standard blood gas analysis was performed using a Bayer Rapidlab 845 blood gas analyzer with co-oximeter (Siemens Medical Diagnostics, Bayer, Tarrytown, NY, USA) to obtain fractions of COHb and tHb concentrations.
To determine the variability of COHb over time and between voluntary periods of apnea and eupnea, we opportunistically sampled six of the nine juvenile elephant seals for up to 12 h. Once sedated, the animals were placed in a holding cage and the extradural vein was percutaneously catheterized with a long-term polyurethane catheter (MILA; 16 gauge × 25 cm), which was affixed to the fur using Loctite glue (Henkel Corporation, Westlake, OH, USA). An extension tube (76 cm) with a three-way stopcock system was attached to the catheter to allow blood collection during periods of voluntary sleep apnea and eupnea, with minimal disturbance. Over the course of 8–12 h, we collected a total of 806 blood samples ranging from 36 to 134 samples per individual.
This work was conducted through the National Marine Fisheries Service permit no. 14636 and was approved by the Institutional Animal Care and Use Committee at Sonoma State University.
Statistical analysis
Data were analyzed using the statistical program JMP 11.0. We examined the effects of age class (adult, juvenile and pup) and tHb concentration on the percentage of COHb in blood samples using a multiple regression analysis. Changes in COHb over the course of several hours were evaluated using a repeated-measures linear mixed model, with individual ID as the random subject effect. All mean values are reported ±s.e.m. Significance was determined at P<0.05.
ACKNOWLEDGEMENTS
We thank the rangers and docents at Año Nuevo State Reserve for their logistical support. E. Ranalli, A. Dallara, V. Farnham, J. Jelincic, J. Vázquez-Medina, C. Champagne and M. Fowler provided valuable assistance in the field. Advice from T. Jue and V. Agarwal on absorption spectra techniques and use of B. Moore's spectropohotometer are greatly appreciated. We also thank G. Kooyman, J. Maresh and B. McDonald for manuscript review, and J. West, who introduced P.J.P. to Pugh's 1959 findings and stimulated this research.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
Funding for this project was provided by the Office of Naval Research [N000141010514 to P.J.P.]. Graduate student support for M.S.T. was provided by National Institutes of Health [NHLBI R01-HL091767 to R.M.O.] and the National Science Foundation [09-44220 to P.J.P.]. Deposited in PMC for release after 12 months.
References
- Åkeson A., Biörck G., Simon R. (1968). On the content of myoglobin in human muscles. Acta Med. Scand. 183, 307-316 [DOI] [PubMed] [Google Scholar]
- Andersen M. R., Simonsen U., Uldbjerg N., Aalkjaer C., Stender S. (2009). Smoking cessation early in pregnancy and birth weight, length, head circumference, and endothelial nitric oxide synthase activity in umbilical and chorionic vessels: an observational study of healthy singleton pregnancies. Circulation 119, 857-864 [DOI] [PubMed] [Google Scholar]
- Andrews R. D., Jones D. R., Williams J. D., Thorson P. H., Oliver G. W., Costa D. P., Le Boeuf B. J. (1997). Heart rates of northern elephant seals diving at sea and resting on the beach. J. Exp. Biol. 200, 2083-2095 [DOI] [PubMed] [Google Scholar]
- Andrews R. D., Costa D. P., Le Boeuf B. J., Jones D. R. (2000). Breathing frequencies of northern elephant seals at sea and on land revealed by heart rate spectral analysis. Respir. Physiol. 123, 71-85 [DOI] [PubMed] [Google Scholar]
- Barañano D. E., Rao M., Ferris C. D., Snyder S. H. (2002). Biliverdin reductase: a major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA 99, 16093-16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell S. B., Le Boeuf B. (1993). Developmental aspects of sleep apnoea in northern elephant seals, Mirounga angustirostris. J. Zool. 231, 437-447 [Google Scholar]
- Bryden M. (1972). Body size and composition of elephant seals (Mirounga leonine): absolute measurements and estimates from bone dimensions. J. Zool. 167, 265-276 [Google Scholar]
- Butler P. J., Jones D. R. (1997). Physiology of diving of birds and mammals. Physiol. Rev. 77, 837-899 [DOI] [PubMed] [Google Scholar]
- Carden D. L., Granger D. N. (2000). Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 190, 255-266 [DOI] [PubMed] [Google Scholar]
- Champagne C. D., Boaz S. M., Fowler M. A., Houser D. S., Costa D. P., Crocker D. E. (2013). A profile of carbohydrate metabolites in the fasting northern elephant seal. Comp. Biochem. Physiol. 8D, 141-151 [DOI] [PubMed] [Google Scholar]
- Cnattingius S., Lambe M. (2002). Trends in smoking and overweight during pregnancy: prevalence, risks of pregnancy complications, and adverse pregnancy outcomes. Semin. Perinatol. 26, 286-295 [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Blakemore W. S., Forster R. E. (1963). Endogenous carbon monoxide production in man. J. Clin. Invest. 42, 1172-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Williams W. J., Kahn S. B. (1966). Endogenous carbon monoxide production in patients with hemolytic anemia. J. Clin. Invest. 45, 460-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D. P., Ortiz C. L. (1982). Blood chemistry homeostasis during prolonged fasting in the northern elephant seal. Am. J. Physiol. 242, R591-R595 [DOI] [PubMed] [Google Scholar]
- Costa D. P., Gales N. J., Goebel M. E. (2001). Aerobic dive limit: how often does it occur in nature? Comp. Biochem. Physiol. 129A, 771-783 [DOI] [PubMed] [Google Scholar]
- Dennery P. A., Seidman D. S., Stevenson D. K. (2001). Neonatal hyperbilirubinemia. N. Engl. J. Med. 344, 581-590 [DOI] [PubMed] [Google Scholar]
- Dierauf L., Dougherty S., Baker B. (1984). Neonatal hyperbilirubinemia in harbor seals (Phoca vitulina richardsi). J. Zoo Animal Med. 15, 55-59 [Google Scholar]
- Hampson N. B. (2007). Carboxyhemoglobin elevation due to hemolytic anemia. J. Emerg. Med. 33, 17-19 [DOI] [PubMed] [Google Scholar]
- Hassrick J. L., Crocker D. E., Teutschel N. M., McDonald B. I., Robinson P. W., Simmons S. E., Costa D. P. (2010). Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. J. Exp. Biol. 213, 585-592 [DOI] [PubMed] [Google Scholar]
- Herrera E. A., Reyes R. V., Giussani D. A., Riquelme R. A., Sanhueza E. M., Ebensperger G., Casanello P., Méndez N., Ebensperger R., Sepúlveda-Kattan E., et al. (2008). Carbon monoxide: a novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano. Cardiovasc. Res. 77, 197-201 [DOI] [PubMed] [Google Scholar]
- Hill R. J., Konigsberg W., Guidotti G., Craig L. C. (1962). The structure of human hemoglobin. I. The separation of the α and β chains and their amino acid composition. J. Biol. Chem. 237, 1549-1554 [PubMed] [Google Scholar]
- Kajimura M., Fukuda R., Bateman R. M., Yamamoto T., Suematsu M. (2010). Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 13, 157-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrew J. C., Bodo G., Dintzis H. M., Parrish R. G., Wyckoff H., Phillips D. C. (1958). A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature 181, 662-666 [DOI] [PubMed] [Google Scholar]
- Kevin L. G., Laffey J. G. (2008). Carbon monoxide: from poison to therapy for cardiopulmonary bypass-induced lung injury? Anesthesiology 108, 977-978 [DOI] [PubMed] [Google Scholar]
- Kooyman G. L. (1989). Diverse Divers: Physiology and Behaviour. Berlin: Springer-Verlag; [Google Scholar]
- Kooyman G., Kooyman T. (1995). Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97, 536-549 [Google Scholar]
- Kooyman G. L., Ponganis P. J. (1998). The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19-32 [DOI] [PubMed] [Google Scholar]
- Kreutzer U., Chung Y., Butler D., Jue T. (1993). 1H-NMR characterization of the human myocardium myoglobin and erythrocyte hemoglobin signals. Biochim. Biophys. Acta 1161, 33-37 [DOI] [PubMed] [Google Scholar]
- Krogh A. (1929). The progress of physiology. J. Physiol. 90, 243-251 [Google Scholar]
- Law M. R., Morris J. K., Watt H. C., Wald N. J. (1997). The dose–response relationship between cigarette consumption, biochemical markers and risk of lung cancer. Br. J. Cancer 75, 1690-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins G. C., Qvist J., Hochachka P. W., Murphy B. J., Creasy R. K., Schneider R. C., Snider M. T., Zapol W. M. (1980). Fetal cardiovascular and metabolic responses to simulated diving in the Weddell seal. J. Appl. Physiol. 49, 424-430 [DOI] [PubMed] [Google Scholar]
- Meir J. U., Champagne C. D., Costa D. P., Williams C. L., Ponganis P. J. (2009). Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am. J. Physiol. 297, R927-R939. [DOI] [PubMed] [Google Scholar]
- Möller P., Sylvén C. (1981). Myoglobin in human skeletal muscle. Scand. J. Clin. Lab. Invest. 41, 479-482 [DOI] [PubMed] [Google Scholar]
- Motterlini R., Otterbein L. E. (2010). The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 9, 728-743 [DOI] [PubMed] [Google Scholar]
- Mustafa A. K., Gadalla M. M., Snyder S. H. (2009). Signaling by gasotransmitters. Sci. Signal. 2, re2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K. R., Whitfield N. L., Bearden S. E., St Leger J., Nilson E., Gao Y., Madden J. A. (2010). Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. 298, R51-R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscai L. B., Williams B. T., Hertig B. A. (1968). Effect of exercise on blood volume. J. Appl. Physiol. 24, 622-624 [DOI] [PubMed] [Google Scholar]
- Ozaki K. S., Kimura S., Murase N. (2012). Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant. Rev. 26, 125-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponganis P. J. (2011). Diving mammals. Compr. Physiol. 1, 447-465 [DOI] [PubMed] [Google Scholar]
- Ponganis P. J., Kooyman G. L., Castellini M. A. (1993). Determinants of the aerobic dive limit of weddell seals: analysis of diving metabolic rates, postdive end tidal PO2's, and blood and muscle oxygen stores. Physiol. Zool. 66, 732-749 [Google Scholar]
- Ponganis P. J., Kooyman G. L., Ridgway S. H. (2003). Comparative diving physiology. In Bennett and Elliott's Physiology and Medicine of Diving, 5th. edn. (Brubakk A. O., Neuman T. S.), pp. 211-226 Edinburgh: Saunders Ltd. [Google Scholar]
- Ponganis P. J., Stockard T. K., Levenson D. H., Berg L., Baranov E. A. (2006). Cardiac output and muscle blood flow during rest-associated apneas of elephant seals. Comp. Biochem. Physiol. 144A, 105-111 [DOI] [PubMed] [Google Scholar]
- Ponganis P. J., Kreutzer U., Stockard T. K., Lin P. C., Sailasuta N., Tran T. K., Hurd R., Jue T. (2008). Blood flow and metabolic regulation in seal muscle during apnea. J. Exp. Biol. 211, 3323-3332 [DOI] [PubMed] [Google Scholar]
- Ponganis P. J., Meir J. U., Williams C. L. (2011). In pursuit of Irving and Scholander: a review of oxygen store management in seals and penguins. J. Exp. Biol. 214, 3325-3339 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R. (2012). Carbon monoxide (CO) and hydrogen sulfide (H2S) in hypoxic sensing by the carotid body. Respir. Physiol. Neurobiol. 184, 165-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh L. G. (1959). Carbon monoxide content of the blood and other observations on Weddell seals. Nature 183, 74-76 [DOI] [PubMed] [Google Scholar]
- Robinson P. W., Costa D. P., Crocker D. E., Gallo-Reynoso J. P., Champagne C. D., Fowler M. A., Goetsch C., Goetz K. T., Hassrick J. L., Hückstädt L. A., et al. (2012). Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE 7, e36728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter S. W., Morse D., Choi A. M. (2007). Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. 36, 175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. G., Gilmartin W. G., Ridgway S. H. (1970). Blood Volume and Other Hematologic Values in Young Elephant Seals (Mirounga angustirostris). Belvoir, VA: Defense Technical Information Center; [PubMed] [Google Scholar]
- Snyder S. H., Jaffrey S. R., Zakhary R. (1998). Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res. Brain Res. Rev. 26, 167-175 [DOI] [PubMed] [Google Scholar]
- Stewart R. D. (1975). The effect of carbon monoxide on humans. Annu. Rev. Pharmacol. 15, 409-423 [DOI] [PubMed] [Google Scholar]
- Stockard T. K., Levenson D. H., Berg L., Fransioli J. R., Baranov E. A., Ponganis P. J. (2007). Blood oxygen depletion during rest-associated apneas of northern elephant seals (Mirounga angustirostris). J. Exp. Biol. 210, 2607-2617 [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. (1987). Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 84, 5918-5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. (1968). The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 61, 748-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson P. H., Le Boeuf B. (1994). Developmental aspects of diving in northern elephant seal pups. In Elephant Seals: Population Ecology, Behavior, and Physiology (ed. Le Boeuf B. J., Laws R. M.), pp. 271-289 Berkeley, CA: University of California Press; [Google Scholar]
- Tift M. S., Houser D. S., Crocker D. E. (2011). High-density lipoprotein remains elevated despite reductions in total cholesterol in fasting adult male elephant seals (Mirounga angustirostris). Comp. Biochem. Physiol. 159B, 214-219 [DOI] [PubMed] [Google Scholar]
- Tift M. S., Ranalli E. C., Houser D. S., Ortiz R. M., Crocker D. E. (2013). Development enhances hypometabolism in northern elephant seal pups (Mirounga angustirostris). Funct. Ecol. 27, 1155-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Tift M. S., Forman H. J., Crocker D. E., Ortiz R. M. (2011). Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. J. Exp. Biol. 214, 4193-4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti C. C., Casselman R., Murphy M. S., Adamson S. L., Sled J. G., Smith G. N. (2013). Chronic carbon monoxide inhalation during pregnancy augments uterine artery blood flow and uteroplacental vascular growth in mice. Am. J. Physiol. 305, R939-R948 [DOI] [PubMed] [Google Scholar]
- Weaver L. K. (2009). Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 360, 1217-1225 [DOI] [PubMed] [Google Scholar]
- Wikström A.-K., Stephansson O., Cnattingius S. (2010). Tobacco use during pregnancy and preeclampsia risk: effects of cigarette smoking and snuff. Hypertension 55, 1254-1259 [DOI] [PubMed] [Google Scholar]
- Wu L., Wang R. (2005). Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57, 585-630 [DOI] [PubMed] [Google Scholar]
- Zenteno-Savín T., Clayton-Hernández E., Elsner R. (2002). Diving seals: are they a model for coping with oxidative stress? Comp. Biochem. Physiol. 133C, 527-536 [DOI] [PubMed] [Google Scholar]
- Zijlstra W. G., Buursma A., van Assendelft O. W. (2000). Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application. Leiden, The Netherlands: VSP BV; [Google Scholar]