Abstract
People constantly face the need to choose one option from among many, such as when selecting words to express a thought. Selecting between many options can be difficult for anyone, and can feel overwhelming for individuals with elevated anxiety. The current study demonstrates that anxiety is associated with impaired selection across three different verbal tasks, and tests the specificity of this finding to anxiety. Anxiety and depression frequently co-occur; thus, it might be assumed that they would demonstrate similar associations with selection, although they also have distinct profiles of symptoms, neuroanatomy, and neurochemistry. Here, we report for the first time that anxiety and depressive symptoms counterintuitively have opposite effects on selection among competing options. Specifically, whereas anxiety symptoms are associated with impairments in verbal selection, depressive symptoms are associated with better selection performance. Implications for understanding the mechanisms of anxiety, depression, and selection are discussed.
Keywords: executive function, selection, anxiety, depression
One of the defining human characteristics is that we can engage executive functions to respond to a given environmental context in a wide variety of ways, rather than being tied to habitual responses. This ability allows us to engage in an almost infinite repertoire of behaviors. This capacity for generativity (the ability to produce an infinite number of variety of responses) has long been considered definitional for the most human behavior of all: language. But like all cognitive abilities, it comes at a cost: with the capacity to generate infinite options comes the difficulty of choosing among them. We constantly face the need to choose one option from among many, such as when we select words to express a thought. For example, when constructing a sentence, a speaker must not only choose the intended message but must also select among multiple words that are all compatible with the intended message. People are slowed, and prefrontal executive function areas are engaged, when selection demands are high, that is, when there is competition between multiple automatically activated representations, which must be resolved in order for the speaker to select a single response for output (e.g., Snyder & Munakata, 2008; Snyder et al., 2010).
Selecting between many options can be difficult for anyone, and can feel overwhelming for individuals with elevated anxiety. People with anxiety disorders find coping with too many options particularly difficult, and struggle with making decisions, indecisiveness, and intolerance of uncertainty (e.g., Rassin, Muris, Franken, Smit, & Wong, 2007). Whereas decision-making deficits in persons with anxiety have previously been shown in complex or affective tasks (e.g., Rassin et al., 2007), the selection deficits that lie at the core of these problems are observed even in a simple language-production task (Snyder et al., 2010).
To explain these and other findings, we have developed a unified, biologically-plausible model of selection among competing options (Snyder et al., 2010). Our model demonstrates how competitive, inhibitory dynamics among neurons in prefrontal cortical networks can support selection between alternatives. Specifically, these competitive dynamics serve to sharpen cognitive representations by amplifying activity in the most active, task-relevant, representations (e.g., the most appropriate word to complete a sentence) and by suppressing competing representations (e.g., for the many other word possibilities; Snyder et al., 2010). Our model demonstrates how reduced inhibitory (i.e., GABAergic) function can lead to reduced competitive dynamics in prefrontal cortical networks, allowing non-winning competitors (alternative responses that are not selected) to become more active and to compete over a longer period, which impairs selection. As predicted by this model, (a) the GABA agonist midazolam improved selection; and (b) greater anxiety, which has been linked to reduced GABAergic function, was associated with more difficulty selecting between competing word options and reduced activation in prefrontal executive function areas during such selection (Snyder et al., 2010).
However, an important question remains as to whether deficits in selection are uniquely related to anxiety, or could be affected by other forms of co-occurring psychopathology. Specifically, anxiety and depression are highly correlated at the symptom level (e.g., Stöber & Joormann, 2001), and frequently comorbid at the disorder level; approximately 60% of individuals with major depressive disorder also have an anxiety disorder (e.g., for review see Rivas-Vasques et al., 2004). Anxiety and depression can begin before, concurrently, or after one another, and often recur throughout the lifespan (Moffitt et al., 2007). Comorbid anxiety and depression often produce worse outcomes than either alone, including more severe symptoms, higher rates of recurrence, worse psychosocial function, and poorer treatment response (e.g., Moffitt et al., 2007; Rivas-Vasques et al., 2004). Some research also suggests that both anxiety and depression can contribute to cognitive deficits; for example, anxiety and depression are each associated with deficits in executive function (EF, e.g., Snyder et al., 2010; Snyder, 2013). Co-occurring anxiety and depression may have additive effects on EF deficits, as evidenced by studies that have found that individuals with comorbid depressive and anxiety disorders have worse performance on some EF tasks than individuals with either depressive or anxiety disorders alone (e.g., Basso et al., 2007).
However, anxiety and depression are also associated with distinct profiles of symptoms and neurobiology: current clinical models and empirical evidence generally agree that whereas anxiety and depression are related constructs with some shared aspects, they are nonetheless distinct, with aspects that are unique to each (e.g., for review see Moffitt et al., 2007). In fact, some evidence suggests that they can have opposing effects on brain and behavior, such as the finding that anxiety symptoms were associated with a greater visual attentional bias towards the right hemisphere (left visual field), and depressive symptoms with greater bias towards the left hemisphere (Keller et al., 2000). These asymmetries only became apparent when controlling for the variance in common between anxiety and depressive symptoms (Keller et al., 2000). Similarly, anxiety and depressive symptoms were associated with opposing patterns of activity in several brain regions during an emotional Stroop task (although there were no behavioral differences; Engels et al., 2007). Finally, participants with social anxiety disorder alone generally had reduced performance on EF tasks under social stress compared to non-stress baseline, whereas those with comorbid social anxiety and depression generally improved their performance under social stress, although the performance of the comorbid group was never significantly better than that of the social anxiety only group (Graver & White, 2007).
Given these distinct mechanisms and effects, it is possible that in some cases anxiety and depressive symptoms could have opposing effects. That is, the neurobiological changes associated with these symptoms could have countervailing effects on specific aspects of EF, such that one is associated with impaired performance, and the other with improved performance. To our knowledge this has never been demonstrated. We examine this issue in the context of the ability to select among competing options, an aspect of EF that is critical for language and decision-making. We assess selection across three different tasks to generate a robust composite measure. Composite scores that aggregate results across multiple tasks provide a more accurate and reliable measure of the intended EF than single tasks, because the non-executive task requirements specific to each task (e.g., visual processing of pictures in blocked cyclic naming vs. sentence reading in the sentence completion task) have less influence (e.g., Miyake et al., 2000). The current study aims to (a) extend the previous finding that anxiety symptoms are associated with impaired selection (Snyder et al., 2010) to this more reliable composite measure and to individuals with clinically relevant levels of anxiety, and (b) test the specificity of selection deficits to anxiety, namely, the possibility that anxiety and depressive symptoms may have opposing effects.
Method
Participants
Participants were 162 native English-speaking young adult undergraduate students from the University of Colorado Boulder (71% female). We used an extreme group design, which allowed us not only to evaluate the association between impaired selection and clinically significant levels of anxiety, but given the expected linear effect of anxiety (Snyder et al., 2010) also provides a more optimized or powerful test of the association between selection and anxiety (McClelland, 1997). Participants were selected based on Penn State Worry Questionnaire scores (PSWQ, Meyer, Miller, Metzger, & Borkovec, 1990), a 16-item scale evaluating symptoms of anxious apprehension. Participants scoring in the top (>48, n = 116) and bottom (<34, n = 46) quartiles (Gillis, Haaga, & Ford, 1995) were included in the current study. The distribution of PSWQ scores for the students completing the pre-screening process closely matched previously published norms (e.g., Gillis et al., 1995). The top quartile score we used for classifying participants into the high anxiety subsample is slightly higher than the cut score recommended for screening for generalized anxiety disorder (Behar, Alcaine, Zuellig & Borkovec, 2003). Depressive symptoms were assessed with the Beck Depression Inventory – Second Edition (BDI-II, Beck, Steer, & Brown, 1996), a 21-item scale evaluating current symptoms of depression. Participants gave informed consent, were treated in accordance with procedures approved by the University of Colorado Boulder Institutional Review Board, and were compensated with course credit or $15.
Materials and Procedure
Trained research assistants tested participants individually in a quite room in a one-hour session. Participants completed three tasks that assessed verbal selection abilities to yield a composite score: verb generation (e.g., Snyder et al., 2010), blocked cyclic naming (e.g., Schnur, Schwartz, Brecher, & Hodgson, 2006) and sentence completion (e.g., Snyder & Munakata, 2008). In each, reaction times (RT) were recorded using a voice-activated microphone. Responses were also audio-recorded and transcribed to remove error trials. In addition, participants completed measures that allowed us to statistically control for psychomotor speed and IQ.
Verb generation
Verb generation was administered as in Snyder et al. (2010). Stimuli were 25 nouns in two conditions: high competition with many possible verb responses (e.g., cat, associated with purr, lick, meow, etc.) and low competition with few possible verb responses (e.g., scissors, associated with cut). Participants saw nouns one at a time and stated the first verb that came to mind (something the noun does or something that could be done with the noun). Data were excluded for 10 participants due to failure to follow task directions (>25% errors, leaving too few valid trials for accurate RT analysis), and data were missing from one participant due to equipment failure.
Blocked cyclic naming
Participants repeatedly named 16 pictures as quickly as possible in two conditions: homogenous blocks of pictures from the same category (e.g., bed, table, bench, crib), and mixed blocks with each picture from a different category (e.g., lion, pajamas, bench, car). The homogenous condition creates high competition among responses due to spreading semantic activation, whereas the mixed condition has low competition (e.g., Schnur et al., 2006). Participants completed eight blocks, each with four pictures repeated six times in different orders. The same pictures appeared in both conditions. Data were missing for one participant due to equipment failure.
Sentence completion
Sentence completion was administered as in Snyder and Munakata (2008). Stimuli were sentences with the final word omitted, with 50 sentences each in two conditions: high competition with many possible endings (e.g., There is something grand about the _____.), and low competition with few possible endings (e.g., He mailed the letter without a______.). Participants read sentences silently as they appeared in segments of 1–2 words (to control reading speed) then said a word aloud to complete the sentence. The final segment always contained one word and the blank. Data were missing for one participant due to equipment failure.
North American Adult Reading Test (NAART)
The NAART is a well-established IQ estimate (Uttl, 2002). Participants read 60 irregular words aloud, which increased in difficulty (e.g., debt to synecdoche). Estimated full scale IQ was calculated from the number of incorrect pronunciations (Uttl, 2002). One participant did not complete the NAART due to experimenter error.
Choice RT
Participants pressed buttons with their left and right hands as fast as possible when presented with left or right pointing triangles. Data were missing for three participants due to equipment failure, and one participant did not complete Choice RT due to experimenter error.
Data Analysis
Data were transformed and dependent variables calculated as in previous research (e.g., Snyder et al., 2010). Incorrect responses (e.g., non-verbs in verb generation) and microphone errors (e.g., failing to trigger) were excluded. Trials with RTs <200 ms, >10,000 ms, or greater than three standard deviations above the participant’s mean RT, were trimmed (excluded from analysis). RTs were log transformed to remove skew and z-transformed within subjects to remove baseline differences in RT. For the verb generation, blocked cyclic naming, and sentence completion tasks, selection cost was calculated as the z RT difference between the high competition and low competition conditions. Selection costs for each task were z-transformed across subjects (to put them on the same scale and thus give them equal weight in the composite score) and averaged into the primary measure of interest, the selection composite score.
To provide a full picture of the effects of anxiety and depressive symptoms separately as well as effects of those aspects of anxiety and depression that are unique to each (controlling for the other variable), data were analyzed with regression analyses testing the effects on selection composite scores (and on selection cost for each task) of (1) PSWQ scores, (2) BDI-II scores, (3) PSWQ and BDI-II scores simultaneously, and (4) PSWQ and BDI-II scores controlling for NAART and choice RT. Outliers were excluded using the standard cut-off of standardized DfBeta > 2/√n.
Results
Participant Characteristics
Overall, the average PSWQ score was 52.15, SD = 17.61, range 16–80, and the average BDI-II score was 11.44, SD = 9.93, range 0–46. PSWQ and BDI-II scores were correlated, r = .56, p < .001, n = 162, consistent with previous studies (e.g., Stöber & Joormann, 2001), and below commonly accepted cut-offs above which collinearity is considered a potential concern in multiple regression (e.g., O’Brien, 2007).
The mean PSWQ score for the high anxiety subsample was 62.21, SD = 8.15, range 49–80, above the 90th percentile (Gillis et al., 1995) and similar to levels reported for participants with anxiety disorders (e.g., Behar et al., 2003). The mean BDI-II score for the high anxiety subsample was 14.34, SD = 10.21, range 0–46, with 47% in the dysphoric to dysphoric/depressed range using the criteria of Dozois, Dobson, and Ahnberg (1998). Thus, although the high anxiety subsample was not clinically diagnosed, their average self-reported levels of anxiety and depressive symptoms are likely of clinical significance. Moreover, there was a wide range of depressive symptoms. Thus, restriction of range was not a concern in analyzing the effects of depressive symptoms in the high anxiety subsample.
IQ estimates on the NAART were in the average range, mean = 107.12, SD = 6.10, range 92–122, and the high anxiety subsample had nearly identical IQ scores, mean = 107.28, SD = 6.06, range 94–121. All participants had IQs in the normal range or above (>90)
Selection
Regression analyses are reported in Table 1. Models 1 and 2 respectively tested the separate effects of anxiety and depressive symptoms. For the primary measure of interest, selection composite scores, there was a significant effect of anxiety, such that as anxiety symptoms (PSWQ) increased, so did selection costs (i.e., performance decreased), β = 0.28, p = .001, but there is no significant effect of depressive symptoms (BDI-II), β = −0.02, p = .83.1 The key model of interest, Model 3, included both anxiety and depressive symptoms, and thus tested the effects of anxiety controlling for depression and depression controlling for anxiety. For the primary measure of interest, selection composite scores, there were significant effects of both anxiety and depressive symptoms. Controlling for level of depressive symptoms (BDI-II), as anxiety symptoms (PSWQ) increased, so did selection costs (i.e., performance decreased), β = 0.43, p < .001 (Figure 1A). However, after removing the variance associated with anxiety, the effects of depression were in the opposite direction: controlling for anxiety symptoms, as depressive symptoms increased, selection costs decreased (i.e., performance increased), β = −0.28, p = .007 (Figure 1B).2 This suggests that it is the variance in depressive symptoms that is not shared in common with anxiety symptoms that predicts improved selection. Importantly, these effects remained significant in the high anxiety subsample. That is, among participants with clinically significant levels of anxiety, those with more severe depressive symptoms had smaller selection costs, β = −0.29, p = .012. Moreover, even within the restricted range of anxiety levels in the high anxiety subsample, higher levels of anxiety symptoms still predicted increased selection costs when controlling for depressive symptoms, β = 0.25, p = .026.
Table 1.
Regression Analyses
| Dependent Variable | Model | Independent Variable | Beta | t | p | Model R2 | Model p |
|---|---|---|---|---|---|---|---|
| Selection Composite a | 1 | PSWQ | 0.282* | 3.44 | .001 | .080 | .001 |
|
| |||||||
| 2 | BDI-II | −0.018 | −0.22 | .829 | .000 | .829 | |
|
| |||||||
| 3 | PSWQ | 0.431* | 4.25 | <.001 | .122 | <.001 | |
| BDI-II | −0.280* | −2.76 | .007 | ||||
|
| |||||||
| 4 | PSWQ | 0.439* | 4.26 | <.001 | .124 | .002 | |
| BDI-II | −0.283* | −2.77 | .006 | ||||
| IQ | 0.025 | 0.30 | .765 | ||||
| Choice RT | −0.035 | −0.41 | .682 | ||||
|
| |||||||
| Selection Composite High Anxiety Subsample b | 1 | PSWQ | 0.119 | 1.16 | .248 | .014 | .248 |
|
| |||||||
| 2 | BDI-II | −0.167 | −1.66 | .101 | .028 | .101 | |
|
| |||||||
| 3 | PSWQ | 0.254* | 2.26 | .026 | .078 | .022 | |
| BDI-II | −0.287* | −2.56 | .012 | ||||
|
| |||||||
| 4 | PSWQ | 0.277* | 2.36 | .021 | .084 | .087 | |
| BDI-II | −0.298* | −2.61 | .010 | ||||
| IQ | 0.700 | 0.66 | .508 | ||||
| Choice RT | −0.023 | −0.23 | .819 | ||||
|
| |||||||
| Sentence Completion c | 1 | PSWQ | 0.169* | 2.02 | .045 | .029 | .045 |
|
| |||||||
| 2 | BDI-II | −0.022 | −0.27 | .792 | .001 | .792 | |
|
| |||||||
| 3 | PSWQ | 0.260* | 2.48 | .014 | .052 | .026 | |
| BDI-II | −0.186# | −1.74 | .085 | ||||
|
| |||||||
| 4 | PSWQ | 0.276* | 2.64 | .009 | .067 | .057 | |
| BDI-II | −0.186# | −1.75 | .082 | ||||
| IQ | 0.083 | 0.95 | .345 | ||||
| Choice RT | −0.108 | −1.23 | .222 | ||||
|
| |||||||
| Verb Generation d | 1 | PSWQ | 0.150# | 1.72 | .087 | .022 | .087 |
|
| |||||||
| 2 | BDI-II | 0.009 | 0.10 | .918 | .000 | .918 | |
|
| |||||||
| 3 | PSWQ | 0.229* | 2.05 | .043 | .033 | .126 | |
| BDI-II | −0.128 | −1.14 | .255 | ||||
|
| |||||||
| 4 | PSWQ | 0.233* | 2.05 | .042 | .035 | .4 | |
| BDI-II | −0.131 | −1.16 | .248 | ||||
| IQ | 0.460 | 0.49 | .624 | ||||
| Choice RT | 0.022 | 0.24 | .814 | ||||
|
| |||||||
| Blocked Cyclic Naming e | 1 | PSWQ | 0.259* | 3.09 | .002 | .067 | .002 |
|
| |||||||
| 2 | BDI-II | 0.002 | 0.02 | .981 | .000 | .981 | |
|
| |||||||
| 3 | PSWQ | 0.341* | 3.25 | .001 | .079 | .004 | |
| BDI-II | −0.137 | −1.31 | .194 | ||||
|
| |||||||
| 4 | PSWQ | 0.303* | 2.89 | .004 | .117 | .003 | |
| BDI-II | −0.122 | −1.18 | .242 | ||||
| IQ | −0.023 | −0.28 | .784 | ||||
| Choice RT | 0.191* | 2.22 | .028 | ||||
Note. For the key selection composite measure, anxiety is associated with impaired selection controlling for depressive symptoms, whereas depressive symptoms are associated with improved selection controlling for anxiety. Effects of anxiety are also significant for all individual tasks controlling for depressive symptoms, whereas those for depressive symptoms controlling for anxiety are in the same direction as for the composite measure but do not reach significance.
n = 133.
n = 96.
n = 135.
n = 125.
n = 134.
p < .05.
p < .10.
Figure 1.
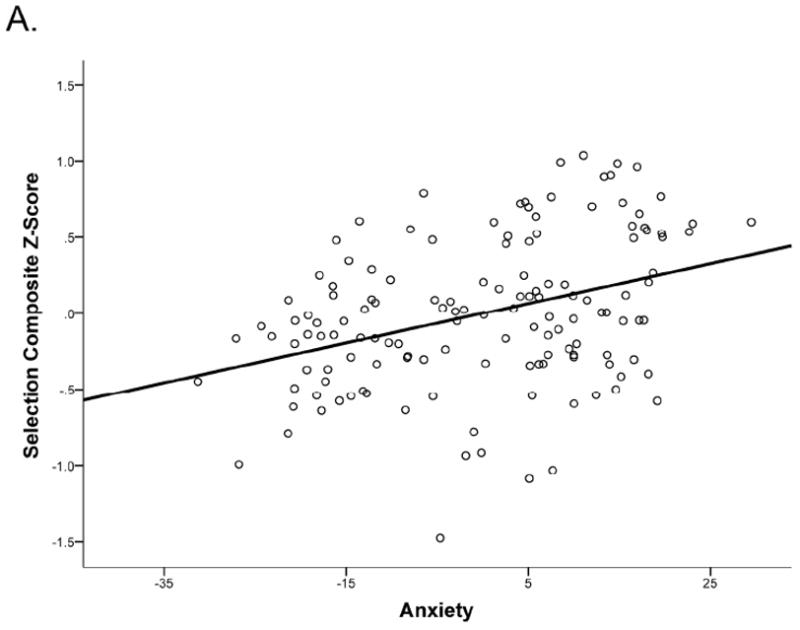
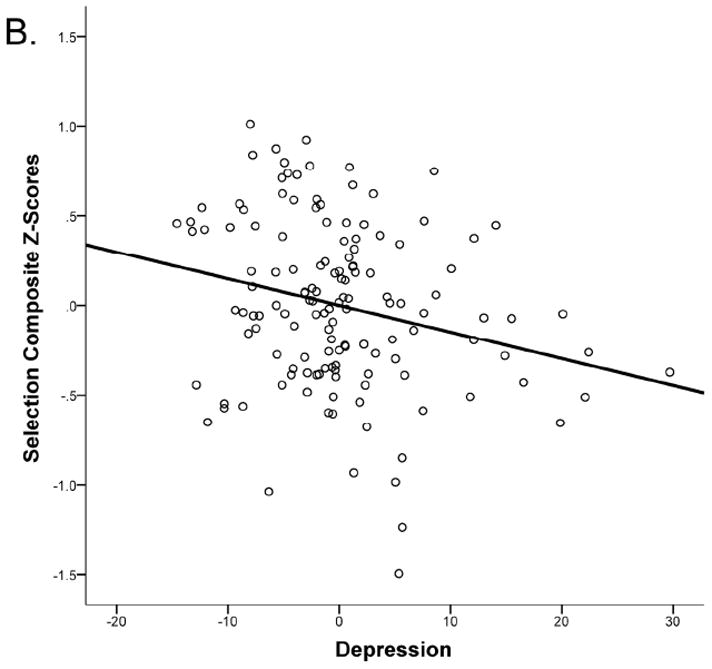
Partial regression plots for selection composite scores. (A) Anxiety predicts higher selection cost (worse performance), controlling for severity of depressive symptoms, IQ, and psychomotor speed (choice RT; residualized anxiety plotted). (B) Increased level of depressive symptoms predicts lower selection costs (better performance) controlling for anxiety, IQ, and psychomotor speed (residualized depression plotted).
Controlling for choice RT and IQ in the regression analyses did not alter any of the results, and choice RT and IQ did not significantly correlate with PWSQ or BDI-II scores (Model 4, ps > .15). Finally, we also ran regression analyses testing for an interaction between anxiety and depressive symptoms; however, the interaction was not significant for the composite measure (p = .83) or any individual task (ps > .23), so we report only main effect analyses.
Discussion
The current study demonstrates that anxiety is associated with impaired selection, supporting our earlier finding in a non-selected sample (Snyder et al., 2010), and extending it to people with more highly elevated anxiety symptoms and to additional tasks measuring verbal selection. Furthermore, the use of an extreme group design in the current study provided a more efficient and statistically powerful test of the association between verbal selection and anxiety relative to the use of an unselected, random sample (McClelland, 1997). Because the current study used selected high and low anxiety groups, conclusions about the linearity of this effect across the middle range of anxiety symptom levels cannot be drawn; however, taken together with the earlier study, these results suggest that verbal selection deficits are significantly and positively associated with anxiety, including clinically significant levels of anxiety symptoms.
These findings are consistent with our model, which posits that reduced neural inhibition associated with anxiety leads to an impaired ability to resolve competition among response options (Snyder et al., 2010). Although the current results demonstrate selection impairments in language production tasks, it is possible that difficulty selecting among competing representations could also play a role in indecisiveness, procrastination, and intolerance of uncertainly associated with anxiety, due to the difficulty of selecting appropriate courses of action and outcome representations. Understanding the core EF deficits involved in these phenomena is important because problems making decisions can interfere with the ability to achieve major life goals, whereas intolerance of uncertainty leads to avoiding many potentially positive experiences, and may promote the maintenance or increase of anxiety (e.g., Chen & Hong, 2010). Future research is needed to determine whether impaired selection on simple non-affective tasks, such as language production tasks, predicts indecisiveness, procrastination, and intolerance of uncertainty, beyond what is predicted by anxiety alone, and what causal role this may play in the development or maintenance of anxiety.
Counterintuitively, the current study found that the unique aspect of depressive symptoms that is not shared with anxiety (i.e., controlling for anxiety) is associated with better selection, as indexed by a composite measure of three verbal selection tasks. This finding is in contrast to theories suggesting that anxiety and depression additively contribute to EF deficits (e.g., Basso et al., 2007), but is in accord with previous evidence for opposite changes in brain and behavior associated with anxiety and depressive symptoms in other domains (e.g., Keller et al., 2000). The current study shows for the first time that anxiety and depressive symptoms have opposite effects on one aspect of EF: verbal selection among competing options. Importantly, these effects also held true for the subsample of participants with clinically significant levels of anxiety, suggesting that the results may generalize to individuals with clinically elevated levels of anxiety with and without co-occurring depression.
Although the current study cannot directly address the reasons for this effect, one intriguing possibility is that anxiety and depression may be related to opposing changes in neural activity in prefrontal areas critical for EF. Specifically, whereas anxiety is associated with reduced function of the major inhibitory neurotransmitter GABA (e.g., for review see Kalueff & Nutt, 2007), depression is associated with reduced function of the major excitatory neurotransmitter glutamate (e.g., for review, see Yüksel & Ongur, 2010).3 Neural network simulations and our previous empirical research have demonstrated how GABAergic interneurons in prefrontal circuits can play a key role in selection, by allowing one representation to more quickly win out over competing options (Snyder et al., 2010). Our preliminary extensions to these neural network simulations suggest that reduced glutamatergic function can improve selection by reducing activation of competing responses. Thus, reduced glutamate associated with increasing depressive symptoms could counteract the effects of reduced GABA associated with increasing anxiety symptoms, leading to improvements in selection. This theory makes testable predictions: for example, depressive symptoms should improve performance only on tasks requiring competitive inhibition, such as selection, and should harm performance on tasks requiring neural excitation, such as working memory maintenance. This prediction is consistent with findings that working memory and active maintenance of task goals are impaired in individuals with depressive disorders (e.g., Snyder, 2013). Future research is needed further test this prediction by carefully differentiate the effects of anxiety and depressive symptoms on different aspects of EF.
One alternative possibility is that individuals with elevated depressive symptoms who are able to cope with their depression effectively enough to attend college might have better pre-existing cognitive function, which both allows them to attend college despite their depression and to do well on selection tasks. However, depressive symptoms did not predict performance on IQ or psychomotor speed tasks, suggesting the groups did not differ in general intellectual function or motivation. Nonetheless, the possibility cannot be ruled out that college students with elevated depressive symptoms are self-selected for high EF in particular. Future research with community samples can address this question.
In sum, we confirm that anxiety is associated with a robust and specific impairment in verbal selection among competing options, while adding the novel finding that depressive symptoms may have opposite effects. This counterintuitive effect could potentially explain mixed evidence for EF deficits associated with anxiety (Castaneda et al., 2008), because previous studies may have varied in the levels of co-occurring depressive symptoms, as well as the sensitivity of their tasks to the effects of depression. Our results emphasize the need to control for co-occurrence and consider the ways that anxiety and depression may differentially affect EF. Further, our results suggest that specific neural mechanisms associated with individual EF processes may be affected differently by anxiety and depression. Future research is needed to investigate these mechanisms and explore the implications for understanding and ameliorating impairments in daily functioning associated with these common mental health problems.
Acknowledgments
This research was supported by grants from the National Institutes of Health (P50-MH079485, F31-MH087073). We thank Bidita Dutta and David Story for assistance with data collection and members of the P50 center on Executive Function and Dysfunction for valuable discussions.
Footnotes
The same pattern holds for all individual tasks: The effect of anxiety alone is significant or marginal for all tasks, whereas the effect of depressive symptoms alone is non-significant and near zero (see Table 1).
For the individual tasks, anxiety symptoms significantly predicted increased selection costs for all tasks, whereas the effects of depressive symptoms were in the same direction as the composite measure, but did not reach significance (see Table 1).
Reduced GABA is also found in depressed patients, who nearly always also have high anxiety (e.g., Kalueff & Nutt, 2007), but reduced glutamate is not associated with anxiety (Phan et al., 2005).
References
- Basso MR, Lowery N, Ghormley C, Combs D, Purdie R, Bornstein R. Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cognitive Neuropsychiatry. 2007;12:437–456. doi: 10.1080/13546800701446517. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, Borkovec TD. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: A receiver operating characteristic analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:25–43. doi: 10.1016/S0005-7916(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Chen CY, Hong RY. Intolerance of uncertainty moderates the relation between negative life events and anxiety. Personality and Individual Differences. 2010;49:49–53. doi: 10.1016/j.paid.2010.03.006. [DOI] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment. 1998;10:83–89. doi: 10.1037//1040-3590.10.2.83. [DOI] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington J, Banich MT, Webb A, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Gillis MM, Haaga DAF, Ford GT. Normative values of the Beck Anxiety Inventory, Fear Questionnaire, Penn State Worry Questionnaire, and Social Phobia and Anxiety Inventory. Psychological Assessment. 1995;7:450–455. doi: 10.1037/1040-3590.7.4.450. [DOI] [Google Scholar]
- Graver CJ, White PM. Neuropsychological effects of stress on social phobia with and without comorbid depression. Behavioral Research and Therapy. 2007;45:1193–1206. doi: 10.1016/j.brat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression and Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Keller J, Nitschke J, Bhargava T, Deldin PJ, Gergen J, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. Journal of Abnormal Psychology. 2000;109:3–10. doi: 10.1037//0021-843X.109.1.3. [DOI] [PubMed] [Google Scholar]
- McClelland GH. Optimal design in psychological research. Psychological Methods. 1997;2:3–19. doi: 10.1037/1082-989X.2.1.3. [DOI] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behavioral Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: Cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity. 2007;41:673–690. doi: 10.1007/s11135-006-9018-6. [DOI] [Google Scholar]
- Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, Moore GJ. Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuro Report. 2005;16:183–186. doi: 10.1097/00001756-200502080-00024. [DOI] [PubMed] [Google Scholar]
- Rassin E, Muris P, Franken I, Smit M, Wong M. Measuring general indecisiveness. Journal of Psychopathology and Behavioral Assessment. 2007;29:60–67. doi: 10.1007/s10862-006-9023-z. [DOI] [Google Scholar]
- Rivas-Vazquez RA, Saffa-Biller D, Ruiz I, Blais MA, Rivas-Vazquez A. Current issues in anxiety and depression: Comorbid, mixed, and subthreshold disorders. Professional Psychology: Research and Practice. 2004;35:74–83. doi: 10.1037/0735-7028.35.1.74. [DOI] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54:199–227. doi: 10.1016/j.jml.2005.10.002. [DOI] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hutchison N, Nyhus E, Curran T, Banich MT, O’Reilly RC, Munakata Y. Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16483–16488. doi: 10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. So many options, so little time: The roles of association and competition in underdetermined responding. Psychonomic Bulletin & Review. 2008;15:1083–1088. doi: 10.3758/PBR.15.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöber J, Joormann J. Worry, procrastination, and perfectionism: Differentiating amount of worry, pathological worry, anxiety, and depression. Cognitive Therapy and Research. 2001;25:49–60. [Google Scholar]
- Uttl B. North American Adult Reading Test: Age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


