Abstract
Most of the literature on serous borderline/atypical proliferative serous tumors (SBT/APSTs) shows no effect of microinvasion or lymph node involvement on outcome. This study is a morphologic and immunohistochemical analysis of the cells comprising SBT/APSTs, microinvasion, lymph node involvement, and low-grade serous carcinoma (LGSC) in an attempt to explain this unusual behavior. We found that the cells in microinvasion and in lymph nodes were morphologically similar to the cells in SBT/APSTs but differed significantly from the cells in LGSCs. In addition, one particular population of cells, those with abundant eosinophilic cytoplasm (eosinophilic cells), in SBT/APSTs, microinvasion, and lymph nodes showed a significant loss of expression of ER, PR, and WT-1 compared to the cuboidal/columnar tumor cells, both in cases of microinvasion (p<0.001 for all three markers) and lymph node involvement (p=<0.001, 0.02, 0.002, respectively). There was a significant decrease in the Ki-67 proliferation index for microinvasion (p=0.004) and a decreasing trend for lymph node involvement (non-significant) compared to the columnar/cuboidal cells. In addition, cells in these tumors showed morphologic evidence of apoptosis which was confirmed by immunostaining with M30, a marker of apoptosis. In contrast, LGSCs lacked eosinophilic cells and showed no loss of expression of ER, PR and WT1. They also had a significantly higher Ki-67 proliferation index than their associated SBT/APSTs (p=0.029). Based on these findings, we propose that the cells comprising microinvasion do not represent an invasive neoplastic process. Instead, in view of the loss of expression of ER, PR, and WT1, evidence of apoptosis, and decrease in the Ki-67 proliferation index, we postulate that they are senescent and terminally differentiated with a subset of cells undergoing apoptosis, which could explain their lack of an adverse effect on outcome.
Keywords: atypical proliferative serous tumors, serous borderline tumors, microinvasion, lymph node involvement
Introduction
Microinvasion was first described by Tavassoli in 1988 as a form of early stromal invasion in serous borderline tumors,1 and further defined by Bell and Scully in 1990 as foci of single cells, nests, or papillae infiltrating the stroma of the tumor, each focus measuring less than 0.3 cm in maximum dimension.2 Other size criteria have also been used, with a maximum dimension of 5 mm and a maximum area of 10mm2.3–6 More recently, McKenney et al have described five patterns of microinvasion (individual eosinophilic cells and clusters, simple and noncomplex branching papillae, inverted macropapillae, cribriform, and micropapillae).7 The first three patterns appear to correspond to the majority of descriptions of classic microinvasion in the literature1, 2, 5, 8, 9 whereas the fourth and fifth patterns (cribriform, micropapillae) corresponds to what we and others regard as a small focus of low-grade serous carcinoma.5, 6, 9 It is of interest that McKenney et al conclude that the alteration featuring micropapillae may represent a comparatively higher-risk lesion (compared to the other patterns) with a clinical course analogous to low-grade serous carcinoma. This has led has led some investigators to propose that this pattern, along with a confluent glandular/cribriform pattern, be designated “microinvasive carcinoma” (i.e., a small focus of low-grade serous carcinoma) to distinguish it from “microinvasion,”5, 6, 9 (Fig. 1). In the present study, the lesion that we refer to as microinvasion corresponds to first three patterns described by McKenney and colleagues and is consistent with the definition used by other investigators.
FIGURE 1.
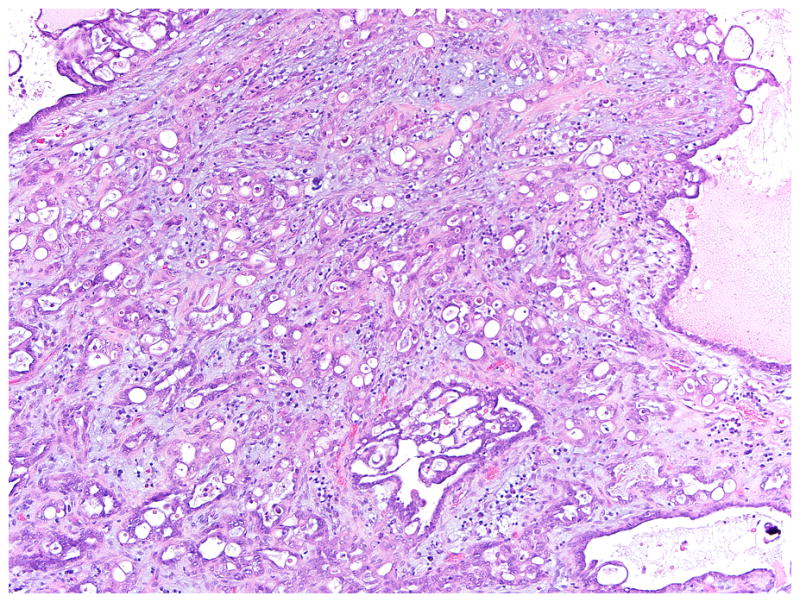
A 4mm focus of low-grade serous carcinoma in an SBT/APST, demonstrating a complex glandular proliferation and stromal desmoplasia. Compare to typical microinvasion in Figure 4.
One of the most striking and consistent features of microinvasion is the presence of large round cells with dense eosinophilic cytoplasm and centrally located, bland nuclei, sometimes with prominent nucleoli (eosinophilic cells), which are present as single cells and/or clusters. Another feature that is somewhat less commonly encountered is glands and papillary structures. Foci of microinvasion are located in the stroma just beneath the basement membrane and are typically surrounded by a clear space, which may be lined by flattened cells resembling a lymphatic channel.1, 2, 5, 8, 9
Lesions in lymph nodes associated with SBT/APSTs are very similar to those classified as microinvasion, namely consisting of eosinophilic cells (singly and in clusters), glands, and papillary structures. In addition, endosalpingiosis is frequently detected either by itself or in association with these other lesions.
Most of the literature shows no significant effect of microinvasion or lymph node involvement on outcome. The present study was undertaken in an effort to find a possible explanation for this unusual behavior by comparing the morphologic and immunohistochemical features of the cells in SBT/APSTs, microinvasion, and lymph nodes to those in low-grade serous carcinomas associated with SBT/APSTs, since SBT/APST is the putative precursor of low-grade serous carcinoma and presumably microinvasion would be the earliest step in progression. Similarly, one would assume that tumor cells in lymph nodes might have some relationship to low-grade serous carcinoma.
Anecdotally, we had observed that one particular population of cells, specifically, the eosinophilic cells in SBT/APSTs and microinvasion, showed a significant loss of expression of ER, PR, and WT-1 compared to the cuboidal/columnar cells lining the papillae of SBT/APSTs. In addition, we noted morphologic evidence of apoptosis and a decrease in the Ki-67 proliferation index in the eosinophilic cells. Therefore, we employed antibodies to detect ER, PR, WT1, apoptosis, and proliferation in the immunohistochemical component of this study to confirm our impressions. Finally, as clusters of cells in foci of microinvasion often appear to lie in a space that resembles a lymphatic channel, we performed immunohistochemistry with CD31 to evaluate that finding.
Materials and Methods
SBT/APSTs and seromucinous borderline/atypical proliferative seromucinous tumors with microinvasion and/or lymph node involvement were retrieved from The Johns Hopkins Gynecologic Pathology Consultation Service and the in-house Gynecologic Pathology service of The Johns Hopkins Hospital, prospectively from 2011–2012 and retrospectively for the preceding 10 years. Seromucinous tumors were included because despite some morphologic differences (mainly the presence of endocervical-type epithelium), their behavior with respect to microinvasion, lymph node involvement, and outcome is similar to their serous counterparts. Cases with micropapillary features were excluded. Microinvasion was defined as individual cells, clusters, glands, or papillae in the stroma below the basement membrane, usually surrounded by a clear space and without a stromal reaction. A size limit of 5mm was used for each focus.3, 5, 6 Lymph node involvement was defined as involvement of lymph node sinuses and/or parenchyma by individual cells and clusters of cells with abundant eosinophilic cytoplasm or by proliferative glands and papillae with an appearance similar to that of the ovarian tumor. In addition, 6 cases of invasive low-grade serous carcinoma (LGSC) arising in association with SBT/APST were collected from the in-house files retrospectively for the preceding 10 years. All cases were reviewed by two authors (KPM and RJK).
Descriptive characteristics of the tumors, namely, laterality and size of ovarian tumors, extent of ovarian tumor sampling, intracystic and/or exophytic growth of the ovarian tumors, the number and size of foci of microinvasion and lymph node involvement, presence or absence of endosalpingiosis, cellular population (cuboidal/columnar and/or eosinophilic cells on the surface of the tumor or detached from it), and architectural/cytologic features of microinvasion and lymph node involvement, were recorded. Endosalpingiosis was defined as simple glands lined by flattened cuboidal to columnar epithelium with cilia. Cases in which the proliferation was more florid but the lining was still mostly comprised of cuboidal to columnar rather than eosinophilic cells were still diagnosed as endosalpingiosis, although the distinction between florid endosalpingiosis and involvement by tumor is ill-defined and likely represents a spectrum of change.
For each case, an immunohistochemical panel consisting of ER, PR, WT-1, and Ki-67 was performed. (See Table 1 for antibody specifications). These antibodies were chosen because they are consistently expressed in SBT/APST but had been anecdotally noted to be lost in some cases of microinvasion and lymph node involvement. All immunostains with the exception of Ki-67 were scored using a modified Remmele score, in which the product of staining intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong) and percentage of cells staining (1 = 0–10% of cells, 2 = 11–50% of cells, 3 = 51–80% of cells, and 4 = >80% of cells) was calculated.10–12 Only nuclear staining was considered positive for ER, PR, WT-1, and Ki-67.
TABLE 1.
Antibody specifications
| Antibody | Clone # | Dilution | Source |
|---|---|---|---|
| ER | 6F11 | Prediluted | Leica Microcystems; Banoockbum, IL |
| PR | 16 | Prediluted | Leica Microcystems; Banoockbum, IL |
| WT-1 | 6F-H2 | Prediluted | Ventana; Tucson, AZ |
| Ki-67 | 30-9 | Prediluted | Ventana; Tucson, AZ |
| CD31 | JC70 | Prediluted | Ventana; Tucson, AZ |
| M30 (CK18-Asp396 neo-epitope) | M30 | 1:600 | Enzo Life Sciences; Farmingdale, NY |
For the Ki-67 proliferation index, individual cells were counted by examining the slide under a microscope at high power, and the proliferation index was calculated as the number of cells with positively staining nuclei divided by the total number of cells counted. At least 500 cells were counted for the cuboidal/columnar tumor cells of the SBT/APSTs and seromucinous tumors, as well as for the carcinomas. An attempt was made to count at least 200 cells to score microinvasion, lymph node involvement, surface and detached eosinophilic cells, and endosalpingiosis. In cases of microinvasion or lymph node involvement in which 200 cells could not be found, all cells qualifying as microinvasion or lymph node involvement in the stained slide were counted.
Finally, in cases with sufficient tissue, we performed immunohistochemistry for apoptosis using the M30 antibody in 16 cases, and to determine whether clusters of microinvasion cells were in vascular/lymphatic channels, we performed immunohistochemistry using CD31 in 19 cases. CD31 positivity was recognized as a membranous/cytoplasmic staining pattern. Positivity for M30, an antibody to an epitope of CK18 exposed early in apoptosis, was characterized by a cytoplasmic staining pattern.13
Statistical analysis for the immunohistochemical scores consisted of descriptive statistics (mean, median, range) as well as Mood’s median test for comparison of medians between groups (for non-parametric data).14 All tests were performed using Microsoft Excel 2010 with the ProcessMA statistical add-in.
Results
Tumors with Microinvasion
Twenty-three SBT/APSTs and seromucinous tumors with microinvasion in which material was available for immunostaining, including one which also demonstrated lymph node involvement, were identified. The age range of the patients was 21–71 years (median 39, mean 41.8). Four of the cases were seromucinous borderline/atypical proliferative seromucinous tumors; the remainder were serous tumors. For the 22 cases in which this information was available, 7 were bilateral and 15 were unilateral. The ovarian tumors ranged from 2.5 to 18 cm in greatest dimension, with a mean size of 8.3 cm. For the 14 cases in which the report specified the presence/absence of surface involvement, 11 were intracystic and 3 had an exophytic component. Sampling of the ovarian tumor was adequate (at least 1 section/cm greatest tumor dimension) in 15 of the 21 cases for which this information was available.
Microscopically, all the tumors displayed the typical hierarchical architecture of an SBT/APST. The epithelium covering the papillae was composed of cuboidal to columnar cells interspersed with rounded cells with abundant, dense, eosinophilic cytoplasm. Cilia were frequently present in all the cell types, and the columnar/cuboidal cells often demonstrated a pseudostratified arrangement. In addition, the seromucinous tumors contained foci of endocervical-type mucinous epithelium. Some of the eosinophilic cells had a hobnail appearance and several appeared to be budding from the surface with clusters of detached eosinophilic cells floating above the surface of the papillae (Fig. 2). A few of these floating single cells as well as those on the surface of the papillae demonstrated morphologic features suggestive of apoptosis (cell shrinkage, nuclear pyknosis), which was confirmed by positive staining with the antibody M30, an apoptotic marker. Specifically, 16 tumors (8 SBT/APSTs with microinvasion and 8 SBT/APSTs with lymph node involvement) from 15 patients (2 tumors were in the same patient) showed a positive reaction with the M30 antibody in approximately 0.5% of the tumor cells (Fig. 3).
FIGURE 2.
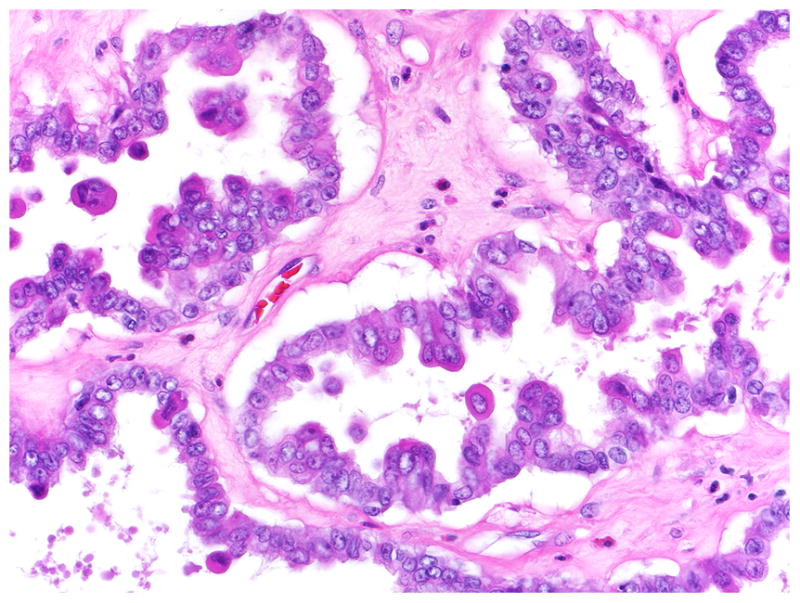
SBT/APST with cuboidal surface epithelial cells interspersed with budding and detached eosinophilic cells.
FIGURE 3.
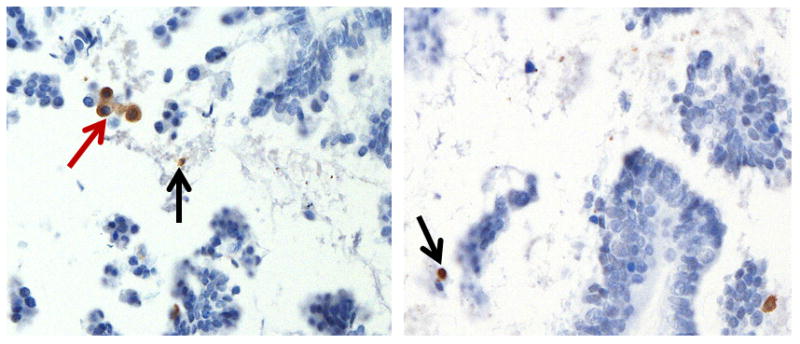
Immunoreactivity of M30 in two representative cases. M30 staining is detected in scattered epithelial cells on the surface of the papillae of a SBT/APST as well as in the detached cells. The “pink cells” (red arrow) and those with morphologic features of apoptosis (black arrows) express M30.
The microinvasive foci ranged from less than 1 mm to 5 mm, with 11 of the cases demonstrating no foci greater than 1 mm in greatest dimension, six of the cases with foci between 1 and 3 mm, and the remainder >3 mm. The foci were composed predominantly and often exclusively of cells with dense eosinophilic cytoplasm and rounded nuclei present singly or in clusters or small glands, surrounded by a clear zone, just below the basement membrane but eliciting no stromal reaction. These cells were virtually identical to the eosinophilic cells on the surface of the papillae or floating above the papillae as detached single cells or clusters of cells (Fig. 4). While most cases displayed multiple small foci of individual cells and clusters, five demonstrated foci with a more florid proliferation (Fig. 5). In eight cases, the eosinophilic cells had areas of glandular architecture, often in association with clusters and single cells. Two cases demonstrated glandular and simple papillary structures comprised of cells that did not have prominent eosinophilic cytoplasm and more closely resembled the cuboidal/columnar surface epithelial cells. One of these two cases also did not demonstrate many detached eosinophilic cells floating above the papillae.
FIGURE 4.
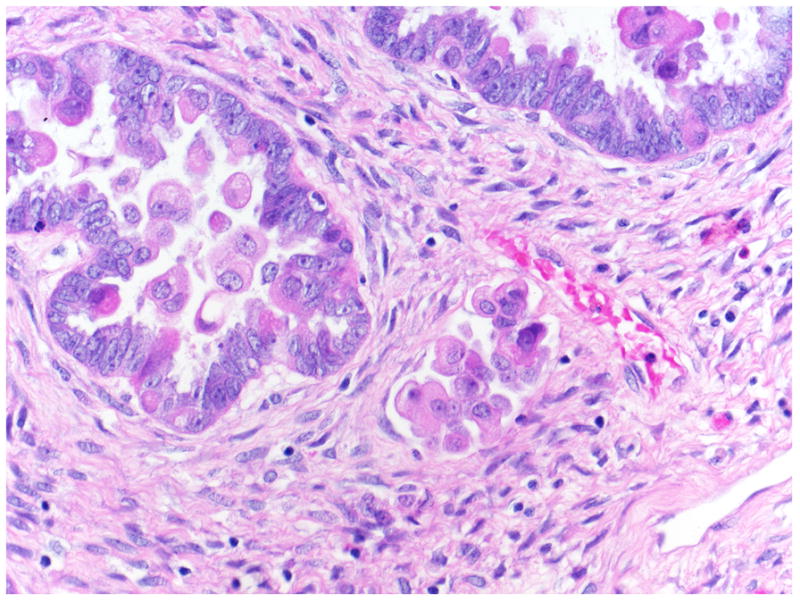
Microinvasion with overlying cuboidal/columnar surface epithelium and eosinophilic cells (on surface and detached).
FIGURE 5.
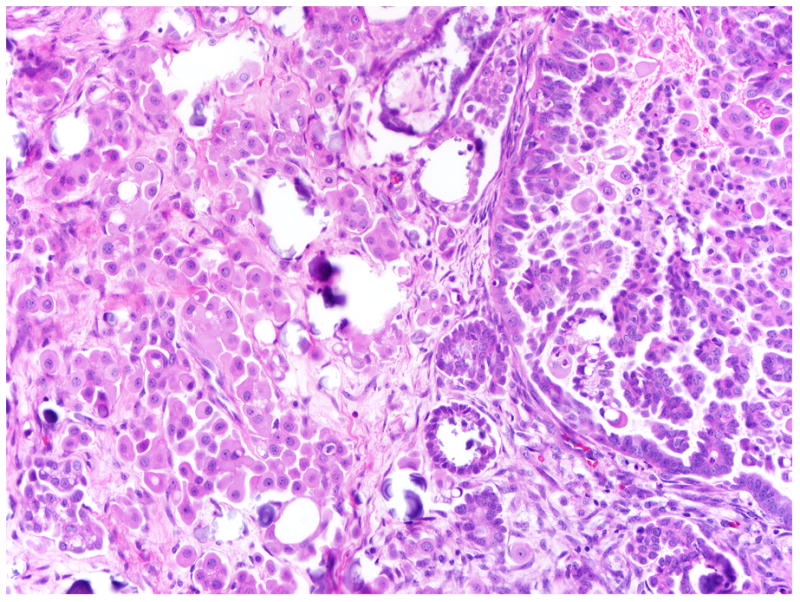
Florid microinvasion composed of clusters of cells with eosinophilic cytoplasm, accompanied by psammoma bodies. Similar eosinophilic cells along with small papillary structures can be seen detached and floating above the surface epithelium.
Sufficient material was present for scoring on all immunostained sections. In all but five cases, at least 200 microinvasive cells were present to score the Ki-67 proliferation index. The eosinophilic cells (detached and on the surface of papillae), the cuboidal/columnar surface epithelial cells, and the microinvasive cells were scored separately for each immunohistochemical marker. The results for each marker in each cell type are presented in Table 2. The medians were compared among the three groups as follows. The microinvasive cells were first compared to the eosinophilic cells (detached and on the surface of papillae), and no significant differences were found between the median immunohistochemical scores for these two groups for ER, PR, WT-1, or the median Ki-67 proliferation indices. Given this finding, the scores for these two groups were combined and then compared to the surface cuboidal/columnar epithelium. The microinvasive cells and eosinophilic cells (detached and on the surface of papillae) had significantly lower median scores for ER, PR, and WT-1, as well as a significantly lower Ki-67 proliferation index, than the cuboidal/columnar tumor cells (Fig. 6). The microinvasive cells were also separately compared to the surface cuboidal/columnar epithelial cells without inclusion of the detached eosinophilic cells, with similar results (data not shown).
TABLE 2.
Ovarian serous borderine/atypical proliferative serous tumors (SBT/APST) with microinvasion: descriptive statistics and comparison of medians
| Immunohistochemical scores
|
Ki-67 index | |||
|---|---|---|---|---|
| ER | PR | WT-1 | ||
|
| ||||
| Cuboidal/columnar surface epithelial cells of SBT/APST | ||||
| Mean | 11 | 7.4 | 10.4 | 7.1% |
| Median | 12 | 6 | 12 | 7% |
| Range | 6 – 12 | 0 – 12 | 0 – 12 | 1–16% |
|
| ||||
| Eosinophilic cells (detached and on the surface of the papillae) | ||||
| Mean | 5.7 | 3.9 | 4.4 | 3.1% |
| Median | 6 | 4 | 3 | 2.5% |
| Range | 2 – 12 | 0 – 12 | 0 – 12 | 0–9% |
|
| ||||
| Microinvasive cells | ||||
| Mean | 5.6 | 3.2 | 5.2 | 3.3% |
| Median | 6 | 2 | 4 | 3% |
| Range | 0 – 12 | 0 – 12 | 0 – 12 | 0–10% |
|
| ||||
| Microinvasive cells vs. eosinophilic cells (detached and on the surface of the papillae) | ||||
| p-value | 0.987 | 0.226 | 0.567 | 0.702 |
|
| ||||
| Cuboidal/columnar surface epithelial cells vs. microinvasive cells + eosinophilic cells | ||||
| p-value | <0.001* | <0.001* | <0.001* | 0.004* |
A p-value of < 0.5 was used to determine significance.
FIGURE 6.
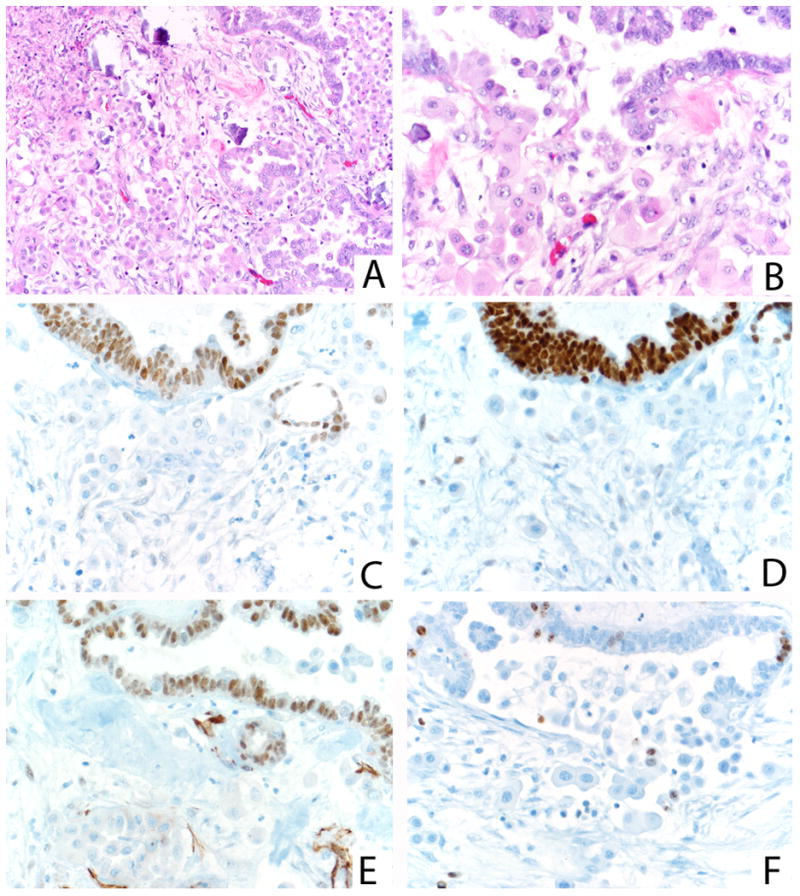
Low-power view of florid microinvasion with psammoma bodies (A). Higher power demonstrates eosinophilic microinvasive cells and overlying cuboidal/columnar surface epithelium with occasional eosinophilic cells (B). In contrast to the surface epithelium, the microinvasive cells demonstrate a loss of ER (C), PR (D), and WT-1 (E). The Ki-67 proliferation index is also lower in the microinvasive cells (F).
As mentioned above, two cases demonstrated a more papillary and glandular architecture in the foci of microinvasion, without many eosinophilic cells. Although these cases were too few to separately analyze and compare, some loss of immunohistochemical marker expression was seen in both cases. In one case, the microinvasive cells demonstrated a PR score of 1 (compared to 12 in the columnar/cuboidal tumor cells) and a Ki-67 proliferation index of 3% (compared to 11% in the columnar/cuboidal tumor cells); no loss of expression of ER or WT-1 was seen. In the second case, cells in the focus of microinvasion demonstrated an ER score of 9 (compared to 12 in the columnar/cuboidal tumor cells) and a PR score of 4 (compared to 6 in the columnar cuboidal cells); no loss of expression of WT-1 or decrease in Ki-67 proliferation index was seen.
Finally, although numerous small vascular spaces in the stroma of 19 cases tested were positive for CD31, none of the foci of micoinvasion showed positive staining around the nests, indicating that they were not in lymphatic channels.
Tumors with lymph node involvement
Fifteen cases with lymph node involvement for which material was available for immunostaining were identified, including one which also demonstrated microinvasion and thus was included in both this and the microinvasion analysis. All of the tumors were SBT/APSTs; none were seromucinous. The age range of the patients was 21–79 years (median 33, mean 39.9). The involved lymph nodes were in the pelvic and/or para-aortic groups; nine showed involvement of pelvic lymph nodes only, two showed involvement of para-aortic nodes only, and four showed involvement of both pelvic and para-aortic nodes. In all but two cases, multiple lymph nodes were involved. Nine of the ovarian tumors were bilateral and 6 were unilateral. Ovarian tumor size ranged from 4.5 to 32 cm, with a mean of 13 cm. For the 14 cases in which the report specified the presence/absence of surface involvement, 4 were intracystic and 10 had an exophytic component. Sampling of the ovarian tumor was adequate (at least 1 section/cm greatest tumor dimension) in 10 of the 14 cases for which this information was available. The size of individual lymph node foci ranged from less than 1 mm to 6 mm, with two cases demonstrating diffuse involvement of the majority of the node. Seven of the 15 cases demonstrated between 1 and 3 discrete foci of involvement, with the remaining 6 non-diffuse cases demonstrating greater than 3 discrete foci each.
In the majority of the involved lymph nodes, individual cells and clusters with abundant eosinophilic cytoplasm involved the lymph node sinuses (Fig. 7), with more florid cases also involving lymph node parenchyma. Ten of the cases also contained areas with a more glandular architecture, generally still comprised of cells with prominent eosinophilic cytoplasm. While some areas of tumor within lymph nodes demonstrated an appearance similar to that of the ovarian tumor, with a mixture of eosinophilic and cuboidal/columnar cells, the eosinophilic cells were the predominant cell type. Endosalpingiosis was present in 12 of the 15 cases. In one additional case, endosalpingiosis was present in a lymph node by report but was not available for review. Endosalpingiosis was often intimately associated with the eosinophilic cells and glands comprising lymph node involvement by tumor (Fig. 8). In some foci, the more florid endosalpingiotic glands contained a large number of detached intraluminal eosinophilic cells, bearing a striking resemblance to the associated ovarian tumor and suggesting a close relationship similar to that of the detached eosinophilic cells to those on the surface of the papillae in the ovarian SBT/APST (Fig. 9). Interestingly, 12 of the cases of lymph node involvement also had large numbers of detached eosinophilic cells in the associated ovarian tumor (Fig. 10).
FIGURE 7.
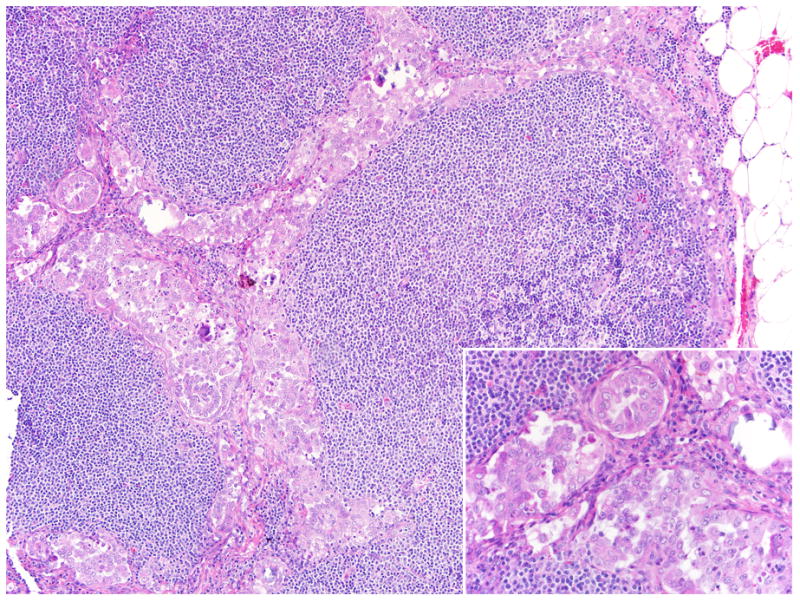
Lymph node sinuses expanded by tumor cells. Higher power (inset) demonstrates clusters and glands of eosinophilic cells.
FIGURE 8.
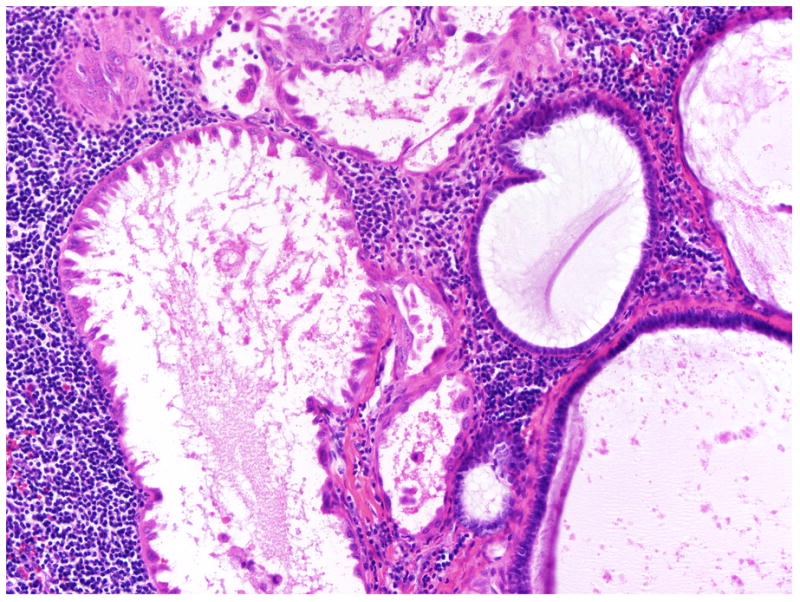
Lymph node involvement by SBT/APST with adjacent endosalpingiosis.
FIGURE 9.
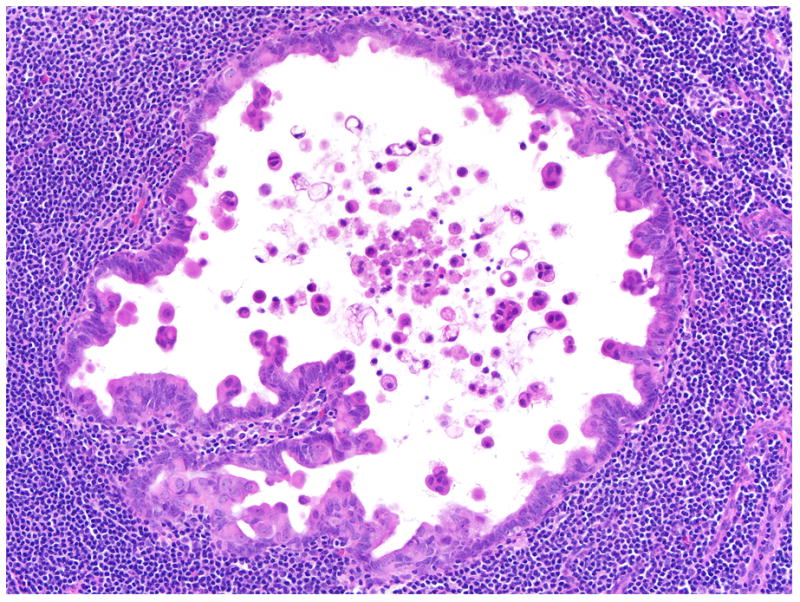
Endosalpingiosis in lymph node with prominent eosinophilic cells interspersed with columnar epithelium lining the endosalpingiosis and detached floating in the lumen. Several cells in the lumen have undergone apoptosis.
FIGURE 10.
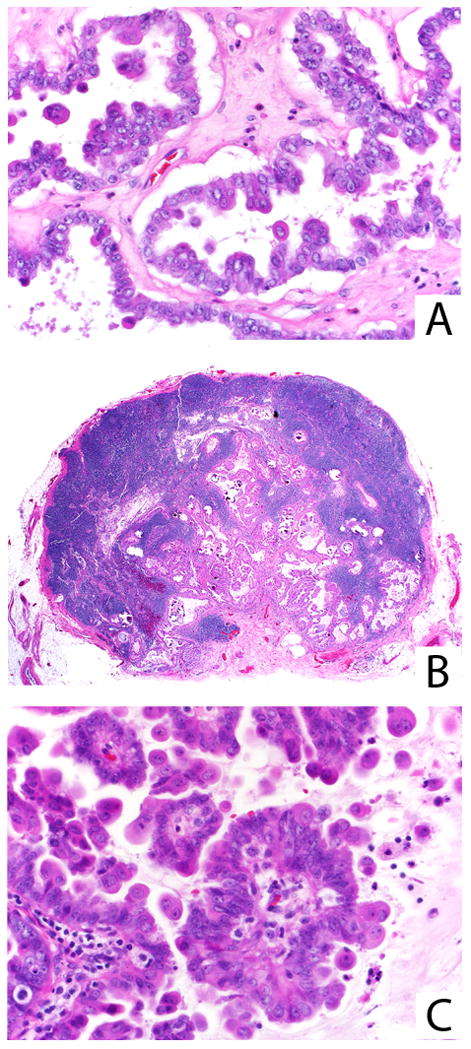
Ovarian SBT/APST with prominent eosinophilic cells (A). The associated lymph node is extensively involved by tumor (B), which on higher power appears identical to the ovarian tumor (C). Both cuboidal/columnar and eosinophilic cells are present in both sites.
In all but three cases, sufficient material was present for scoring on all immunostained sections, and in all but three cases, at least 200 cells of tumor in lymph node were present to score the Ki-67 proliferation index. The eosinophilic cells (detached and on the surface of papillae), the cells comprising lymph node involvement by tumor, the cuboidal/columnar surface epithelial cells on the papillae of the SBT/APSTs, and the cuboidal/columnar cells in endosalpingiosis (when present) were scored separately for each immunohistochemical marker. The results for each marker in each cell type are presented in Table 3. The medians were compared among the groups as follows. The cells comprising the lymph node involvement were first compared to the eosinophilic cells in the ovarian tumor (detached and on the surface of the papillae), and no significant differences between the median immunohistochemical scores were found for ER, PR, WT-1, or the median Ki-67 proliferation indices. Given this finding, the scores for these two groups were combined and then compared to the surface cuboidal/columnar epithelium. The lymph node involvement and eosinophilic cells (detached and on the surface of papillae) had significantly lower median scores for ER, PR, and WT-1, compared to the surface cuboidal/columnar epithelium (Fig. 11); the median and mean Ki-67 proliferation index was also lower in the cells comprising lymph node involvement and the eosinophilic cells compared to the cuboidal/columnar cells, but this difference was not statistically significant. The lymph node involvement was also separately compared to the surface cuboidal/columnar epithelial cells without inclusion of the surface/detached eosinophilic cells, with similar results (data not shown). The lymph node involvement was additionally compared to the associated endosalpingiosis, and found to have significantly lower median scores for ER, PR, and WT-1. The Ki-67 proliferation index, however, was significantly higher in the lymph node involvement by tumor compared to endosalpingiosis.
TABLE 3.
Ovarian serous borderline/atypical proliferative serous tumors (SBT/APST) with lymph node involvement: descriptive statistics and comparison of medians
| Immunohistochemical scores
|
Ki-67 index | |||
|---|---|---|---|---|
| ER | PR | WT-1 | ||
|
| ||||
| Cuboidal/columnar surface epithelial cells of SBT/APST | ||||
| Mean | 10.8 | 8.3 | 10.6 | 9.3% |
| Median | 12 | 9 | 12 | 9% |
| Range | 6 – 12 | 1 – 12 | 6 – 12 | 0–23% |
|
| ||||
| Eosinophilic cells (detached and on the surface of the papillae) | ||||
| Mean | 7.2 | 5.3 | 7.2 | 3.8% |
| Median | 7 | 5 | 6 | 4% |
| Range | 2 – 12 | 1 – 12 | 4 – 12 | 0–9% |
|
| ||||
| Tumor cells in lymph node(s) | ||||
| Mean | 8.6 | 4.7 | 8.3 | 6.6% |
| Median | 9 | 4 | 9 | 6% |
| Range | 2 – 12 | 0 – 12 | 0 – 12 | 0–18% |
|
| ||||
| Endosalpingiosis | ||||
| Mean | 11.6 | 7.6 | 12 | 4.6% |
| Median | 12 | 9 | 12 | 1.5% |
| Range | 8 – 12 | 0 – 12 | 12 | 0–19% |
|
| ||||
| Tumor in lymph node vs. eosinophilic cells (detached on the surface of the papillae) | ||||
| p-value | 0.223 | 0.381 | 0.892 | 0.299 |
|
| ||||
| Cuboidal/columnar surface epithelial cells vs. tumor in lymph node + eosinophilic cells | ||||
| p-value | <0.001* | 0.020* | 0.002* | 0.334 |
|
| ||||
| Tumor in lymph node vs. Endosalpingiosis | ||||
| p-value | 0.005* | 0.026* | <0.001* | 0.013* |
A p-value of < 0.5 was used to determine significance.
FIGURE 11.
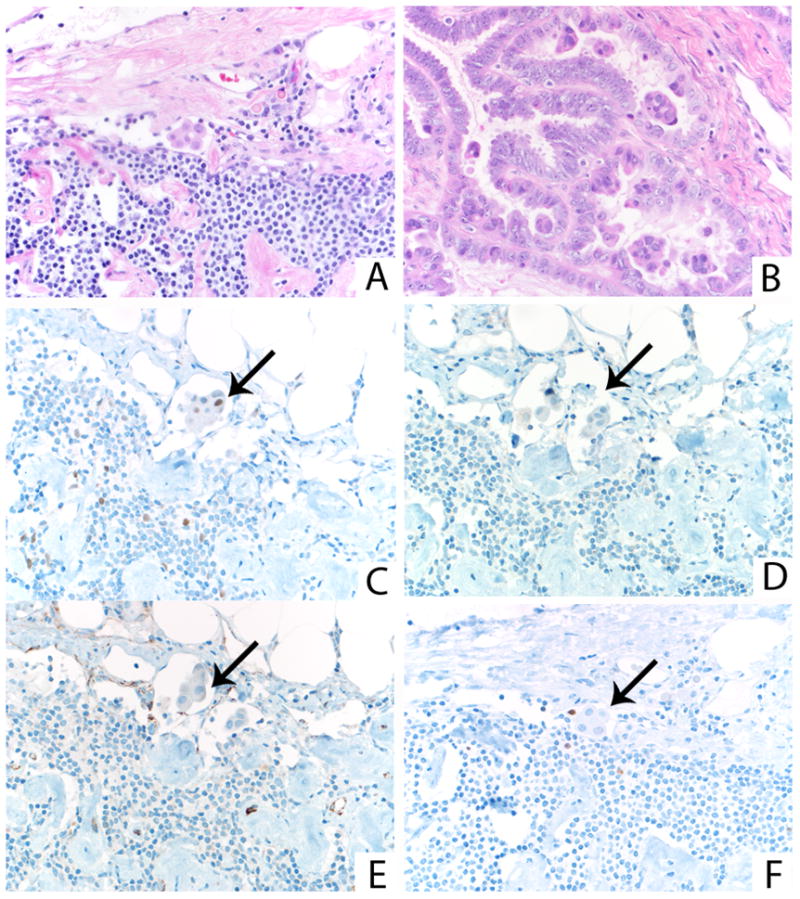
Small cluster of eosinophilic tumor cells in subcapsular lymph node sinus (A), similar in appearance to budding/detached clusters of eosinophilic cells in accompanying ovarian SBT/APST (B). The cells comprising lymph node involvement by tumor demonstrate only focal expression of ER (C, arrow) and no expression of PR (D, arrow) or WT-1 (E, arrow). The Ki-67 proliferation index is low (F, arrow).
In addition to the above cases, one case displayed diffuse involvement of multiple pelvic and para-aortic lymph nodes by SBT/APST but without an identifiable ovarian SBT/APST. Both ovaries in this case demonstrated a serous cystadenoma (25.5 and 26 cm) without any areas qualifying as SBT/APST, although sampling was less than 1 section per centimeter greatest tumor dimension. The lymph nodes in this case also demonstrated endosalpingiosis. Diffuse strong staining (score of 12) was seen in both the tumor and the endosalpingiosis for ER and WT-1. The tumor did not stain for PR, while the endosalpingiosis had a score of 2. The Ki-67 proliferation index for the tumor was 3%, and that for the endosalpingiosis was 1%.
Low-grade serous carcinomas
There were 6 cases of invasive ovarian low-grade serous carcinoma arising in association with SBT/APST. In contrast to the focal eosinophilic cells seen in cases of microinvasion, these tumors displayed solid and cribriform growth patterns, destructive stromal invasion, a higher nuclear-to-cytoplasmic ratio with greater nuclear atypia compared to the cells in microinvasion, and an absence of eosinophilic cells. Sufficient material was present for scoring on all immunostained sections for these cases. The immunohistochemical scores of the carcinomas were compared to the associated SBT/APSTs. No significant differences were found in median scores for ER, PR, or WT-1, but the carcinomas had a significantly higher Ki-67 proliferation index (Fig. 12). See Table 4 for a summary of these results.
FIGURE 12.
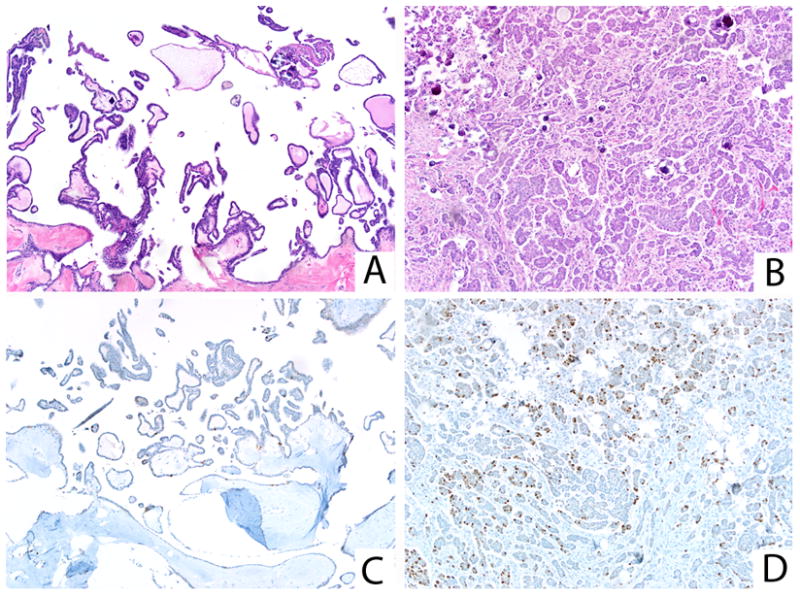
SBT/APST (A) and associated low-grade serous carcinoma (B), with a significantly lower Ki-67 proliferation index in the SBT/APST (C) vs. the carcinoma (D).
TABLE 4.
Low-grade serous carcinomas (LGSC) arising in serous borderline/atypical proliferative serous tumors (SBT/APST): descriptive statistics and comparison of medians
| Immunohistochemical scores
|
Ki-67 index | |||
|---|---|---|---|---|
| ER | PR | WT-1 | ||
|
| ||||
| SBT/APST | ||||
| Mean | 10.3 | 6.5 | 11.3 | 4.3% |
| Median | 12 | 7.5 | 12 | 4% |
| Range | 6 – 12 | 2 – 9 | 8 – 12 | 1–10% |
|
| ||||
| LGSC | ||||
| Mean | 11 | 4.5 | 10.7 | 15% |
| Median | 12 | 3 | 12 | 16% |
| Range | 6 – 12 | 0 – 12 | 8 – 12 | 4–20% |
|
| ||||
| SBT/APST vs. LGSC | ||||
| p-value | 0.505 | 0.558 | 0.505 | 0.021* |
A p-value of < 0.5 was used to determine significance.
Discussion
The vast majority of studies evaluating clinical outcome in patients whose tumors display microinvasion and/or lymph node involvement have not shown an adverse effect on either overall survival or recurrence-free survival,1, 2, 8, 15–18 and a review article encompassing 97 studies and 4129 patients, with 101 cases of microinvasion, reported an overall survival of 100%.3 It has also been noted that microinvasion is frequently overlooked and still has no adverse effect on outcome.3, 8 These studies led participants in the Bethesda Borderline Ovarian Tumor Workshop in 2003 to conclude that patients with well-sampled serous borderline tumors of low stage have a 5-year survival close to 100% and a very low recurrence rate regardless of the presence or absence of microinvasion.5 A subsequent study of microinvasion by McKenney and colleagues7 reported an adverse effect on survival but this is attributable to inclusion of a pattern of microinvasion with micropapillae that the authors acknowledge resembles low-grade serous carcinoma, a pattern not included in the present analysis and one that many authors do not regard as classic microinvasion.5, 6, 9
As numerous studies have evaluated clinical outcome in SBT/APSTs with microinvasion and/or lymph node involvement, our aim was not to analyze outcome but to evaluate the morphologic and immunohistochemical features that might explain why microinvasion and lymph node involvement do not contribute to poor outcome. We compared the morphologic and immunohistochemical characteristics of the cells comprising microinvasion and lymph node involvement with those found in SBT/APSTs and low-grade serous carcinomas, reasoning that if microinvasion is a step in progression to low-grade serous carcinoma, the cells that constitute microinvasion should display some features that resemble those in the carcinoma. Similarly, one would expect that tumor cells involving pelvic and para-aortic lymph nodes might be related to carcinoma.
SBT/APSTs contain a heterogeneous population of cells which include cuboidal to columnar cells that line the surface of papillae as well as eosinophilic cells that are interspersed among them. All the different cell types can contain cilia. The eosinophilic cells are also present individually and in detached clusters floating above the surface of the papillae. The same cell types were observed in foci of microinvasion and in lymph nodes, and the dominant population of cells in these lesions was the eosinophilic cells. The nuclei in all the cell types are bland and mitotic activity is low. In contrast, although some low-grade serous carcinomas display features similar to SBT/APSTs, with florid cellular proliferation and mild atypia, they generally demonstrate a more uniform population of rounded cells, lacking dense eosinophilic cytoplasm, with nuclei showing moderate atypia. Cilia are not present and mitotic activity is greater than that seen in a typical SBT/APST.
The immunohistochemical findings also showed marked differences. Compared to the cuboidal/columnar cells, the eosinophilic cells in the primary SBT/APSTs, microinvasion, and involved lymph nodes showed a statistically significant lower expression of ER, PR and WT1. In addition, the Ki-67 labeling index was lower in the eosinophilic cells. In contrast, the low-grade serous carcinomas showed no loss of expression of ER, PR, and WT1, and had a significantly higher Ki-67 labeling index than their associated SBT/APSTs.
In general, the presence of tumor cells within the stroma of a neoplasm is considered evidence of true invasion. However, in the case of SBT/APSTs with microinvasion, several findings suggest that this may not be the case. To begin with, foci of microinvasion show no associated reactive change or destructive growth. Secondly, invasion is generally heralded by a morphologic alteration of the invasive cells which differs from the noninvasive tumor and more closely resembles the invasive carcinoma. This is not the case with microinvasion and lymph node involvement. The cells comprising microinvasion and those in involved lymph nodes resemble the cells of the SBT/APST, particularly the eosinophilic cells, rather than the cells of low-grade serous carcinoma. One might argue that the loss of the markers that we observed signifies loss of differentiation, as occurs with poorly differentiated malignant tumors, but this is inconsistent with the observation that the microinvasive cells are bland and have a much lower Ki-67 proliferation index compared to low-grade serous carcinoma. Moreover, the low-grade serous carcinomas did not lose marker expression.
Finally, it has been reported by Sangoi and associates that in 60% of their cases of SBTs with microinvasion, lymph-vascular space invasion was present.19 This finding contrasts with the report of Tavassoli1 and our study in which none of the cases of microinvasion demonstrated lymph-vascular invasion. The Sangoi study utilized the reagent D2–40, which specifically identifies lymphatic channels, but also reacts with mesothelium.20 Our study failed to show evidence of lymph-vascular invasion employing the reagent CD31, which identifies both vascular and lymphatic channels. It is therefore possible that the flattened lining surrounding the eosinophilic cells in the stroma described by Sangoi et al19 is composed of mesothelial cells resulting from tangential sectioning of a cleft in which the surface cells have invaginated into the stroma (Fig. 13). In any event, all these studies showed a very low association of microinvasion and lymph node involvement. Specifically, Sangoi et al reported one positive lymph node among 20 cases of microinvasion,19 Tavassoli one lymph node among 18 cases,1 and in the present study one lymph node among 23 cases of microinvasion (5% for all three studies). Furthermore, most cases with lymph node involvement are not associated with microinvasion and outcome is not adversely affected by its presence. The precise mechanism by which the cells in microinvasion enter the stroma is unknown but in our opinion, microinvasion does not represent true invasion in the sense of an initial step in progression to invasive low-grade serous carcinoma. It is of interest that the investigators who were among the first to describe microinvasion in SBTs share this view. Specifically, Katzenstein and associates did not consider the presence of unattached eosinophilic cells in the stroma of papillae as indicative of an invasive process21 and Tavassoli noted that the behavior of tumors with microinvasion more closely paralleled SBTs than well-differentiated serous carcinomas.1
FIGURE 13.
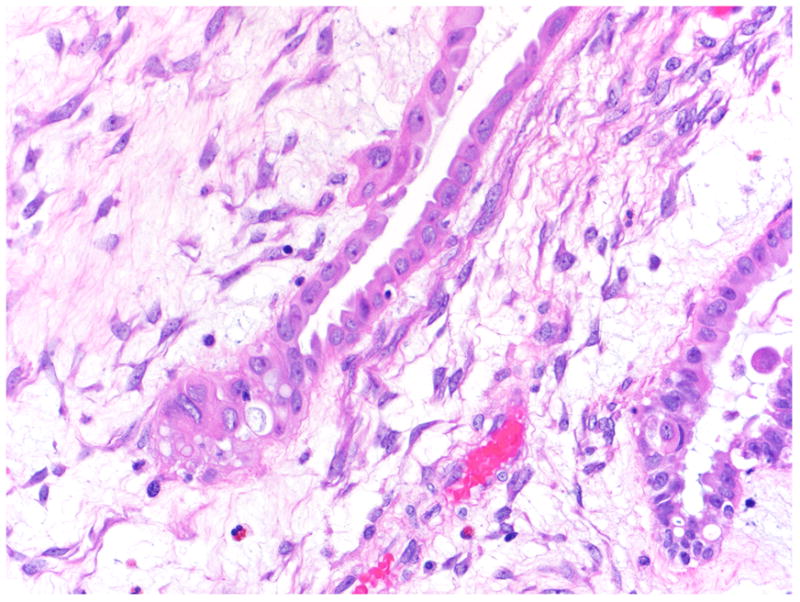
A glandular cleft containing eosinophilic cells, which may result in “microinvasion” when cut tangentially.
Most studies of lymph node involvement in SBT/APST, like those describing microinvasion, have found no negative effect on overall survival,15, 18, 22–26 disease-specific survival, or recurrence rates.25, 27 In the review conducted by Seidman and Kurman, 63 cases with lymph node involvement were reported, of which 43 had follow-up data, and the survival was found to be 98% for these patients after a mean follow-up of 6.5 years.3 McKenney et al found that the presence of discrete nodular tumor aggregates greater than 1 mm in the lymph nodes was significantly associated with decreased disease-specific survival; morphologically, this type of lymph node involvement was also found to be associated with a micropapillary architecture and a desmoplastic stromal response, features similar to low-grade serous carcinoma and distinct from the usual type of lymph node involvement in SBT/APSTs.22
It appears that lymph node involvement by SBT/APST may occur by different mechanisms. Some are derived from an ovarian SBT/APST and some may arise from endosalpingiosis. In the former scenario, we concur with the conclusions of Fadare et al28 that in many cases, eosinophilic cells from the ovarian tumor detach from the surface and enter the peritoneal cavity, where they can access the lymphatic system and pass through lymph node sinuses without destroying lymphoid tissue like a malignant tumor. Interestingly, in our study, over 70% of tumors in which there was lymph node involvement displayed exophytic growth on gross examination.
In the present study, no significant differences were found in the median immunohistochemical scores for ER, PR, and WT-1, as well as in the Ki-67 proliferation indices of the eosinophilic cells (detached and on the surface of the papillae) and the lymph node involvement, supporting the interpretation that these are, in fact, the same cells. The significantly decreased expression of ER, PR, and WT-1 in the cells of microinvasion as well as those in lymph node tumor versus the cuboidal/columnar tumor cells, suggests that the eosinophilic cells are undergoing terminal differentiation and possibly senescence, with a subset of cells undergoing apoptosis (based on morphologic and immunohistochemical findings). This could explain why they do not have an adverse effect on outcome. Another possible explanation for why lymph node involvement in particular does not adversely affect outcome is that the tumor in the lymph node arises independently. Several studies have found a higher frequency of endosalpingiosis in patients with serous borderline tumors,5, 26, 29 leading to the proposal that they develop independently, so-called “field effect.” Additionally, foci of endosalpingiosis are frequently seen in association with lymph node involvement,26, 30–32 as was also demonstrated in the current study. One report described a morphologic “transition” between lymph node involvement by tumor and endosalpingiosis,33 while another group reported cases of serous carcinoma arising in a lymph node with morphologic transition from clearly benign areas to borderline-like areas to frank carcinoma.34 Indeed, in the present study, one case of lymph node involvement was identified associated with endosalpingiosis but without an identified ovarian SBT/APST, suggesting that the tumor in the lymph node arose in that location from endosalpingiosis (although an unsampled focal ovarian SBT/APST cannot be entirely excluded).
In summary, the cells in microinvasion and lymph nodes have the same appearance as those lining the papillae of SBT/APSTs. The predominant cellular population is composed of eosinophilic cells which, compared to the cuboidal and columnar cells on SBT/APSTs, demonstrate a significant reduction in ER, PR, WT1, and Ki-67 labeling. The cells are bland, often contain cilia, and show evidence of apoptosis. We believe these findings indicate that the eosinophilic cells are undergoing terminal differentiation and senescence, which could explain why microinvasion and lymph node involvement do not affect clinical outcome. The eosinophilic cells are loosely cohesive as evidenced by their presence as detached single cells and clusters floating above the surface of the papillae. This lack of cohesion may explain their presence in the stroma of papillae as foci of microinvasion, and since SBT/APSTs are frequently exophytic, the detached cells have access to the peritoneal cavity and are filtered by regional lymph nodes. The morphologic and immunohistochemical features of all of the cellular populations differ from the cells that comprise low-grade serous carcinoma, which lack eosinophilic cells and are instead composed of a relatively uniform population of rounded cells with a high nuclear:cytoplasmic ratio that show a moderate degree of nuclear atypia. The low-grade serous carcinoma cells retain ER, PR and WT1 and have a significantly higher Ki-67 labeling index than the cells in their associated SBT/APSTs. It therefore appears that microinvasion is not an early step in progression to low-grade serous carcinoma and that the cells in lymph nodes also have no relationship to low-grade serous carcinoma. In view of these findings, as well as the documented lack of an adverse effect on outcome shown by nearly all studies, we suggest that the presence of microinvasion probably does not need to be reported and that lymph node involvement by these cells should not be diagnosed as “metastasis” to avoid overtreatment. Consideration should also be given to revising the FIGO staging system so that it does not result in upstaging of these tumors.
Acknowledgments
This study is in part supported by the NIH/NCI grant RO1CA116184, and by DoD Ovarian Consortium OC100517.
References
- 1.Tavassoli FA. Serous tumor of low malignant potential with early stromal invasion (serous LMP with microinvasion) Mod Pathol. 1988;1:407–414. [PubMed] [Google Scholar]
- 2.Bell DA, Scully RE. Ovarian serous borderline tumors with stromal microinvasion: a report of 21 cases. Hum Pathol. 1990;21:397–403. doi: 10.1016/0046-8177(90)90201-f. [DOI] [PubMed] [Google Scholar]
- 3.Seidman JD, Kurman RJ. Ovarian serous borderline tumors: A critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31:539–557. doi: 10.1053/hp.2000.8048. [DOI] [PubMed] [Google Scholar]
- 4.Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors. Histologic features and prognosis. Cancer. 1988;62:2212–2222. doi: 10.1002/1097-0142(19881115)62:10<2212::aid-cncr2820621024>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg SG, Bell DA, Kurman RJ, et al. Borderline ovarian tumors: key points and workshop summary. Hum Pathol. 2004;35:910–917. doi: 10.1016/j.humpath.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Seidman JD, Soslow RA, Vang R, et al. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol. 2004;35:918–933. doi: 10.1016/j.humpath.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.McKenney JK, Balzer BL, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol. 2006;30:1209–1221. doi: 10.1097/01.pas.0000213299.11649.fa. [DOI] [PubMed] [Google Scholar]
- 8.Nayar R, Siriaunkgul S, Robbins KM, et al. Microinvasion in low malignant potential tumors of the ovary. Hum Pathol. 1996;27:521–527. doi: 10.1016/s0046-8177(96)90156-2. [DOI] [PubMed] [Google Scholar]
- 9.Silva EG, Kurman RJ, Russell P, et al. Symposium: ovarian tumors of borderline malignancy. Int J Gynecol Pathol. 1996;15:281–302. [PubMed] [Google Scholar]
- 10.Remmele W, Hildebrand U, Hienz HA, et al. Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol. 1986;409:127–147. doi: 10.1007/BF00708323. [DOI] [PubMed] [Google Scholar]
- 11.Schlosshauer PW, Deligdisch L, Penault-Llorca F, et al. Loss of p16INK4A expression in low-grade ovarian serous carcinomas. Int J Gynecol Pathol. 2011;30:22–29. doi: 10.1097/PGP.0b013e3181ed89b3. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich M, Villena-Heinsen C, Reitnauer K, et al. Malignancies of the uterine corpus and immunoreactivity score of the DNA “mismatch-repair” enzyme human Mut-S-homologon-2. J Histochem Cytochem. 1999;47:113–118. doi: 10.1177/002215549904700112. [DOI] [PubMed] [Google Scholar]
- 13.Leers MPG, Kölgen W, Björklund V, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Brown GW, Mood AM. On Median Tests for Linear Hypotheses. Proc Second Berkeley Symp on Math Statist and Prob. 1951:159–166. [Google Scholar]
- 15.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy AW, Hart WR. Ovarian papillary serous tumors of low malignant potential (serous borderline tumors). A long-term follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer. 1996;78:278–286. doi: 10.1002/(SICI)1097-0142(19960715)78:2<278::AID-CNCR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Casey AC, Bell DA, Lage JM, et al. Epithelial ovarian tumors of borderline malignancy: long-term follow-up. Gynecol Oncol. 1993;50:316–322. doi: 10.1006/gyno.1993.1218. [DOI] [PubMed] [Google Scholar]
- 18.Morice P, Camatte S, Rey A, et al. Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann Oncol. 2003;14:592–598. doi: 10.1093/annonc/mdg173. [DOI] [PubMed] [Google Scholar]
- 19.Sangoi AR, McKenney JK, Dadras SS, et al. Lymphatic vascular invasion in ovarian serous tumors of low malignant potential with stromal microinvasion: a case control study. Am J Surg Pathol. 2008;32:261–268. doi: 10.1097/PAS.0b013e318141fc7a. [DOI] [PubMed] [Google Scholar]
- 20.Hyun TS, Barnes M, Tabatabai ZL. The diagnostic utility of D2–40, calretinin, CK5/6, desmin and MOC-31 in the differentiation of mesothelioma from adenocarcinoma in pleural effusion cytology. Acta cytologica. 2012;56:527–532. doi: 10.1159/000339586. [DOI] [PubMed] [Google Scholar]
- 21.Katzenstein AL, Mazur MT, Morgan TE, et al. Proliferative serous tumors of the ovary. Histologic features and prognosis. Am J Surg Pathol. 1978;2:339–355. doi: 10.1097/00000478-197812000-00001. [DOI] [PubMed] [Google Scholar]
- 22.McKenney JK, Balzer BL, Longacre TA. Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): pathology, prognosis, and proposed classification. Am J Surg Pathol. 2006;30:614–624. doi: 10.1097/01.pas.0000194743.33540.e6. [DOI] [PubMed] [Google Scholar]
- 23.Longacre TA, McKenney JK, Tazelaar HD, et al. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707–723. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 24.Leake JF, Rader JS, Woodruff JD, et al. Retroperitoneal lymphatic involvement with epithelial ovarian tumors of low malignant potential. Gynecol Oncol. 1991;42:124–130. doi: 10.1016/0090-8258(91)90331-x. [DOI] [PubMed] [Google Scholar]
- 25.Lesieur B, Kane A, Duvillard P, et al. Prognostic value of lymph node involvement in ovarian serous borderline tumors. Am J Obstet Gynecol. 2011;204:438.e431–438.e437. doi: 10.1016/j.ajog.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Camatte S, Morice P, Atallah D, et al. Lymph node disorders and prognostic value of nodal involvement in patients treated for a borderline ovarian tumor: an analysis of a series of 42 lymphadenectomies. J Am Coll Surg. 2002;195:332–338. doi: 10.1016/s1072-7515(02)01250-4. [DOI] [PubMed] [Google Scholar]
- 27.Buttin BM, Herzog TJ, Powell MA, et al. Epithelial ovarian tumors of low malignant potential: the role of microinvasion. Obstet Gynecol. 2002;99:11–17. doi: 10.1016/s0029-7844(01)01675-1. [DOI] [PubMed] [Google Scholar]
- 28.Fadare O, Orejudos MP, Jain R, et al. A comparative analysis of lymphatic vessel density in ovarian serous tumors of low malignant potential (borderline tumors) with and without lymph node involvement. Int J Gynecol Pathol. 2008;27:483–490. doi: 10.1097/PGP.0b013e3181742d7c. [DOI] [PubMed] [Google Scholar]
- 29.Moore WF, Bentley RC, Berchuck A, et al. Some mullerian inclusion cysts in lymph nodes may sometimes be metastases from serous borderline tumors of the ovary. Am J Surg Pathol. 2000;24:710–718. doi: 10.1097/00000478-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Tan LK, Flynn SD, Carcangiu ML. Ovarian serous borderline tumors with lymph node involvement. Clinicopathologic and DNA content study of seven cases and review of the literature. Am J Surg Pathol. 1994;18:904–912. doi: 10.1097/00000478-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Bell DA, Scully RE. Clinicopathologic features of lymph node (LN) involvement with ovarian serous borderline tumors (OSBT) Mod Pathol. 1992;5:61A. [Google Scholar]
- 32.Shiraki M, Otis CN, Donovan JT, et al. Ovarian serous borderline epithelial tumors with multiple retroperitoneal nodal involvement: metastasis or malignant transformation of epithelial glandular inclusions? Gynecol Oncol. 1992;46:255–258. doi: 10.1016/0090-8258(92)90267-m. [DOI] [PubMed] [Google Scholar]
- 33.Kadar N, Krumerman M. Possible metaplastic origin of lymph node “metastases” in serous ovarian tumor of low malignant potential (borderline serous tumor) Gynecol Oncol. 1995;59:394–397. doi: 10.1006/gyno.1995.9955. [DOI] [PubMed] [Google Scholar]
- 34.Prade M, Spatz A, Bentley R, et al. Borderline and malignant serous tumor arising in pelvic lymph nodes: evidence of origin in benign glandular inclusions. Int J Gynecol Pathol. 1995;14:87–91. doi: 10.1097/00004347-199501000-00015. [DOI] [PubMed] [Google Scholar]


