Abstract
BACKGROUND
Previous studies suggest cross-sectional associations between a diagnosis of chronic obstructive pulmonary disease (COPD) and mild cognitive impairment (MCI). However, few studies have assessed whether COPD, a potentially modifiable factor, is associated with an increased risk of MCI and if the relation is specific to type of MCI.
OBJECTIVE
To investigate whether a diagnosis of COPD, and COPD duration, is associated with an increased risk of incident MCI, and MCI subtypes (amnestic MCI (a-MCI) and non-amnestic MCI (na-MCI)).
DESIGN
Mayo Clinic Study on Aging, a prospective population-based cohort study.
SETTING
Olmsted County, Minnesota.
PARTICIPANTS
The study included 1425 cognitively normal individuals aged 70–89 years, who were randomly selected from Olmsted County, MN, on October 1, 2004, using the medical records linkage system.
METHODS
At baseline and every 15 months thereafter, participants were assessed with a nurse interview, neurological examination, and neuropsychological testing. A diagnosis of COPD was confirmed via medical record chart review. A baseline diagnosis of COPD and disease duration were examined as risk factors for MCI and MCI-subtypes using Cox proportional hazards models and adjusting for demographic variables and medical comorbidities, using age as the time scale.
MAIN OUTCOME MEASURES
Incident MCI, amnestic MCI, non-amnestic MCI
RESULTS
Of 1425 cognitively normal subjects at baseline, 370 developed incident MCI. The median duration of follow-up was 5.1 years (Interquartile Range [IQR], 3.8–5.4 years). COPD significantly increased the risk of na-MCI by 83% (HR 1.83; 95% CI, 1.04–3.23), but not any MCI or a-MCI in multivariate analyses. There was a dose-response relationship such that individuals with COPD duration of 5 years or longer at baseline had the greatest risk of both MCI (HR 1.58, 95% CI:1.04, 2.40) and na-MCI (HR 2.58, 95% CI:1.32–5.06).
CONCLUSIONS AND RELEVANCE
COPD was associated with an increased risk of MCI, particularly na-MCI. There was a dose-response relationship between COPD duration and risk of MCI. These findings highlight the importance of COPD as a risk factor for MCI and may provide a substrate for early intervention to prevent or delay the onset and progression of MCI, particularly na-MCI.
Chronic Obstructive Pulmonary Disease (COPD) is a progressive, but potentially treatable and preventable, disease characterized by the chronic airflow limitation and associated with an abnormal inflammatory response of the lungs to noxious particles or gases.1 Current surveillance data show that more than 13.5 million adults aged 25 years and older suffer from COPD in the United States.2 Chronic airflow limitation can result in hypoxemia and hypercapnia, which may predispose these patients to an increased risk of cognitive dysfunction.3,4
Previous studies suggest COPD is associated with hypoxemia and cognitive impairment.4–6 Cross-sectional studies have also reported a higher frequency of mild cognitive impairment (MCI) in individuals with, versus without, a diagnosis of COPD7,8. In the absence of a curative therapy for dementia, the early identification of modifiable risk factors for MCI, the earliest symptomatic phase of dementia, is important for preventing or delaying the onset of cognitive impairment. One recent longitudinal study reported that persons with COPD and asthma were at increased risk of MCI/dementia.9 However, this study utilized self-reported diagnoses and did not examine MCI subtypes (amnestic MCI (a-MCI) and non-amnestic MCI (na-MCI), which may provide insights into the underlying MCI etiology. In the present study, we examined the association between medical-record confirmed diagnoses of COPD, COPD duration, and risk of MCI and MCI subtypes in cognitively normal individuals enrolled and followed in the Mayo Clinic Study of Aging (MCSA).
Methods
The MCSA is a prospective, population-based study designed to identify the prevalence, incidence, and risk factors of MCI. The details of the study design have been published.10 In brief, from an enumeration of Olmsted County, MN residents, aged 70–89 years on October 1, 2004 (n = 9953), identified using the Rochester Epidemiology Project (REP) medical records linkage system,11 a sample of 5233 was randomly selected by age-and sex-stratification. The study cohort was evaluated for eligibility: residency in Olmsted County, MN, absence of dementia (determined through medical record review by a behavioral neurologist), and not terminally ill or in hospice. Out of the 4398 eligible individuals, 2719 agreed to participate (2050 were evaluated in person and 669 were evaluated via telephone interview). After excluding prevalent cases of MCI, and individuals who died or dropped out prior to any follow-up, the present analysis included 1425 cognitively normal subjects at baseline (eFigure 1). The study was approved by the Institutional Review Boards of the Mayo Clinic and of Olmsted Medical Center (OMC). Written informed consent was obtained from all participants.
Assessment of Cognitive Status
All participants were interviewed by a nurse or study coordinator, had a neurologic evaluation by a physician, and completed neuropsychological testing administered by a psychometrist.10 The nurse interview included questions about memory to both the participant and an informant using the Clinical Dementia Rating scale.12 The physician examination included a medical history review, a complete neurological examination, and administration of the Short Test of Mental Status13 and the Unified Parkinson’s Disease Rating Scale.14 The neuropsychological battery consisted of 9 cognitive tests used to assess function in four domains (memory, language, executive function and visuospatial skills). For the purpose of determining impairment for a MCI diagnosis, the raw scores on each test were age- and education-adjusted and scaled using normative data from the Mayo’s Older American Normative Studies.15 Within each domain, the scaled test scores were summed and scaled to obtain the final global and domain-specific z-scores.10 A domain-specific score less than 1.0 standard deviation (SD) below the age-specific mean among the general population was considered as possible cognitive impairment. A decision about impairment in a cognitive domain was made taking into consideration occupation. A diagnosis of normal cognition, MCI, or dementia was made according to published criteria and was based on a consensus agreement between the interviewing nurse, examining physician, and the neuropsychologist taking into account all the information collected.10,16 At follow-up visits, cognitive diagnoses from previous evaluations were blinded. MCI cases were further classified into amnestic (a-MCI) or non-amnestic (na-MCI) MCI depending on whether the memory domain was impaired.
Ascertainment of COPD Diagnosis
We identified potential cases of COPD using two sources of information: a) automated digital algorithms,17 and b) ascertainment of diagnoses through the REP medical records linkage system.11
a. Automated digital algorithm
The automated digital algorithm is a highly sensitive automatic method of extracting comorbidities, including COPD, from the electronic medical records (EMR) using Boolean combinations of clinical variables and natural language processing data feeds.17 The implementation of an automatic note search strategy to extract COPD from the EMR is advantageous in that it facilitates fast recognition of COPD cases with both high sensitivity (>98%) and specificity (>99%).17,18 However, some providers in Olmsted County do not have medical records that can be accessed by the digital algorithms. As a result, we also used the International Classification of Diseases [ICD-9] and Hospital International Classification of Diseases Adapted [HICDA] codes from the REP and manual medical record data abstraction to identify individuals with COPD.
b. Medical records ascertainment
The REP compiles the medical records and residency status of each person who visited any health care provider in Olmsted County since January 1, 1966; this primarily includes the Mayo Clinic, Olmsted Medical Center, and their affiliated clinics.11 Using the REP records linkage system, we identified all MCSA subjects with any of the following primary ICD or HICDA codes indicative of possible COPD: 491.xx - chronic bronchitis, 492.xx - emphysema, or 496.xx - chronic airway obstruction, not elsewhere classified.19
After the identification of potential participants with COPD, the clinical records of the subjects were reviewed to make a confirmatory diagnosis. Patients were determined to have COPD if there was a physician diagnosis of COPD in the medical record (eg, documented diagnosis of chronic bronchitis, emphysema, COPD in the admission note, progress note, or discharge summary from the index hospitalization) and/or use of COPD medication therapy (eg, beta2-agonists, anticholinergics, or methylxanthines). Of the 1425 cognitively normal individuals at baseline, 171 individuals were confirmed to have a diagnosis of COPD. The Kappa for both the automated digital algorithms and identification of COPD cases using the REP codes was excellent (Kappa = 0.8, with 94% agreement).
To assess the reliability of abstracting information for a COPD diagnoses, two reviewers independently abstracted the EMR of 50 randomly selected subjects (10 COPD and 40 non-COPD cases). The inter-reviewer agreement was also excellent, Kappa = 0.9 (94% agreement).
Assessment of Covariates
Demographics (age, sex, education) were assessed by interview at the baseline visit. Participants were asked to bring all medications to the study visit and the names of medications were recorded. Information related to smoking history (former, current or never smokers), cardiovascular comorbidities, hypertension, coronary artery disease (angina, myocardial infarction, coronary revascularization, or coronary artery bypass grafting), and diabetes were abstracted from a review of the EMR. Depressive symptoms were assessed using the Beck Depression Inventory II Scale.20 Evidence of a stroke was assessed at baseline by the study physician and further validated using the medical records. The study coordinator measured height and weight for computing body mass index (BMI). APOE genotyping was performed using standard methods.
Statistical Analysis
Continuous variables were reported as medians with the interquartile range (IQR), and categorical variables as counts with percentages. Differences in the baseline demographic and health-related characteristics between subjects with and without MCI, and with and without COPD, were examined using chi-square tests for categorical variables and Wilcoxon Rank Sum tests for continuous variables. The date of onset of MCI was defined as the midpoint between the last evaluation when the subject was cognitively normal and the first evaluation when the subject received a diagnosis of MCI. For the 16 subjects who progressed directly from normal cognition at one visit to dementia at the next visit, the date of onset of MCI was defined as the midpoint between the last evaluation when the subject was cognitively normal and first evaluation when the subject was diagnosed as having dementia. Subjects who refused further participation, were lost to follow up, or died were censored at their last evaluation.
Multivariate Cox proportional hazard models were used to investigate the association between COPD and incident MCI and MCI subtypes, with age as a time scale, directly standardized by age and sex to the Olmsted County population on October 1, 2004, and adjusted for nonparticipation at baseline using reciprocal probability weighting in Poisson regression models. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI). Three models were evaluated, each building upon the previous model. Model 1 adjusted for age, education, and sex. Model 2 controlled for the variables in Model 1 plus BDI-II depression scores (as a categorical variable, <13 and ≥13) and history of stroke. Model 3 controlled for the variables in Model 2 and also included APOE genotype (any ε4 vs no ε4), smoking (ever vs never smoker), diabetes, hypertension, coronary artery disease, BMI and baseline global z-score. The proportional hazard assumption for COPD at enrollment, COPD duration at baseline, and COPD duration levels defined at baseline (no COPD, duration ≤5 years, >5 years), were valid. COPD duration was dichotomized at the median of 5 years and the same models were run. There was no significant interaction between age and COPD or sex and COPD. All the statistical tests were performed at the conventional 2-tailed alpha level of 0.05 using JMP 9.0.1 and SAS (SAS Institute, Cary, NC) software.
RESULTS
Of the 1425 cognitively normal subjects with at least one follow-up visit, 370 developed incident MCI: 230 (62.2%) had a-MCI, 97 (26.2%) had na-MCI, 27 (7.3%) had MCI of unknown type, and 16 (4.3%) went from being cognitively normal at one visit to dementia at the next (Figure 1). The median duration of follow-up was 5.1 years (IQR, 3.8–5.4 years). Compared to participants included in the subsequent analyses, those who were lost to follow-up had lower education (median of 12 vs 13 years, P = .016) but were similar in sex, frequency of stroke, cardiovascular comorbidities, diabetes and COPD. At baseline, participants who developed incident MCI were significantly (p<0.05; Table 1) older, less educated, and had a higher frequency of stroke, diabetes, depression, and APOE ε4 genotype compared to those who remained cognitively normal.
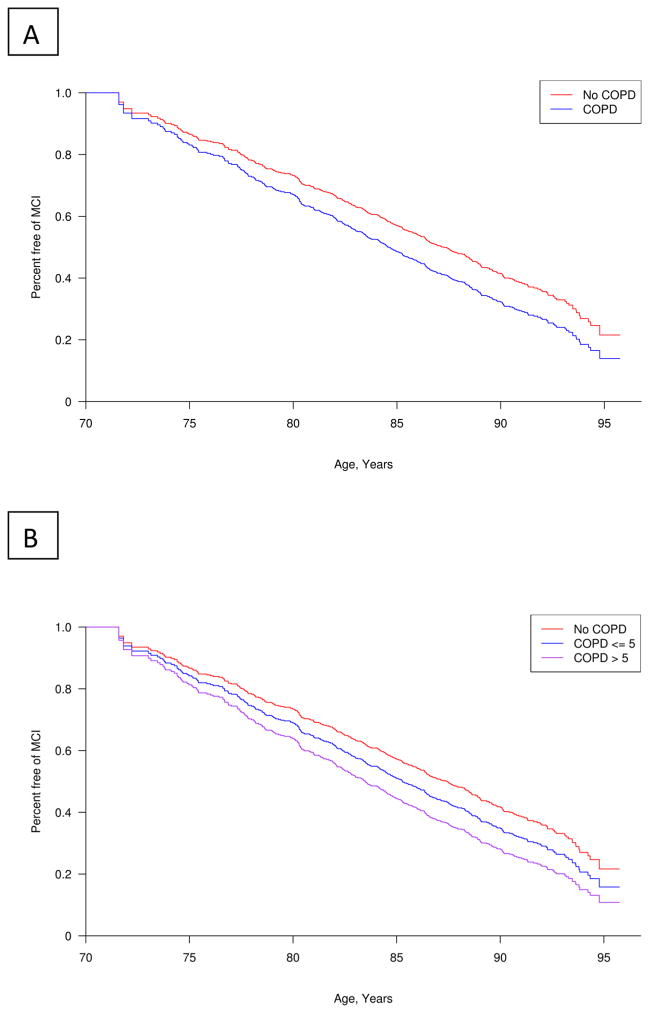
Figure 1. Adjusted Kaplan-Meier plots of COPD and risk of MCI.
Figure 1A shows the relationship between COPD (Yes/No) and percent-free survival of MCI. Figure 1B shows the relationship between COPD duration (>5 years vs. less) or no COPD and percent-free survival of MCI with age as the timescale.
Table 1.
Baseline Characteristics of the Study Participants by Cognitive Status
| Baseline Variables | MCI (n=370) | Normal cognition (n=1055) | P Value |
|---|---|---|---|
| Male sex, N (%) | 186 (50.27) | 529 (50.14) | .9662 |
| Age (years) -- median (IQR) | 82.08 (77.42, 85.16) | 78.33 (74.37, 82.73) | <.0001 |
| Education (years) -- median (IQR) | 12 (12, 15) | 13 (12, 16) | <.0001 |
| APOE E4 allele (E24/34/44 vs. 22/23/33), N (%)a | 109 (29.54) | 239 (22.78) | .0095 |
| Hypertension, N (%) | 290 (78.38) | 794 (75.26) | .2265 |
| Stroke, N (%) | 48 (12.97) | 87 (8.25) | .0076 |
| BDI-II Depression (≥13), N (%)b | 42(11.80) | 65 (6.38) | .0010 |
| BMI, median (IQR)c | 27.27 (24.04, 30.23) | 27.20 (24.49, 30.34) | .5075 |
| Smokers | .8700 | ||
| Never | 193 (52.16) | 539 (51.09) | |
| Former | 162 (43.78) | 477 (45.21) | |
| Current | 15 (4.05) | 39 (3.70) | |
| Coronary artery disease, N (%) | 161 (43.51) | 415 (39.34) | .1589 |
| Diabetes Mellitus, N (%) | 78 (21.08) | 165 (15.64) | .0166 |
| COPD, N (%) | 52 (14.05) | 119(11.28) | .1576 |
| Memory z score – median (IQR) | −0.28 (−0.83, 0.26) | 0.45 (−0.14, 1.03) | <.0001 |
| Language z score – median (IQR) | −0.23 (−0.90, 0.35) | 0.37 (−0.18, 0.93) | <.0001 |
| Attention z score – median (IQR) | −0.22 (−0.83, 0.37) | 0.46 (−0.10, 0.94) | <.0001 |
| Visual Spatial z score – median (IQR) | −0.18 (−0.90, 0.38) | 0.38 (−0.24, 0.92) | <.0001 |
| Global z score – median (IQR) | −0.38 (−0.87, 0.16) | 0.51 (−0.04, 1.01) | <.0001 |
Notes: Data are n (%) or median (IQR). Abbreviations: IQR, interquartile ratio; BMI, body mass index; BDI, Beck Depression Inventory; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
7 patients missing APOE status (6 Normal, 1 MCI), percentage was based on non-missing data.
50 patients missing BMI (36 Normal, 14 MCI), percentage was based on non-missing data.
26 patients missing BDI-II Depression (18 Normal, 8 MCI), percentage was based on non-missing data.
There were 171 individuals with a COPD diagnosis at baseline (Table 2). Compared to subjects without a COPD diagnosis, those with a diagnosis were more often (p<0.05) men, older, had a higher frequency of coronary artery disease (CAD), hypertension and stroke, and were more likely to report former or current smoking.
Table 2.
Baseline Characteristics of the Study Participants According to the COPD Status
| Baseline Variables | COPD (n=171) | No-COPD (n=1254) | P Value |
|---|---|---|---|
| Male sex, N (%) | 100 (58.48) | 615 (49.04) | 0.0206 |
| Age (years) -- median (IQR) | 80.79 (75.89 – 83.87) | 79.10 (74.86 – 83.34) | 0.0499 |
| Education (years) -- median (IQR) | 13 (12–16) | 13 (12–16) | 0.1094 |
| APOE E4 allele (E24/34/44 vs 22/23/33), N (%)a | 42 (24.85) | 306 (24.50) | 0.9204 |
| Hypertension, N (%) | 143 (83.63) | 941 (75.04) | 0.0136 |
| Coronary artery disease, N (%) | 108 (63.16) | 468 (37.32) | <0.0001 |
| Myocardial infarction, N (%) | 35 (20.47) | 178 (14.19) | 0.0309 |
| Diabetes Mellitus, N (%) | 28 (16.37) | 215 (17.15) | 0.8015 |
| Stroke, N (%) | 24 (14.04) | 111 (8.85) | 0.0299 |
| BDI-II Depression (≥13), N (%)b | 14 (8.38) | 93 (7.70) | 0.7569 |
| BMI, median (IQR)c | 27.52 (24.33–30.85) | 27.19 (24.40–30.18) | 0.2007 |
| Smokers | <0.0001 | ||
| Never | 34 (19.88) | 704 (56.14) | |
| Current | 32 (18.71) | 22 (1.75) | |
| Former | 105 (61.40) | 528 (42.11) | |
| Domain z score | |||
| Memory -- median (IQR)d | 0.03 (−0.48, 0.58) | 0.29 (−0.35, 0.92) | 0.0029 |
| Language -- median (IQR)e | 0.12 (−0.57, 0.67) | 0.25 (−0.31, 0.81) | 0.0164 |
| Attention -- median (IQR)f | 0.002 (−0.55, 0.59) | 0.34 (−0.27, 0.84) | 0.0004 |
| Visual Spatial -- median (IQR)g | 0.05 (−0.61, 0.63) | 0.27 (−0.37, 0.80) | 0.0189 |
| Global -- median (IQR h | 0.05 (−0.61, 0.66) | 0.33 (−0.25, 0.90) | 0.0007 |
Notes: Data are n (%) or median (IQR). Abbreviations: IQR, interquartile ratio; BMI, body mass index; BDI, Beck Depression Inventory; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
7 patients missing APOE status (2 COPD, 5 no- COPD), percentage was based on non-missing data.
50 patients missing BDI-II Depression (4 COPD, 46 no-COPD).
26 patients missing BMI (5 COPD, 21 no-COPD).
17 patients missing memory z score (12 Normal, 5 MCI).
56 patients missing language z score (40 Normal, 16 MCI).
72 patients missing attention z score (47 Normal, 25 MCI).
71 patients missing visual spatial z score (49 Normal, 22 MCI).
101 patients missing global z score (69 Normal, 32 MCI).
A diagnosis of COPD was associated with an increased risk of MCI (HR 1.43; 95% CI, 1.07–1.91) adjusting for covariates in Model 2, but the result was attenuated and borderline-non significant after additional adjustment for variables in Model 3 (HR 1.33; 95% CI: 0.96–1.84) (Table 3). When examining MCI subtypes, COPD was associated with almost a two-fold risk of na-MCI (HR 1.83; 95% CI, 1.04–3.23) in the fully adjusted Model 3. COPD was not associated with an increased risk of a-MCI.
Table 3.
Association of COPD with Incident MCI and MCI Subtypes
| Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MCI Type | Incident MCI/At risk, N | HR (95% CI) | Incident MCI/At risk, N | HR (95% CI) | Incident MCI/At risk, N | HR (95% CI) |
| Any MCI | 370/1,425 | 1.37 (1.03, 1.83) | 356/1,375 | 1.43 (1.07, 1.91) | 318/1,250 | 1.33 (0.96, 1.84) |
| Amnestic MCI | 230/1,425 | 0.87 (0.57, 1.35) | 222/1,375 | 0.88 (0.57, 1.37) | 203/1,250 | 0.97 (0.61, 1.54) |
| Non-amnestic MCI | 97/1,425 | 2.01 (1.23, 3.28) | 92/1,375 | 2.22 (1.36, 3.64) | 83/1,250 | 1.83 (1.04, 3.23) |
Abbreviations: COPD, chronic obstructive pulmonary disease; MCI, mild cognitive impairment.
No-COPD is the reference group [HR 1.00] for all the analyses. Numbers in bold denotes significant association.
Model 1 adjusted for education as a continuous variable, sex where applicable, and age at baseline as time variable.
Model 2 additionally adjusted for BDI-II Depression, and history of stroke.
Model 3 includes model 2 variables, with additional adjustment for APOEe4 genotype, smoking, diabetes, hypertension, coronary artery disease, z-scores and BMI.
COPD Duration and Association with MCI
There was a dose-response relationship between duration of COPD and risk of any MCI and na-MCI (Table 4). The overall HR for any MCI increased from 1.11 (95% CI, 0.70–1.74) in subjects with a COPD duration of ≤ 5 years at baseline to 1.58 (95% CI, 1.04–2.40) in subjects with a COPD duration > 5 years. Similarly, the overall HR for na-MCI increased from 1.14 (95% CI, 0.46–2.79) in subjects with a COPD duration at baseline of ≤ 5 years to 2.58 (95% CI, 1.32–5.06) in subjects with COPD > 5 years. We did not observe a dose-response effect when examining duration of COPD and a-MCI.
Table 4.
Hazard Ratios for Association of Mild Cognitive Impairment by COPD Duration
| Model 1a | Model 2b | Model 3c | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| COPD duration ≤ 5 years | COPD duration > 5 years | COPD duration ≤ 5 years | COPD duration > 5 years | COPD duration ≤ 5 years | COPD duration > 5 years | ||||
|
| |||||||||
| MCI Type | Incident MCI/At risk, N | HR (95% CI) | HR (95% CI) | Incident MCI/At risk, N | HR (95% CI) | HR (95% CI) | Incident MCI/At risk, N | HR (95% CI) | HR (95% CI) |
| Any MCI | 370/1,425 | 1.22 (0.79, 1.88) | 1.49 (1.04, 2.14) | 356/1,375 | 1.26 (0.82, 1.95) | 1.57 (1.09, 2.26) | 318/1,250 | 1.11 (0.70, 1.74) | 1.58 (1.04, 2.40) |
| Amnestic MCI | 230/1,425 | 1.23 (0.72, 2.10) | 0.58 (0.29, 1.15) | 222/1,375 | 1.26 (0.74, 2.15) | 0.56 (0.27, 1.15) | 203/1,250 | 1.23 (0.71, 2.13) | 0.67 (0.32, 1.42) |
| Non- Amnestic MCI | 97/1,425 | 1.21 (0.51, 2.90) | 2.63 (1.51, 4.58) | 92/1,375 | 1.30 (0.54, 3.13) | 2.97 (1.70, 5.18) | 83/1,250 | 1.14 (0.46, 2.79) | 2.58 (1.32, 5.06) |
Abbreviations: COPD, chronic obstructive pulmonary disease; MCI, mild cognitive impairment.
No-COPD is the reference group [HR 1.00] for all the analyses. Numbers in bold denotes significant association.
Model 1 adjusted for education as a continuous variable, sex where applicable, and age at baseline as time variable.
Model 2 additionally adjusted for BDI-II Depression, and history of stroke.
Model 3 includes model 2 variables, with additional adjustment for APOEe4 genotype, smoking, diabetes, hypertension, coronary artery disease, z-scores and BMI.
Discussion
In this population-based, prospective study of individuals aged 70 and older, COPD was associated with an increased risk of na-MCI and borderline non-significant for incident MCI. Further, there was a dose-response relationship such that the greatest risk of MCI and na-MCI was among individuals with a COPD duration of five years or more. These findings highlight the importance of COPD as a risk factor for MCI and may provide a substrate for early intervention, or to more aggressively treat COPD at earlier timepoints, to prevent or delay the onset and progression of MCI, particularly na-MCI.
Previous studies have shown that COPD is associated with poor performance in tests of attention, memory and executive function, especially in severe COPD patients.5,21,22 However, only two cross-sectional studies have investigated the association between COPD and MCI using standard criteria. One study compared the frequency of MCI among 45 patients with moderate to severe COPD and 50 healthy controls referred from an outpatient pulmonary clinic.8 The moderate to severe COPD group had a higher frequency of MCI, especially na-MCI, than the healthy controls. In the MCSA, we also recently reported a higher frequency of COPD in persons with MCI compared to cognitively normal individuals.7 However, only one previous longitudinal study from Finland examine whether COPD was associated with risk of MCI.9 The authors reported that a diagnosis of COPD in mid-life (aged 39–64), but not in late-life (aged 65–80), was associated with an increased risk of MCI (HR 1.85; 95% CI, 1.05–3.28). In fact, there was a trend for individuals with COPD in late-life to have a reduced risk of MCI. In contrast to this study, we found that a diagnosis of COPD was associated with an increased risk of both MCI and na-MCI, and that the risk increased with COPD duration. There are several potential explanations for the incongruent results. First, the prior study incorporated self-reported COPD while we reviewed the medical records to confirm a diagnosis of COPD. Second, in the Finland study,9 investigators used a multistage evaluation such that only subjects who screened positive were fully evaluated for a MCI diagnosis, which may have missed some individuals. In the MCSA, we comprehensively evaluated every subject and the consensus-based approach provided diagnoses of MCI and MCI subtypes.23 Last, the cognitive status of subjects in Finland study9 was evaluated several years after baseline (> 10 years), while the MCSA evaluated the study participants at 15-month intervals. COPD is associated with increased risk of mortality, especially in the elderly. Therefore, a long interval between the baseline and follow-up visit could have led to a survival bias and resulted in an inverse association among COPD and MCI.
The increased risk of na-MCI in patients with COPD indicates a potential role of inflammation and vascular disease in the pathogenesis of na-MCI. COPD patients have increased systemic inflammatory markers including interleukin-6, C-reactive protein, leukotriene B4, cytokine TNF-Alpha, and interleukin-8.24–26 These inflammatory markers have been associated with cognitive impairment and na-MCI.27–29 COPD is also associated with an increased risk of cardiovascular diseases (CVD),30 and CVD is a risk factor for MCI.31–33 Importantly, even after adjustment for vascular diseases and factors, the association between COPD and MCI persisted suggesting that COPD is an independent predictor of MCI risk.
The association between COPD and MCI risk could also be mediated by the increased hypoxic insults to the brain, which may lead to the generation of free radicals, inflammation, neuronal damage, and glial activation.34 Furthermore, hypoxia in COPD could lead to impairment in executive tasks requiring attention allocation,35 possibly explaining the association between COPD and na-MCI in our study. Patients with COPD have altered6 and depressed cerebral perfusion,36 which along with decreased oxygen saturation, could have a significant effect on cognitive function. Indeed, the deterioration in cerebral perfusion and cognitive performance has been shown to be greater in the hypoxemic COPD patients than non-hypoxemic patients.6 Plasma clusterin (ie, apolipoprotein-J), may be another mediator of the COPD-MCI relationship. Clusterin has been shown to be elevated in patients with COPD3 and associated with cognitive decline.37
This study has several strengths. First, the MCSA is a populated-based, prospective-cohort study, designed to investigate the association between COPD and MCI. The subjects were an elderly cohort of subjects aged 70–89 years, who were randomly selected using the REP medical records-linkage system. Second, the diagnosis of the MCI was made by a comprehensive clinical evaluation and by a consensus decision among the examining physician, nurse, and a neuropsychologist, thus enhancing the reliability of the MCI diagnosis. Third, the investigators assessing the medical records for the identification of COPD cases were blinded to the diagnosis of MCI, thus avoiding diagnostic suspicion bias.
Limitations also warrant consideration. First, we defined COPD as “physician-diagnosed COPD” and did not base the definition on spirometry, which is the recommended diagnostic test for COPD but was not routinely available in our population. In the absence of spirometry, previous studies have shown that COPD, particularly mild COPD, is under-diagnosed in the population.2,38 However, physician-diagnosed COPD may also over-diagnose COPD if not confirmed by spirometry.39,40 Second, the Olmsted County population, aged 70 and older, is primarily white and of European ancestry, potentially reducing the generalizability of the study results to other populations. However, the findings from the previous studies conducted in Olmsted County have been shown to be generalizable to the Upper Midwest population,41 and thereby provide important information regarding various diseases, and are consistent with national data.41 Third, we did not have adequate data for smoking duration and could not adjust for years of smoking. Instead, we characterized smokers as ever vs. never smokers at baseline. Controlling for smoking status had little effect on the models suggesting that the association between COPD and MCI is independent of smoking status.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health (P50 AG016574, U01 AG006786 [under which study participants were enrolled], K01 MH068351, and K01 AG028573), the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, by Clinical and Translational Science Award UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the NIH and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Footnotes
Author Contributions:
Study concept and design: Singh, Mielke, Roberts, Geda, Scanlon, Petersen.
Acquisition of data: Singh, Parsaik, Geda, Scanlon, Petersen
Analysis and Interpretation of data: Singh, Mielke, Roberts, Cha, Christianson, Pankratz, Petersen.
Drafting of the manuscript: Singh, Mielke, Parsaik, Roberts, Petersen.
Critical revision of the manuscript for important intellectual content: Singh, Mielke, Roberts, Geda, Parsaik, Christianson, Cha, Pankratz, Scanlon, Petersen.
Statistical analysis: Singh, Cha, Mielke, Christianson, Pankratz.
Study supervision: Mielke, Roberts, Scanlon and Petersen
Data Access, Responsibility, and Analysis: Drs. Singh and Mielke had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Statistical analyses were conducted by: Balwinder Singh, Ruth Cha, Teresa J. Christianson, and V. Shane Pankratz, Mayo Clinic, Rochester, MN, USA
Conflict of Interest Disclosures: Dr. Mielke receives research support from the NIH/NIA and has served as a consultant for Eli Lilly. Dr. Roberts receives research support from the NIH/NIA and from Abbvie Health Economics and Outcomes Research. Dr. Scanlon has participated as an investigator in clinical trials funded by Boehringer Ingelheim, GlaxoSmithKline, Forest, Novartis and Pearl Therapeutics, and has served as a consultant to GlaxoSmithKline and Merck. Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Elan Pharmaceuticals, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA. Drs. Singh, Parsaik, Geda, Pankratz, Ms. Cha, and Ms. Christianson report no disclosures.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance--United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Huang Y, Fei GH. The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration. 2013;85(2):98–105. doi: 10.1159/000342970. [DOI] [PubMed] [Google Scholar]
- 4.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476. [PubMed] [Google Scholar]
- 5.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 6.Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med. 2006;20(2):99–106. doi: 10.1007/BF02985621. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Parsaik AK, Mielke MM, et al. Chronic obstructive pulmonary disease is associated with mild cognitive impairment: the Mayo Clinic Study of Aging. Mayo Clin Proc. 2013;88(11):1222–1230. doi: 10.1016/j.mayocp.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villeneuve S, Pepin V, Rahayel S, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012;142(6):1516–1523. doi: 10.1378/chest.11-3035. [DOI] [PubMed] [Google Scholar]
- 9.Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Curr Alzheimer Res. 2013;10(5):549–555. doi: 10.2174/1567205011310050011. [DOI] [PubMed] [Google Scholar]
- 10.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 14.Fahn S, Elton R . Committee MotUD. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Caine D, Lieberman A, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 15.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6(Supplement):1–30. [Google Scholar]
- 16.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsara A, Warner DO, Li G, Herasevich V, Gajic O, Kor DJ. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86(5):382–388. doi: 10.4065/mcp.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 21.Schou L, Ostergaard B, Rasmussen LS, Rydahl-Hansen S, Phanareth K. Cognitive dysfunction in patients with chronic obstructive pulmonary disease--a systematic review. Respir Med. 2012;106(8):1071–1081. doi: 10.1016/j.rmed.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Incalzi RA, Gemma A, Marra C, Muzzolon R, Capparella O, Carbonin P. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis. 1993;148(2):418–424. doi: 10.1164/ajrccm/148.2.418. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RO. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 25.Hill AT, Bayley D, Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999;160(3):893–898. doi: 10.1164/ajrccm.160.3.9901091. [DOI] [PubMed] [Google Scholar]
- 26.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RO, Geda YE, Knopman DS, et al. Association of C-reactive protein with mild cognitive impairment. Alzheimer’s Dement. 2009;5(5):398–405. doi: 10.1016/j.jalz.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts RO, Geda YE, Knopman DS, et al. Metabolic syndrome, inflammation, and nonamnestic mild cognitive impairment in older persons: a population-based study. Alzheimer Dis Assoc Disord. 2010;24(1):11–18. doi: 10.1097/WAD.0b013e3181a4485c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 30.Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular Comorbidity in COPD: Systematic Literature Review. Chest. 2013;144(4):1163–1178. doi: 10.1378/chest.12-2847. [DOI] [PubMed] [Google Scholar]
- 31.Roberts RO, Geda YE, Knopman DS, et al. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA Neurol. 2013;70(3):374–382. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ettorre E, Cerra E, Marigliano B, et al. Role of cardiovascular risk factors (CRF) in the patients with mild cognitive impairment (MCI) Arch Gerontol Geriatr. 2012;54(2):330–332. doi: 10.1016/j.archger.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Wysocki M, Luo X, Schmeidler J, et al. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriatr Psychiatry. 2012;20(2):179–187. doi: 10.1097/JGP.0b013e31820ee833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Torre JC. Critical threshold cerebral hypoperfusion causes Alzheimer’s disease? Acta Neuropathol. 1999;98(1):1–8. doi: 10.1007/s004010051044. [DOI] [PubMed] [Google Scholar]
- 35.Stuss DT, Peterkin I, Guzman DA, Guzman C, Troyer AK. Chronic obstructive pulmonary disease: effects of hypoxia on neurological and neuropsychological measures. J Clin Exp Neuropsychol. 1997;19(4):515–524. doi: 10.1080/01688639708403741. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli Incalzi R, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease--a neuropsychological and spect study. J Neurol. 2003;250(3):325–332. doi: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 37.Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011;305(13):1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 38.Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- 39.Prieto Centurion V, Huang F, Naureckas ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73. doi: 10.1186/1471-2466-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD. 2006;3(2):95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 41.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.