Abstract
A range of emotional and motivation impairments have long been clinically documented in people with schizophrenia, and there has been a resurgence of interest in understanding the psychological and neural mechanisms of the so-called “negative symptoms” in schizophrenia, given their lack of treatment responsiveness and their role in constraining function and life satisfaction in this illness. Negative symptoms comprise two domains, with the first covering diminished motivation and pleasure across a range of life domains and the second covering diminished verbal and non-verbal expression and communicative output. In this review, we focus on four aspects of the motivation/pleasure domain, providing a brief review of the behavioral and neural underpinnings of this domain. First, we cover liking or in-the-moment pleasure: immediate responses to pleasurable stimuli. Second, we cover anticipatory pleasure or wanting, which involves prediction of a forthcoming enjoyable outcome (reward) and feeling pleasure in anticipation of that outcome. Third, we address motivation, which comprises effort computation, which involves figuring out how much effort is needed to achieve a desired outcome, planning, and behavioral response. Finally, we cover the maintenance emotional states and behavioral responses. Throughout, we consider the behavioral manifestations and brain representations of these four aspects of motivation/pleasure deficits in schizophrenia. We conclude with directions for future research as well as implications for treatment.
Keywords: Schizophrenia, Motivation, Pleasure, Neural substrates, Effort, Anticipation
1. Introduction
A range of emotional and motivation impairments have long been clinically documented in people with schizophrenia, and there has been a resurgence of interest in understanding the psychological and neural mechanisms of the negative symptoms in schizophrenia, given their lack of treatment responsiveness and their role in constraining function and life satisfaction in this illness. Negative symptoms comprise five consensus-based domains: anhedonia, asociality, avolition, alogia, and blunted affect (Kirkpatrick et al., 2006). However, conceptual and empirical reviews of newer (e.g., CAINS; (Kring et al., 2013) or BNSS (Strauss et al., 2012)) and older (e.g., SANS; (Andreasen, 1982)) interview-based measures of negative symptoms indicate that a two-factor model more parsimoniously describes the negative symptoms (Blanchard and Cohen, 2006; Kimhy et al., 2006; Messinger et al., 2011). The first factor reflects diminished motivation and pleasure across a range of life domains; the second factor reflects diminished verbal and non-verbal expression and communicative output. Newer measurement techniques for these two domains–motivation/pleasure and expression–may better capture the underlying mechanisms giving rise to negative symptoms (Blanchard and Cohen, 2006; Kring et al., 2013) and help in developing more effective treatments. In this review, we focus on the motivation/ pleasure domain, providing a brief review of the behavioral and neural underpinnings of this domain.
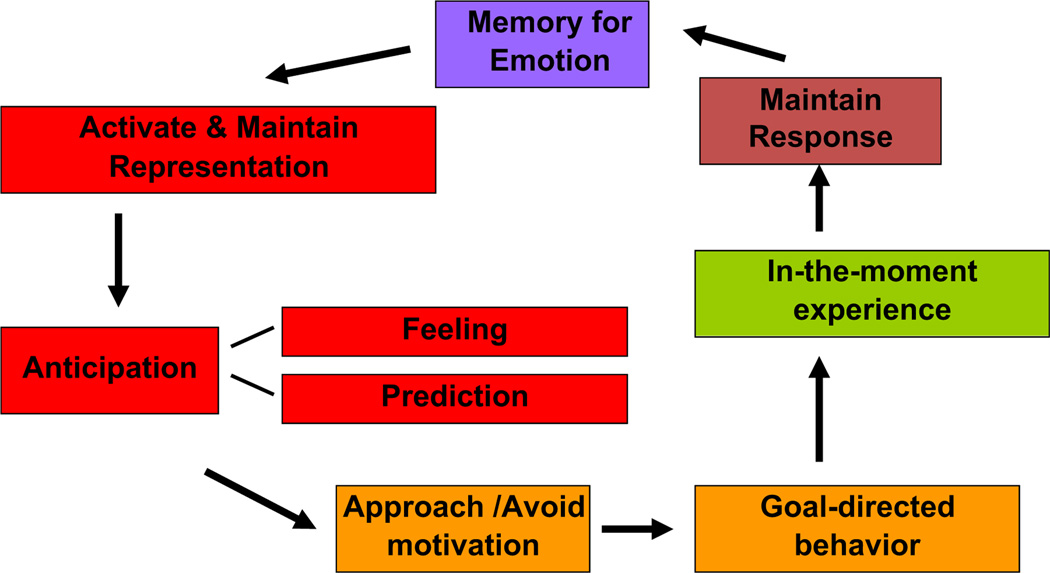
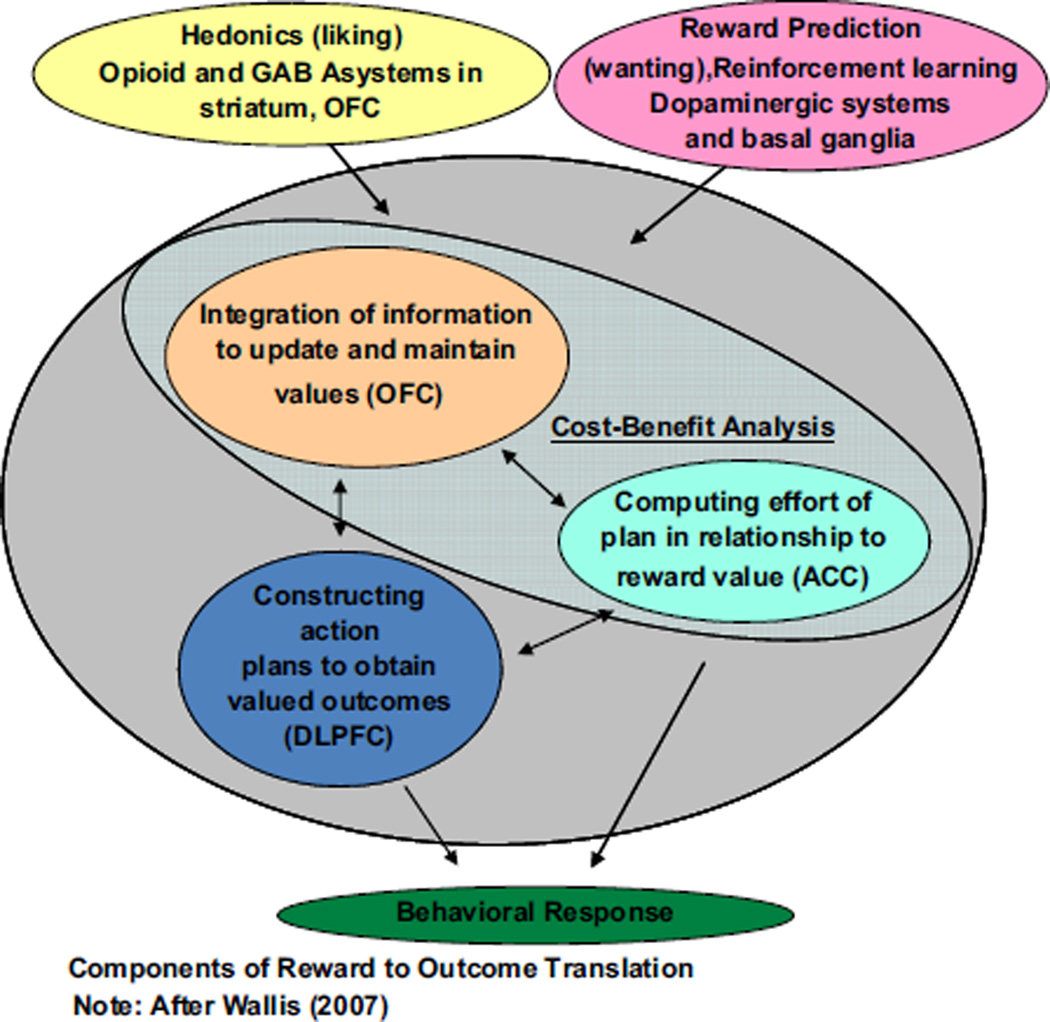
We have argued elsewhere that adopting a translational approach to research on negative symptoms has and will continue to give us the greatest purchase toward more clearly identifying mechanisms of negative symptoms and developing more effective and targeted treatments (Barch and Dowd, 2010; Kring and Elis, 2013). Indeed, adopting the methods, theories, and measures of affective science, as well as translating from the animal and human affective and cognitive neuroscience, has propelled us ever closer to identifying core deficits that give rise to motivation/pleasure deficits in schizophrenia and other disorders. Independently, we have developed theoretical frameworks to guide our research on the emotional and motivational underpinnings of negative symptoms, highlighting how these deficits necessarily also involve behavior and brain regions that are crucial for cognitive control. Both of us have translated animal and human cognitive and affective neuroscience research to our models of motivation/pleasure deficits in schizophrenia. Kring and colleagues have argued for the importance of characterizing the time course of emotion to distinguish among anticipatory, consummatory (“in-the-moment”), and maintenance of emotion responding (see Fig. 1). Relatedly, Barch and colleagues have argued for the importance of representations about motivationally salient incentives necessary to develop and actively maintain behavioral plans that are necessary to achieve desired pleasurable outcomes (see Fig. 2).
Fig. 1.
Temporal experience of emotion.
Fig. 2.
Reward to outcome translation.
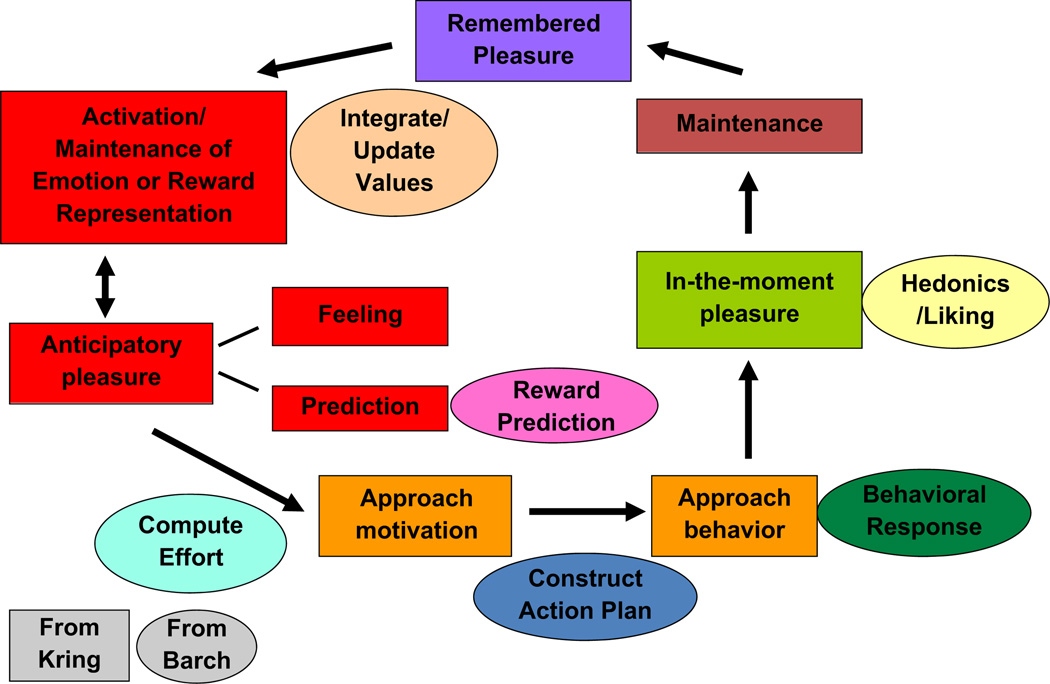
In this review, we integrate our approaches to highlight the latest advances in our understanding of behavioral and brain systems that contribute to diminished motivation and pleasure in schizophrenia (see Fig. 3). Specifically, we focus on four common aspects of our models. First, we cover liking or in-the-moment pleasure, which involves immediate responses to pleasurable stimuli. Second, we cover anticipatory pleasure or wanting, which involves a prediction about a future enjoyable outcome (reward) and/or a feeling of pleasure in anticipation of that outcome. Third, we address motivation, which includes a calculation of how much effort is needed to achieve a desired outcome (reward), a plan of how to obtain that outcome, and the actual behavioral response to get the reward. Finally, we cover the maintenance of emotional states and behavioral responses. Throughout, we consider the behavioral manifestations and brain representations of these four aspects of motivation/pleasure deficits in schizophrenia.
Fig. 3.
Integrating the temporal experience of emotion with reward to outcome translation (Kring & Barch).
2. In-the-moment emotional experience in schizophrenia
In-the-moment experience in our models refers to the responses people provide when presented with putatively positive things. That is, how do people report feeling when presented with funny pictures, tasty foods, or reward of $5? This in-the-moment experience is called consummatory pleasure or “liking” in some human and animal models (see Table 1). In this section, we review the evidence on the behavioral and brain responses people with schizophrenia exhibit when presented with positive stimuli.
Table 1.
Key terms and concepts.
| Term | Definition |
|---|---|
| In-the-moment pleasure experience (“liking” or “consummatory pleasure”) |
The responses people provide when presented with putatively positive things, such as how they people report feeling when presented with funny pictures, tasty foods, or reward of $5, or how their brain responds when presented with such stimuli |
| Anticipatory pleasure (“wanting”) |
A prediction about a future enjoyable outcome (reward) and/or a feeling of pleasure in anticipation of that outcome |
| Prediction error | An increase in striatal (presumably dopaminergic) responses to unexpected rewards and a decrease in striatal responses when predicted rewards do not occur |
| Effort computation | How much effort is needed to achieve a desired outcome (reward) |
| Value computation | How valuable a potential reward or desired outcome is, given the current state of the person (e.g., how valuable is a bottle of spring water when I am very thirsty versus when I just drank a bottle of water) |
| Reversal learning | When presented with a choice between two stimuli (e.g., door 1 or door 2), learning that a previously rewarded stimulus (e.g., door 1) is no longer associated with reward, and that the other stimulus is now associated with reward (e.g., door 2) |
| Goal-directed behavior | Selection and execution of actions necessary to obtain potentially desirable or rewarding outcomes that fulfill a goal (e.g., the experience of pleasure, or a special outcome) |
| DLPFC | Dorsolateral prefrontal cortex |
| OFC | Orbital frontal cortex |
| DA | Dopamine |
| ACC | Anterior cingulate cortex |
Although people with schizophrenia often report limited experiences of positive emotion during interview based clinical assessments (Horan et al., 2006), behavioral research tells a different story. Specifically, one of the most well-replicated findings in schizophrenia research is that people with schizophrenia report experiencing similar (or slightly less) amounts of positive emotion compared to those without schizophrenia in the presence of emotionally evocative stimuli (Cohen and Minor, 2010; Kring and Moran, 2008; Llerena et al., 2012) and in daily life (Gard et al., 2007; Myin-Germeys et al., 2000; Oorschot et al., 2009, 2012), regardless of changes in medication status (Kring et al., 1999a, 1999b). Interestingly, a number of studies have found that people with schizophrenia report experiencing more negative emotion to putatively positive stimuli or events in daily life compared to people without schizophrenia (Kring and Elis, 2013; Strauss and Gold, 2012; Tremeau et al., 2009; Ursu et al., 2011) (see Cohen and Minor (2010) for meta-analysis). In short, people with schizophrenia generally report liking or enjoying positive things as much as do people without schizophrenia even as they may also report disliking them a bit more.
At least some aspects of liking or in-the-moment experience appears also appear to be intact at the level of the brain, though the findings are more variable and complicated by methodological differences across studies. Brain imaging studies examining striatal responses to the receipt of expected monetary rewards in schizophrenia have shown a consistent pattern of intact responses, regardless of typical or atypical antipsychotic medication status (Dowd and Barch, 2012; Kirsch et al., 2007; Simon et al., 2009; Walter et al., 2009). However, some studies have found abnormal cortical responses to reward receipt (Schlagenhauf et al., 2009; Walter et al., 2010; Waltz et al., 2010), particularly when the reward is unexpected (see also section on prediction error responses in the striatum). Further, a more mixed picture has arisen in non-monetary studies in schizophrenia (Crespo-Facorro et al., 2001; Paradiso et al., 2003), with some evidence for reduced activation of the insula (Plailly et al., 2006; Schneider et al., 2007), orbital frontal cortex (OFC) (Plailly et al., 2006) and striatum (Dowd and Barch, 2010; Grimm et al., 2012; Waltz et al., 2009).
Two recent meta-analyses that focused on responses to emotional stimuli (Anticevic et al., 2010; Taylor et al., 2012) reported consistent findings in regards to brain responses to emotion in schizophrenia, despite using different meta-analytic techniques. Both meta-analyses included studies that had the following characteristics: assessed in-the-moment responses to evocative stimuli; included contrasts between conditions (emotion condition–neutral condition as well emotion condition–baseline); included between group comparisons, and used standardized coordinates. Anticevic et al. focused primarily on the amygdala, whereas Taylor et al. examined whole brain activation. With respect to amygdala activation, an interesting and consistent result was found across the two meta-analyses. People without schizophrenia showed greater amygdala activation compared to people with schizophrenia, but only for contrasts that subtracted activation during a neutral condition from activation during a negative condition (Anticevic et al., 2010; Taylor et al., 2012). In contrast, when examining direct emotion contrasts (i.e. positive condition–baseline), Anticevic et al. found no between group differences in amygdala activation. Results from both meta-analyses suggest (but cannot fully demonstrate) that under-activation of amygdala in people with schizophrenia in response to evocative stimuli may reflect over-activation to the neutral stimuli, and suggest that responses to negative stimuli themselves are relatively intact. It remains important to examine responses to positive stimuli given that most studies included in the meta-analyses to date have included only negative and neutral stimuli.
Taylor et al. (2012) revealed other regions of activation that distinguished people with and without schizophrenia, including less activation in people with schizophrenia in dorsal medial and dorsolateral PFC, regions activated during emotion and cognitive tasks. Less activation was also observed in more posterior brain regions (occipital pole, fusiform gyrus) among people with schizophrenia, but this may reflect neural correlates of processing complex facial stimuli more than emotion per se. Taylor et al. also examined the potential moderating effects of task type on observed brain activation differences. As such, they compared implicit tasks (e.g., rate the gender of face) versus explicit tasks (e.g., rate your emotional experience), and found that the reduced activation among people without schizophrenia in amygdala and medial prefrontal cortex (PFC) was found primarily in implicit tasks. This raises the question as to whether these non-emotion focused aspects of the task may have contributed to the different patterns of activation.
To summarize, brain imaging studies suggest that people with and without schizophrenia show comparable brain activation in striatum to expected reward and amygdala (more so for negative stimuli relative to baseline) to people without schizophrenia. Despite these similarities, there is also evidence for differences in the insula, orbital frontal cortex (OFC) and striatum to positive non-reward stimuli, though these results are quite mixed with an equal number of studies finding intact responses to positive stimuli in these regions. There also appear to be differences in activation of regions that are also associated with cognitive control, including anterior cingulate cortex (ACC) and the dorsolateral prefrontal cortex (DLPFC).
If in-the-moment pleasure or liking at the level of self-report and the brain are relatively intact in schizophrenia, at least to expected stimuli, where might things go awry? Research from both of our groups as well as other laboratories suggests that more pronounced deficits emerge at the intersection of emotion, cognition, and behavior. In the next sections, we review the fairly consistent behavioral and neural evidence for deficits in anticipation, maintenance, and motivated behavior in schizophrenia.
3. Anticipatory pleasure
Much of what is enjoyable or pleasurable about life is derived from thinking about the future. Will I enjoy having sushi for lunch? Will I be happy with my new job? Will I enjoy the family reunion? Thinking about pleasure or enjoyment in the future is broadly referred to as anticipatory pleasure or “wanting.” Anticipatory pleasure involves a prediction component (how much will I enjoy dinner with my family?) as well as a feeling of pleasuring knowing that something positive is going to happen in the future (I am so excited, I can’t wait to start my new job next week). It is in the domain of anticipatory pleasure that people with schizophrenia appear to have particular trouble.
Indeed, we have argued for and demonstrated anticipatory pleasure deficits in schizophrenia (Barch and Dowd, 2010; Dowd and Barch, 2010, 2012; Gard et al., 2007; Kring and Caponigro, 2010; Wynn et al., 2010). In the animal literature, anticipatory pleasure has been characterized as “wanting” and distinguished from “liking” or in-the-moment pleasure experience (Berridge, 2004; Berridge and Robinson, 1998). Compared to controls, people with schizophrenia are less likely to anticipate or predict that future events will be pleasurable, are less likely to experience pleasure in anticipation of things to come, and thus may be less motivated to seek out pleasurable experiences (see Fig. 3).
Neuroscience research on how animals and people translate these predictions and feelings about possible future rewards or enjoyable things into behavioral responses (Berridge, 2004; Schultz, 2004, 2007; Schultz et al., 1997; Wallis, 2007) has informed our work in schizophrenia. Specifically, animal work has demonstrated that the mid-brain dopamine (DA) system, particularly the projections to ventral and dorsal striatum mediates reward prediction and wanting (Berridge, 2004; Schultz, 2007). Many DA neurons in the substantia nigra and ventral tegmental area respond to stimuli that predict reward, as well as to the rewarding stimuli themselves, with the degree of response depending on reward predictability. If the reward was not predicted, then DA neurons fire strongly (positive prediction error); if a predicted reward does not occur, then there is a transient depression in DA neuron firing (negative prediction error) (Schultz, 1992, 2004, 2007, 1993, 1997). When neutral cues are repeatedly associated with rewards, over time DA neurons begin to fire to the predictive cues rather than to rewards themselves (Schultz, 2007). Human fMRI studies have found activation of ventral and dorsal striatum, major targets of midbrain dopaminergic neurons, in response to cues that predict expected reward (Knutson et al., 2001, 2000) as well as both positive and negative prediction error responses (Abler et al., 2006; McClure et al., 2003). A prominent, though slightly different theory, emphasizes the role of the DA-learning process in transferring incentive salience (i.e., the positive feelings in human terms) from the reward itself (e.g., tasty dinner) to the reward-predicting cues (restaurant review in the paper), thus imbuing these cues with motivational properties themselves (e.g., a “wanting” response (Berridge, 2004)).
fMRI studies of the prediction part of anticipatory pleasure in schizophrenia have reported reduced ventral striatum activity to cues predicting reward in unmedicated people with schizophrenia (Esslinger et al., 2012; Juckel et al., 2006b; Kirsch et al., 2007; Nielsen et al., 2012; Schlagenhauf et al., 2009), as well as in people taking typical, but not atypical antipsychotics (Juckel et al., 2006a; Schlagenhauf et al., 2008; Simon et al., 2009; Walter et al., 2009). Importantly, a number of these studies also showed a relationship between negative symptom severity and deficits in ventral striatal activity to cues predicting reward (Dowd and Barch, 2012; Juckel et al., 2006b; Waltz et al., 2010).
Prediction errors
A number of studies have also examined the role of the striatum in reward prediction by looking at prediction error responses–an increase in striatal (presumably dopaminergic) responses to unexpected rewards and a decrease in striatal responses when predicted rewards do not occur. Some studies have found reduced prediction error responses in the striatum to rewards among people with schizophrenia spectrum disorders (Jeon and Polich, 2003; Morris et al., 2012; Murray et al., 2008), coupled with enhanced prediction error responses to neutral stimuli (Murray et al., 2008) or to unsurprising rewarding stimuli not expected to elicit prediction errors (Morris et al., 2012). By contrast, Gradin found reduced prediction error responses in the caudate, but increased activation associated with expected reward value in the ventral striatum (Gradin et al., 2011). Waltz et al. (2009) found evidence for reduced positive prediction error responses in a range of regions that included the striatum (dorsal and ventral) as well as insula, but relatively intact negative prediction errors in these same regions. Dowd and Barch (2012) found intact prediction error responses in schizophrenia, but in cortical regions rather than striatal regions during a Pavlovian task (neither the controls nor the people with schizophrenia showed prediction error responses in the striatum). Taken together, these findings suggest evidence for reduced prediction error responses to unexpected outcomes in some striatal regions. It is not clear whether these abnormal responses reflect altered computations of the likelihood that stimuli will lead to reward (e.g., more of a problem anticipating the likelihood that a stimulus will lead to a reward) or a problem in updating the values associated with a stimulus at the time that the reward occurs. Further work will be needed to tease these apart and to clarify the reasons for abnormal prediction error responses in schizophrenia.
In summary, the literature on anticipatory pleasure in schizophrenia suggests more evidence for impairment than the literature on in-the-moment “liking” responses. People with schizophrenia self-report a reduction in the anticipation of pleasure in future events. Further, they show reduced responses in the striatum to cues that predict the future occurrence of reward, as well as abnormal responses to the receipt of rewards that are unexpected. A key question is then how this might influence the selection of behaviors that may or may not lead to future rewards.
4. Approach motivation and behavior: computing value, computing effort, and goal-directed behavior
Why are people motivated to seek out some rewards or positive outcomes but not others? Barch and Dowd (2010) have argued for the importance of distinguishing two key components of motivation. First, people compute the value of a reward or desirable outcome (value computation: do I really enjoy or desire that cup of tea?), and second, people compute how much effort or “work” it will take to get that reward or outcome (is it worth walking the two blocks to get the tea?). These two components of motivation then support goal-directed behavior–that is, the action or behavior need to achieve a desired goal or outcome/reward. In this section, we review the behavioral and neuroscience evidence for deficits in value computation, effort computation, and goal-directed behavior. First, we describe the current animal and human neuroscience findings on brain regions supporting theses motivation processes.
Neuroscience research has found that value computation is mediated in part by orbitofrontal cortex (OFC) (Padoa-Schioppa and Cai, 2011; Rudebeck and Murray, 2011). In animal work, value computation includes the positive/ rewarding properties of an outcome as well as the internal state of the organism (e.g., value of juice when thirsty versus not) (Rolls et al., 1989), which is akin to the human work on feeling pleasure in anticipation of a desired outcome (Gilbert and Wilson, 2007). Value computation also includes the delay before an outcome occurs (Roesch and Olson, 2005; Rudebeck et al., 2006b), the available outcomes (e.g., tea versus wine after a hard day) (Padoa-Schioppa, 2007; Padoa-Schioppa and Assad, 2006), and the changing contingencies associated with a stimulus (a previously rewarded response is now punished, such as when the rules change in a game you are playing) (Cools et al., 2002; Dias et al., 1996). Some researchers have described the OFC as being involved in “working memory for value”, or the ability to maintain, update, and integrate different sources of information about value over a short period of time (Frank and Claus, 2006; Wallis, 2007).
Effort computation (i.e., determining the cost of engaging in whatever actions it will take to obtain an outcome) involves the dorsal anterior cingulate cortex (ACC) with contributions from DA input from nucleus accumbens and related forebrain circuitry (Botvinick et al., 2009; Croxson et al., 2009; Salamone, 2007; Salamone et al., 2007). For example, research has shown that ACC lesions, as well as depletions of accumbens DA, lead animals to choose low effort but low reward options over higher reward, but higher effort options (Rudebeck et al., 2006a, 2007, 2006b; Rushworth et al., 2007; Salamone, 2007; Salamone et al., 2007; Walton et al., 2007).
Integrating these computations alongside anticipatory pleasure propels the ability to generate and execute action plans necessary to achieve the valued outcome. A number of researchers have discussed the role of the lateral PFC (Braver and Cohen, 1999; Miller and Cohen, 2001; Wallis, 2007) in relation to goal-directed behavior (i.e., behavior that is enacted in the service or obtaining a goal or reward, such as walking the two blocks for an afternoon coffee). Indeed, other research and theory on DLPFC function has indicated that the DLPFC plays a role in top-down control of cognitive processing and provides a bias signal that helps to facilitate goal-directed behavior (Miller and Cohen, 2001). In addition, people who have lateral prefrontal lesions show impairments in developing an “action plan” for enacting goal-directed behavior (Manes et al., 2002; Zalla et al., 2001). Finally, other studies show that increases in DLFPC activity mediate “motivated” cognitive control enhancements that occur with the provision of incentives in both animals (Kobayashi et al., 2006; Krawczyk et al., 2007; Sakagami and Watanabe, 2007; Watanabe, 1996), and humans (Beck et al., 2010; Jimura et al., 2010; Savine and Braver, 2010; Tsujimoto and Sawaguchi, 2005). In short, intact DLPFC function may be necessary to translate information about value into goal representations and to maintain such information so that is can be implemented as goal-directed behavior to achieve the desired outcome.
In schizophrenia, behavioral studies indicate that people with schizophrenia have deficits in value computation (Brown et al., 2013; Gold et al., 2012, 2008; Heerey et al., 2008). In addition, indirect evidence for deficits in value computation comes from studies showing deficits on two tasks that rely upon the OFC: (1) reversal learning (Ceaser et al., 2008; Elliott et al., 1995; Oades, 1997; Pantelis et al., 1999; Turnbull et al., 2006; Tyson et al., 2004; Waltz and Gold, 2007) but see (Hutton et al., 1998; Jazbec et al., 2007; Joyce et al., 2002) for exceptions; and (2) the Iowa Gambling task (Kester et al., 2006; Kim et al., 2009; Lee et al., 2007; Martino et al., 2007; Premkumar et al., 2008; Sevy et al., 2007; Shurman et al., 2005; Yip et al., 2009), but some exceptions (Evans et al., 2005; Rodriguez-Sanchez et al., 2005; Turnbull et al., 2006; Wilder et al., 1998). Both tasks require the integration of information about rewards and punishments across trials to then update value representations appropriately. There is also evidence for structural and functional changes in OFC in schizophrenia (Baare et al., 1999; Bertollo et al., 1996; Gur et al., 2000; Pantelis et al., 2003; Plailly et al., 2006), some of which are related to negative symptoms (Baare et al., 1999; Gur et al., 2000). In sum, there is growing evidence from the behavioral literature on schizophrenia for deficits in value computation in schizophrenia, as well as good evidence from the behavioral literature for deficits in tasks thought to reflect OFC function in schizophrenia, and at least some data suggesting that OFC changes may be related to negative symptoms.
Research in schizophrenia is just beginning to examine effort computation. For example, Gold et al. (2013) presented people with and without schizophrenia a computer task that allowed participants to choose between a more effortful response for a bigger monetary reward or a less effortful response for a smaller monetary reward while also varying the likelihood of receiving a reward (50% versus 100%). Interestingly, people with schizophrenia who had more negative symptoms were less likely than controls to expend more effort when the reward was certain (i.e., 100%). The authors speculated that the certainty of reward may have also made the effort expenditure more salient. In short, people with schizophrenia may have computed that the effort was not worth it when it was most salient. Barch et al. (in preparation) used a similar task developed by Treadway et al. (2009) to study effort computation as well. They also found that people with schizophrenia were less likely to exert greater effort as reward magnitude increased and as rewards became more likely. Further, they found that a reduction in the willingness to exert effort was associated with poor community and work function among people with schizophrenia.
Goal-directed behavior has been studied more frequently in schizophrenia, alongside deficits in DLPFC function. As noted earlier, an experience sampling study found that people with schizophrenia anticipated that goal-directed activities would be less enjoyable than did people without schizophrenia and they subsequently engaged in these types of activities less often (Gard et al., 2007). Thus some of the motivational impairments observed in schizophrenia may reflect, at least in part, problems in translating computations of value and effort into goal representations that can be used and maintained in DLPFC to guide goal-directed behavior. One way of examining this question is to determine how motivational incentives impact cognitive performance, potentially via modulation of DLPFC activity. Several studies suggest that people with schizophrenia are not able to improve their performance on cognitive tasks when offered monetary incentives (Green et al., 1992; Hellman et al., 1998; Roiser et al., 2009; Vollema et al., 1995); while an equal number suggest at least some evidence for improvement with reward (Kern et al., 1995; Penn and Combs, 2000; Rassovsky et al., 2005). There is also older clinical work on the use of token economies in schizophrenia that suggests functioning can be improved through an explicit reward system, though token economies provide a number of “external” supports for maintaining reward related information that could compensate for deficits in the ability to translate reward information into action plans. Further, there is strong evidence from a variety of sources for impairments in DLPFC function in schizophrenia (Glahn et al., 2005; Minzenberg et al., 2009; Van Snellenberg et al., 2006). However, to date, there are no fMRI studies examining whether or not anticipated reward or incentives modulate DLPFC activity during cognitive control or working memory tasks in schizophrenia.
5. Maintenance, integration, and updating
Not only is maintenance crucial for enacting goal-directed behavior, but also it is important for later encoding of pleasurable experiences. Indeed, human neuroscience findings in healthy people suggest that our ability to anticipate the future relies upon our ability to remember the past (Schacter et al., 2007, 2008), with a core network of brain regions, including areas of the medial prefrontal and medial temporal cortex, supporting both abilities. Thus, when we predict whether or not we will enjoy the latest novel by a favorite author, we likely draw upon our past experiences with the authors’ novels to guide our predictions. Maintaining emotional experiences as they occur likely facilitates the development of memories for these experiences (Kring and Caponigro, 2010). There is a very robust literature suggesting that people with schizophrenia have deficits in active maintenance of non-emotional information in working memory, with a number of meta-analyses summarizing this evidence (Forbes et al., 2009b; Lee and Park, 2005). As such, it is should not be surprising that there is also evidence from psychophysiological and fMRI studies suggesting that people with schizophrenia have difficulty holding on to prior emotional experiences. For example, people with and without schizophrenia showed comparable and differentiated eyeblink responses to a startle probe during picture viewing (i.e., larger blinks to negative pictures, smaller blinks to positive pictures), yet just 2.5 s after the picture was removed from view, the schizophrenia group no longer showed this differentiated pattern of blink responses, though the control group did (Kring et al., 2011). Similarly, in an fMRI study, people with and without schizophrenia exhibited comparable activation of visual, amygdala, and prefrontal cortical regions, consistent with intact in-the-moment emotional experience. However, during the 12.5 s period after pictures were removed from view, there were marked differences between groups across prefrontal and limbic regions, and reduced activity in the DLPFC was positively correlated with anhedonia (Ursu et al., 2011). Such findings are consistent with the hypothesis that people with schizophrenia may have difficulties actively maintaining emotional experiences. Further, an event related potential study found that people with schizophrenia exhibited comparable responses early and mid-way during picture viewing but the schizophrenia group differed late in the picture viewing window, suggesting that maintenance deficits may begin even before stimuli are removed from view (Horan et al., 2010). An important unanswered issue for future research is whether deficits in the maintenance of emotional or rewarding experiences are simply another manifestation of deficits in working memory more broadly.
6. Conclusions and unanswered questions, and future directions
Taken together, results from our review are consistent with the notion that “in-the-moment” experience of pleasure is relatively intact in schizophrenia. That is, people with schizophrenia report experiencing as much pleasure as do people without schizophrenia in the presence of putatively positive stimuli, and they show comparable brain activation, at least in response to expected positive stimuli or outcomes. Nevertheless, people with schizophrenia also report experiencing more negative emotion in response to positive stimuli and they also show different versus activation in response to unexpected outcomes suggesting that all is not completely intact when it comes to in-the moment responding or the liking aspect of our model. In addition, there is evidence that value computation is impaired in schizophrenia. Further, there is growing and consistent evidence that people with schizophrenia have deficits in both behavior and neural responses associated with the anticipation of future rewarding or pleasurable experiences, as well as with responses to unexpected rewards. In addition, they have impairments in value and effort computation and goal-directed behavior that are related to functioning in the world. There is suggestive evidence that the active maintenance of emotional information and the ability to use prior emotional experience to guide current behavior is also impaired, though the linkages between this and other cognitive deficits (i.e., working memory) have yet to be worked out.
To illustrate the ways in which breakdowns in the interplay among generation and anticipation of goals, value and effort computations, and maintenance may contribute to impairments in goal-directed behavior in schizophrenia, consider the following scenario. A person with schizophrenia may report enjoying brownies and show both behavioral and neural evidence of consummatory pleasure while eating brownies. However, she may not be able to engage in the behaviors necessary to obtain or make more brownies (Barch and Dowd, 2010; Kring and Moran, 2008). Planning, purchasing, preparing or baking the brownies requires ongoing maintenance or retrieval of the prior emotional experience of pleasure while consuming a brownie, as well as maintenance of contextual or cue information that should trigger associations about the food's rewarding properties. Such anticipation and maintenance of emotional information should guide the volitional pursuits and actions over time that may lead to such a reward (in this case intake of delicious food). An intact reward valuation and prediction system is necessary for this set of behaviors to take place, and deficits in these functions in schizophrenia may reduce the ability of appetitive or pleasure cues to drive behavior. However, these functions also depend on the intact ability to maintain pleasure cues, experiences or context over time–a process that may be reliant on working memory and cognitive control, which are compromised in schizophrenia (Barch and Dowd, 2010; Ursu et al., 2011). Thus, deficits in reward prediction may be made worse by deficits in the ability actively maintain information about rewards or pleasure, which may extend beyond simple appetitive stimuli to more abstract rewards.
What is needed next is research that directly tests the ability to use and maintain internal representations of emotional experience and reward information to modulate behavior and brain function in schizophrenia, along with work that characterizes links between deficits in these abilities and everyday function in this illness. Further, more work is needed to understand the role that effort computations may also play in motivation impairments in schizophrenia. It may be that even when people with schizophrenia are able to generate or maintain representations of emotion or motivation, alternations in computing effort to reward valuation may inhibit the development of goal directed action plans that people need in order to pursue and obtain desired outcomes. Of course, these mechanisms may interact–difficulties in the anticipation of pleasurable outcomes may reduce the “reward” part of the value or effort computation, skewing such computations towards less willingness to exert effort if there is less perception of rewards due to representation and maintenance impairment. Further, much work is still needed to understand how these various deficits interact with medications and the course of illness. There is good evidence that negative symptoms are present in currently unmedicated people and in people who have never taken medication. However, it has been more difficult to conduct the experimental studies parsing mechanisms in unmedicated people, and thus future research is needed to disentangle the effects of the disease versus the effects of medication on these different mechanisms.
Another direction for future research is to examine how the components of the model we articulate here are or are not deficient in depression. The temporal course of reward processing impairments in schizophrenia and depression may be different, with more evidence that abnormal reward processing may be more trait-like in schizophrenia and more state-like in depression (Blanchard et al., 2001). Further, there is a growing evidence that the nature of reward processing abnormalities in depression may be different that those found in schizophrenia. For example, there is much more evidence for abnormal responses to the receipts of rewards in depression (Epstein et al., 2006; Eshel and Roiser, 2010; Forbes and Dahl, 2012; Forbes et al., 2009a; Pizzagalli et al., 2008). However, there is also evidence for some similar abnormalities, such as reduced effort computation (Treadway et al., 2012). Unfortunately, very few studies have directly compared different aspects of reward processing in schizophrenia and depression (though see Gradin et al. (2011)), and this will be a critical path for future research that will help us understand the structure of motivational impairments in relationship to varying manifestations of psychopathology.
Nonetheless, the intriguing research coming from the work on the mechanisms driving negative symptoms in schizophrenia is beginning to point towards specific processing and neural systems that could be ripe targets for interventions, both at a pharmacological and a psychological level. It is interesting to speculate as to possible interventions that may help to remediate such impairments in reward prediction, effort allocation, and maintenance of information about emotional and/or reward information, with the hope of potentially improving quality of life and work and community function. There may be a range of pharmacological interventions that could manipulate the dopamine system in ways that could enhance reward processing, though the challenge is to ensure that such pharmacological approaches do not also increase positive symptoms, given the role of dopamine in the pathophysiology of the disorder. Further, there may be behavioral interventions that could be effective as well. Indeed, Grant et al. (2012) modified cognitive therapy for schizophrenia to emphasize goals, including changing beliefs that are associated with negative symptoms (Grant and Beck, 2009) and interfere with goal attainment as well as practice in action planning to achieve long- and short-term goals. Results from a randomized trial indicated that people with schizophrenia who received cognitive therapy had lower avolition at the end of treatment as well as better overall functioning. Other behavioral interventions have shown promise in increasing anticipatory pleasure (Favrod et al., 2010) and maintenance (Johnson et al., 2011), but these studies were very small, open trials that are in need of replication with larger samples and in randomized trials.
Additional treatment development work is needed, however, to more fully translate the findings we have reviewed here into behavioral interventions. For example, as described above, the work of Kring and colleagues suggests that emotional responses in schizophrenia may not be sustained when stimuli are not present in the immediate environment (Kring et al., 2011; Ursu et al., 2011) and other work suggests that people with schizophrenia have difficulties understanding which cues predict future rewards when stimuli. In other words, people with schizophrenia seem to have problems bridging the gap between the occurrence of stimuli that predict reward and the future obtainment that reward through the use of internal representations that can support temporally extended goal directed activities, suggesting a common mechanism that may be contributing to a range of impairments in motivation in this disorder. If so, then environmental supports or cues that remind people with this illness about rewarding outcomes over time may increase the likelihood of increased effort allocation to engage in the behaviors necessary to eventually achieve such goals. While speculative, such hypotheses help translate these experimental findings into interventions for motivational impairments in schizophrenia.
Acknowledgments
None.
Role of funding source
Completion of this manuscript was supported in part by NIMH Grant 1R01MH082890 awarded to Ann Kring; the NIMH had no further role in preparation or completion of this review.
Footnotes
Contributors
Ann Kring and Deanna Barch contributed equally to this manuscript. We flipped a coin to determine which author name would appear first.
Conflict of interest
Neither Ann Kring nor Deanna Barch has any conflicts or competing interests to report.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch. Gen. Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baare WF, Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol. Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment (in preparation) 2013. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PloS One. 2010;5:e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neu-roscience. Physiol. Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bertollo DN, Cowen MA, Levy AV. Hypometabolism in olfactory cortical projection areas of male patients with schizophrenia: an initial positron emission tomography study. Psychiatry Res. 1996;60:113–116. doi: 10.1016/0165-1781(96)02619-4. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr. Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. J. Abnorm. Psychol. 2001;110:363–371. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cogn. Affect. Behav. Neurosci. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Progr. Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Brown JK, Waltz JA, Strauss GP, McMahon RP, Frank MJ, Gold JM. Hypothetical decision making in schizophrenia: the role of expected value computation and “irrational” biases. Psychiatry Res. 2013 doi: 10.1016/j.psychres.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceaser AE, Goldberg TE, Egan MF, McMahon RP, Weinberger DR, Gold JM. Set-shifting ability and schizophrenia: a marker of clinical illness or an intermediate phenotype? Biol. Psychiatry. 2008;64:782–788. doi: 10.1016/j.biopsych.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LLB, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia. J. Am. Med. Assoc. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J. Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dowd E, Barch DM. Subjective emotional experience in schizophrenia: neural and behavioral markers. Biol. Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PloS One. 2012;7:e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol. Med. 1995;25:619–630. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbers-weig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biol. Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Englisch S, Inta D, Rausch F, Schirmbeck F, Mier D, Kirsch P, Meyer-Lindenberg A, Zink M. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr. Res. 2012 doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Evans CE, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task: the key role of emotional experience in schizophrenia. J. Clin. Exp. Neuropsychol. 2005;27:656–664. doi: 10.1081/13803390490918354. [DOI] [PubMed] [Google Scholar]
- Favrod J, Giuliani F, Ernst F, Bonsack C. Anticipatory pleasure skills training: a new intervention to reduce anhedonia in schizophrenia. Perspect. Psychiatr. Care. 2010;46:171–181. doi: 10.1111/j.1744-6163.2010.00255.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research review: altered reward function in adolescent depression: what, when and how? J. Child. Psychol. Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009a;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol. Med. 2009b;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol. Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum. Brain. Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Collins AG, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch. Gen. Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr. Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Reid I, Hall J, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain: J. Neurol. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr. Bull. 2009;35:798–806. doi: 10.1093/schbul/sbn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch. Gen. Psychiatry. 2012;69:121–127. doi: 10.1001/archgenpsychiatry.2011.129. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Ganzell S, Vaclav JF. Wisconsin card sorting test performance in schizophrenia: remediation of a stubborn deficit. Am. J. Psychiatry. 1992;149:62–67. doi: 10.1176/ajp.149.1.62. [DOI] [PubMed] [Google Scholar]
- Grimm O, Vollstadt-Klein S, Krebs L, Zink M, Smolka MN. Reduced striatal activation during reward anticipation due to appetite-provoking cues in chronic schizophrenia: a fMRI study. Schizophr. Res. 2012;134:151–157. doi: 10.1016/j.schres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch. Gen. Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol. Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman SG, Kern RS, Neilson LM, Green MF. Monetary reinforcement and Wisconsin Card Sorting performance in schizophrenia: why show me the money? Schizophr. Res. 1998;34:67–75. doi: 10.1016/s0920-9964(98)00088-7. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr. Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. J. Abnorm. Psychol. 2010;119:18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-episode schizophrenia. Psychol. Med. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: impact of distractors. Schizophr. Res. 2007;89:339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophy-siology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc. Natl. Acad. Sci. USA. 2010;107:8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, Penn DL, Fredrickson BL, Kring AM, Meyer PS, Catalino LI, Brantley M. A pilot study of loving-kindness meditation for the negative symptoms of schizophrenia. Schizophr. Res. 2011;129:137–140. doi: 10.1016/j.schres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Joyce E, Hutton S, Mutsatsa S, Gibbins H, Webb E, Paul S, Robbins T, Barnes T. Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br. J. Psychiatry. 2002;(Suppl. 43):s38–s44. doi: 10.1192/bjp.181.43.s38. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berlin) 2006a;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Vill-ringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006b;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Goldstein MJ. Modification of performance on the span of apprehension, a putative marker of vulnerability to schizophrenia. J. Abnorm. Psychol. 1995;104:385–389. doi: 10.1037//0021-843x.104.2.385. [DOI] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr. Res. 2006;85:113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee KU, Lee SJ. Deficit in decision-making in chronic, stable schizophrenia: from a reward and punishment perspective. Psychiatry Invest. 2009;6:26–33. doi: 10.4306/pi.2009.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr. Bull. 2006;32:274–278. doi: 10.1093/schbul/sbi064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40:196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nomoto K, Watanabe M, Hikosaka O, Schultz W, Sakagami M. Influences of rewarding and aversive outcomes on activity in macaque lateral prefrontal cortex. Neuron. 2006;51:861–870. doi: 10.1016/j.neuron.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kring AM, Caponigro JM. Emotion in schizophrenia: where feeling meets thinking. Curr. Dir. Psychol. Sci. 2010;19:255–259. doi: 10.1177/0963721410377599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Earnst KS, Germans MK. Microexpressive facial activity and psychophysiological deficits in schizophrenia (unpublished manuscript) 1999a [Google Scholar]
- Kring AM, Kerr SL, Earnst KS. Schizophrenic patients show facial reactions to emotional facial expressions. Psycho-physiology. 1999b;36:186–192. [PubMed] [Google Scholar]
- Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu. Rev. Clin. Psychol. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. J. Abnorm. Psychol. 2011;120:79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am. J. Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J. Abnorm. Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim YT, Seo E, Park O, Jeong SH, Kim SH, Lee SJ. Dissociation of emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry. Res. 2007;152:113–120. doi: 10.1016/j.psychres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr. Res. 2012;142:65–70. doi: 10.1016/j.schres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Bucay D, Butman JT, Allegri RF. Neurop-sychological frontal impairments and negative symptoms in schizophrenia. Psychiatry. Res. 2007;152:121–128. doi: 10.1016/j.psychres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human stria-tum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Messinger JW, Tremeau F, Antonius D, Mendelsohn E, Prudent V, Stanford AD, Malaspina D. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin. Psychol. Rev. 2011;31:161–168. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Vercammen A, Lenroot R, Moore L, Langton JM, Short B, Kulkarni J, Curtis J, O’Donnell M, Weickert CS, Weickert TW. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol. Psychiatry. 2012;17(235):280–289. doi: 10.1038/mp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry. 2008;13:267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, Marten W. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr. Bull. 2000;26:847–853. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, Glenthoj B. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol. Psychiatry. 2012;71:898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Oades RD. Stimulus dimension shifts in patients with schizophrenia, with and without paranoid hallucinatory symptoms, or obsessive compulsive disorder: strategies, blocking and monoamine status. Behav. Brain Res. 1997;88:115–131. doi: 10.1016/s0166-4328(97)02304-8. [DOI] [PubMed] [Google Scholar]
- Oorschot M, Kwapil T, Delespaul P, Myin-Germeys I. Momentary assessment research in psychosis. Psychol. Assess. 2009;21:498–505. doi: 10.1037/a0017077. [DOI] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Wichers M, Myin-Germeys I. Mobile assessment in schizophrenia: a data-driven momentary approach. Schizophr. Bull. 2012;38:405–413. doi: 10.1093/schbul/sbr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Orbitofrontal cortex and the computation of economic value. Ann. N. Y. Acad. Sci. 2007;441:223–226. doi: 10.1196/annals.1401.011. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Cai X. The orbitofrontal cortex and the computation of subjective value: consolidated concepts and new perspectives. Ann. N. Y. Acad. Sci. 2011;1239:130–137. doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr. Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am. J. Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Penn DL, Combs D. Modification of affect perception deficits in schizophrenia. Schizophr. Res. 2000;46:217–229. doi: 10.1016/s0920-9964(00)00005-0. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J, d’Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006;29:302–313. doi: 10.1016/j.neuroimage.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Simmons A, Frangou S, Kumari V. Emotional decision-making and its dissociable components in schizophrenia and schizoaffective disorder: a behavioural and MRI investigation. Neuropsychologia. 2008;46:2002–2012. doi: 10.1016/j.neuropsychologia.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. Am. J. Psychiatry. 2005;162:1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Crespo-Facorro B, Perez-Iglesias R, Gonzalez-Blanch C, Alvarez-Jimenez M, Llorca J, Vazquez-Barquero JL. Prefrontal cognitive functions in stabilized first-episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophr. Res. 2005;77:279–288. doi: 10.1016/j.schres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity in primate orbitofrontal cortex reflects the value of time. J. Neurophysiol. 2005;94:2457–2471. doi: 10.1152/jn.00373.2005. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Stephan KE, den Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol. Med. 2009;39:199–209. doi: 10.1017/S0033291708003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur. J. Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006a;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat. Neurosci. 2006b;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci.: Off. J. Soc. Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BH, Shirley E, Rush-worth MF, Bannerman DM. Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur. J. Neurosci. 2007;26:2315–2326. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends. Cogn. Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Ann. N. Y. Acad. Sci. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berlin) 2007;191:389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berlin) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30:10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann. N. Y. Acad. Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berlin) 2008;196:673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Juckel G, Gallinat J, Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol. Psychiatry. 2009;65:1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry. Res. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Schultz W. Activity of dopamine neurons in the behaving primate. Semin. Neurosci. 1992;4:129–138. [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics, and behavioral ecology. Curr. Opin. Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr. Res. 2007;92:74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr. Res. 2005;72:215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. Neural correlates of reward processing in schizophrenia–relationship to apathy and depression. Schizophr. Res. 2009 doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr. Res. 2012;142:88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry. 2012;71:136–145. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Cacioppo JT, Ziwich R, Jalbrzikowski M, Saccente E, Silipo G, Butler P, Javitt D. In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophr. Res. 2009;107:223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Context-dependent representation of response-outcome in monkey prefrontal neurons. Cereb. Cortex. 2005;15:888–898. doi: 10.1093/cercor/bhh188. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Evans CE, Kemish K, Park S, Bowman CH. A novel set-shifting modification of the iowa gambling task: flexible emotion-based learning in schizophrenia. Neuropsychology. 2006;20:290–298. doi: 10.1037/0894-4105.20.3.290. [DOI] [PubMed] [Google Scholar]
- Tyson PJ, Laws KR, Roberts KH, Mortimer AM. Stability of set-shifting and planning abilities in patients with schizophrenia. Psychiatry. Res. 2004;129:229–239. doi: 10.1016/j.psychres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am. J. Psychiatry. 2011;168:276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Vollema MG, Geurtsen GJ, van Voorst AJ. Durable improvements in Wisconsin Card Sorting Test performance in schizophrenic patients. Schizophr. Res. 1995;16:209–215. doi: 10.1016/0920-9964(94)00079-n. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Walter H, Heckers S, Kassubek J, Erk S, Frasch K, Abler B. Further evidence for aberrant prefrontal salience coding in schizophrenia. Front. Behav. Neurosci. 2010;3:62. doi: 10.3389/neuro.08.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berlin) 2009;206:121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM, Rushworth MF. Calculating the cost of acting in frontal cortex. Ann. N. Y. Acad. Sci. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr. Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, Rose EJ, McClure SM, Stein EA. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, Gold JM, Stein EA. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neu-ropsychopharmacol. 2010;35:2427–2439. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr. Res. 1998;30:169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, Green MF. Impaired anticipatory event-related potentials in schizophrenia. Int. J. Psychophysiol.: Off. J. Int. Organ. Psychophysiol. 2010;77:141–149. doi: 10.1016/j.ijpsycho.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Sacco KA, George TP, Potenza MN. Risk/ reward decision-making in schizophrenia: a preliminary examination of the influence of tobacco smoking and relationship to Wisconsin Card Sorting Task performance. Schizophr. Res. 2009;110:156–164. doi: 10.1016/j.schres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalla T, Plassiart C, Pillon B, Grafman J, Sirigu A. Action planning in a virtual context after prefrontal cortex damage. Neuropsychologia. 2001;39:759–770. doi: 10.1016/s0028-3932(01)00019-7. [DOI] [PubMed] [Google Scholar]