Abstract
Overgeneral autobiographical memory (OGM) is a key memory deficit in major depressive disorder (MDD). Much research has examined cognitive mechanisms underlying OGM, but little work has investigated potential neurobiological influences. There is preliminary evidence that a genetic serotonergic vulnerability coupled with depressive symptoms may be associated with other memory impairments, and experimental research suggests a role for serotonin in OGM. We investigated whether a polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) was associated with OGM in interaction with a lifetime history of MDD in 370 young adults in a longitudinal study of risk for emotional disorders. There was a significant interaction between 5-HTTLPR genotype and lifetime history of MDD in predicting OGM. Among S allele homozygotes, MDD history was associated with greater OGM, whereas no significant relationship between MDD history and OGM emerged among L carriers. Furthermore, there was evidence that a greater number of S alleles was associated with greater memory specificity in individuals without a history of MDD. Implications for understanding cognitive and biological risk for depression are discussed.
Keywords: Overgeneral autobiographical memory, Autobiographical memory specificity, 5-HTTLPR, Depression, Genetic association
Over the past 25 years, the degree of specificity with which individuals recall their personal past has been established as a cognitive phenomenon of significance for depression (see Williams et al., 2007, for a review). When asked to retrieve a specific autobiographical memory in response to a cue word, individuals with depression tend to be less specific or more overgeneral in their recollections than healthy controls. Rather than recalling a specific event that occurred at a particular time and place and lasted for less than one day (e.g., “My first day of school”) as instructed, individuals with depression are more likely to recall memories that are summaries or classes of events (categoric memories, e.g., “school dances”) or memories for events lasting longer than one day (extended memories, e.g., “My trip to Hawaii”). Overgeneral autobiographical memory (OGM) not only has a robust association with current depression, but it is also predictive of the course of current depression, as well as future depressive episodes. For example, greater OGM predicts more prolonged depressive episodes and recurrence of depressive episodes (e.g., Sumner et al., 2011; Williams et al., 2007).
Given evidence that OGM may predict a worse prognosis of depression over time, researchers have been interested in elucidating the factors that contribute to this phenomenon. Most research on the mechanisms underlying OGM has focused on cognitive processes, such as rumination, cognitive avoidance, or impairments in executive control (see Sumner, 2012; Williams et al., 2007, for reviews). However, relatively little is known about neurobiological mechanisms underlying OGM, especially genetic influences. There are no published twin studies estimating the heritability of OGM, but there is evidence for at least a moderate genetic basis for memory recall more broadly. Genetic factors are estimated to contribute to 45–50% of the variance in verbal and nonverbal memory recall, with the remainder of the variance being explained by nonshared/unique environmental factors (e.g., Finkel & McGue, 1998). Thus, behavioral or molecular genetic approaches to studying the neurobiological mechanisms of OGM may be fruitful.
Serotonergic Vulnerability, Episodic Memory, and Depression
As summarized by Gibb, Beevers, and McGeary (2012), one promising approach for identifying genetic systems that may contribute to cognitive biases in depression is to utilize knowledge of the biological systems that underlie these phenotypes and then investigate genes associated with those biological systems. Based on this approach, serotonin system dysregulation may be one mechanism that contributes to associations between OGM and depression. It is well established that serotonin is involved in episodic memory (which includes autobiographical memory; e.g., Sambeth et al., 2007) and depression (e.g., Albert, Benkelfat, & Descarries, 2012). Support for a role for serotonin in episodic memory and depression comes, in part, from studies employing the acute tryptophan depletion (ATD) paradigm. ATD is thought to lower brain serotonin functioning by experimentally reducing the availability of tryptophan, the amino acid precursor of serotonin (e.g., Moreno et al., 1999). ATD produces impairments in both immediate and delayed declarative episodic memory in humans compared to sham depletion control conditions (Sambeth et al., 2007). Furthermore, ATD has been found to induce depressive symptoms compared to sham depletion (see Booij et al., 2002, for a review).
Theory suggests that a history of MDD may be a proxy, in part, for an underlying serotonergic vulnerability (e.g., Moreno et al., 1999), and those with prior MDD may thus be especially likely to respond to a serotonergic insult, such as ATD. Consistent with this prediction, the effects of ATD on memory and mood are enhanced in individuals with a history of MDD. For example, individuals with a history of MDD exhibited worse initial memory recall on an auditory-verbal learning task than healthy controls after ATD (but not after sham depletion; Hayward, Goodwin, Cowen, & Harmer, 2005). Also, those with a history of MDD generally report increased depressive symptoms compared to healthy controls following ATD (but not after sham depletion; Booij et al., 2002). Indeed, a history of recurrent depressive episodes is a powerful predictor of mood decrements after ATD (Booij et al., 2002). One potential explanation for these findings is that ATD may primarily produce depressogenic behavioral responses when it challenges an already vulnerable serotonergic system (e.g., as indicated by a history of depression) beyond a certain threshold.
Given the associations between episodic memory, depression, and the serotonin system, researchers have begun to examine associations between serotonin and OGM in particular. For example, Haddad, Williams, McTavish, and Harmer (2009) randomized women with a history of MDD to either ATD or sham depletion, and OGM was assessed before and after the manipulation. They found a greater reduction in memory specificity in participants who underwent ATD compared to those who underwent sham depletion. Although preliminary, the results of Haddad et al. (2009) are consistent with a role for serotonin in OGM.
Genetic Influences on Serotonergic Vulnerability and OGM
To our knowledge, little research has examined the relationship between genetic influences on serotonergic vulnerability and OGM. One candidate gene is the common, functional 44-base pair insertion/deletion polymorphism (5-HTTLPR) in the promoter region of the serotonin transporter gene (SLC6A4). The serotonin transporter is involved in the regulation of reuptake of serotonin to the presynaptic neuron. The short (S) allele of 5-HTTLPR confers reduced reuptake capacity compared to the long (L) allele, thus resulting in greater synaptic serotonin concentrations and postsynaptic insensitivity to serotonin. Also, the S allele serves as a risk factor for depression in the context of elevated life stress relative to the long (L) allele (see Karg, Burmeister, Shedden, & Sen, 2011, for a review).
There is evidence from several studies that S allele carriers exhibit biased attention for emotional stimuli, greater negative attributional styles, and more negative self-referent memory biases (see Gibb et al., 2012, for a review). These biases have been observed in emotional disorders, and Gibb et al. (2012) review evidence suggesting that the genetic association with these information processing biases may be amplified in the context of depressed mood. In other words, genetic influences and certain aspects of emotional experience may interact in contributing to information processing biases. Furthermore, there is initial support that 5-HTTLPR genotype and depression contributed to episodic memory recall in interaction. Specifically, Price et al. (2013) found that among S carriers, depressive symptoms were inversely related to immediate episodic memory in young adults, whereas there was a slight positive relationship in L homozygotes. Although Price et al.’s (2013) study did not examine OGM specifically, these findings nevertheless suggest that 5-HTTLPR genotype coupled with depression may be associated with at least certain aspects of memory recall.
To date, only one study has examined 5-HTTLPR and OGM. In a sample of healthy undergraduates, Lemogne et al. (2009) found that S carriers exhibited a similar degree of OGM compared to L homozygotes, and the relationship between 5-HTTLPR genotype and OGM was not significant. However, these results should be interpreted with caution, given the relatively small sample size (N = 60) and consequent low power for detecting genetic effects. Additionally, Lemogne et al. (2009) did not consider potential associations in those with depression.
Aims of the Current Study
The goal of the current study was to evaluate the association between 5-HTTLPR and OGM in 370 young adult participants in the Northwestern-UCLA Youth Emotion Project (YEP), a longitudinal study of risk for emotional disorders (see Zinbarg et al., 2010 for details). We hypothesized that OGM would be greatest in those with a genetic serotonergic vulnerability (i.e., those with the S allele of 5-HTTLPR) and a history of MDD. This prediction was based on findings supporting a role for serotonin in OGM (e.g., Haddad et al., 2009) and that the genetic association with information processing biases may be amplified within the context of depression (Gibb et al., 2012), in addition to the theory that a history of MDD may represent, in part, an underlying serotonergic vulnerability.
Method
Participants
Participants were from a larger sample of young adults in a 10-year longitudinal study (baseline plus 7–9 years of follow-up, depending on participant cohort) of risk for emotional disorders (the Youth Emotion Project, YEP; see Zinbarg et al., 2010, for details). High school juniors in suburban Chicago or Los Angeles were recruited in three cohorts from 2003–2005. At screening, participants completed the Eysenck Personality Questionnaire neuroticism scale (EPQ-R-N; Eysenck & Eysenck, 1975), and were categorized by tertiles as low-, medium- or high-scorers. High-EPQ-R-N-scorers were oversampled to obtain a high-risk sample for the development of emotional disorders (59% of the original sample of 627 participants were high-EPQ-R-N-scorers).
Beginning in the sixth year of the YEP, participants were invited to provide a DNA sample and complete an assessment of OGM. A total of 407 individuals consented to and completed both of these assessments. Participants who completed the OGM assessment but did not provide a DNA sample (n = 59) did not differ significantly from those who completed both assessments in terms of gender, race/ethnicity, neuroticism at baseline, age at OGM assessment, OGM, or lifetime history of MDD, all ps > .05. Participants were excluded from analyses a priori if they were diagnosed with a current or past history of clinically significant bipolar disorder I or II (n = 6), posttraumatic stress disorder (n = 13), acute stress disorder (n = 1), major depression due to a general medical condition (n = 1), substance-induced mood disorder (n = 1), dysthymia (n = 16), or psychotic symptoms (n = 4).1 Thirty-seven individuals met one or more of these exclusion criteria, resulting in a final sample size of 370.
Similar to in the entire sample, participants in this subsample were predominantly female (69%) and racially and ethnically diverse (12.2% African American, 4.6% Asian American, 0.8% Pacific Islander, 49.5% Caucasian, 14.3% Hispanic/Latino, 12.7% Multiracial, and 5.9% Other). Participants were 20 to 24 years of age (M = 22.4, SD = 0.9) at the time of OGM assessment. Power to detect a significant Genotype × Depression interaction effect was estimated to be .77 based on a small effect size for the interaction effect (f2 = .02), an alpha level of .05, and a sample size of 370.
Measures
Autobiographical Memory Test (AMT)
The AMT (Williams & Broadbent, 1986) uses a cuing paradigm to elicit autobiographical memories. The AMT was administered twice: once approximately 9 months after the baseline assessment of the study and once approximately 5 years after the baseline assessment (see Griffith et al., 2009, and Sumner et al., 2011, for published data from the first AMT and Sumner et al., 2013, for published data from the first and second AMTs). Only a subsample of participants was invited to complete the first AMT (n = 333, out of whom 245 later provided a DNA sample), whereas all participants were offered the opportunity to complete the second AMT. In this study, we report data from the second AMT because of the larger sample, therefore providing a more powerful test of the relationship between 5-HTTLPR, history of MDD, and OGM.
The second AMT was administered over the telephone by trained undergraduate students, graduate students, and Bachelor’s-level research assistants. Participants were instructed to retrieve a specific autobiographical memory in response to cue words and had 30 seconds to respond on each trial. Up to seven practice items were administered; participants were required to either retrieve two consecutive specific memories or complete all seven practice trials before proceeding to the test trials. Feedback was only given on practice items. There were 12 test trials that alternated between positive (safe, ambitious, peace, hope, brave, interested) and negative (disappoint, inferior, hurt, frustrated, tense, regret) cues. Responses were made orally and audio-recorded.
Responses were scored as specific memories (events that occurred at a particular time and place and lasted less than one day), extended memories (events lasting more than one day), categoric memories (summaries/classes of events), semantic associates (responses containing general semantic information but no personal memory), or omissions (no response). Inter-rater reliability of this scoring approach was good overall; mean kappas for within-site (N = 46) and cross-site (N = 46) reliability were .77. We used the proportion of specific memories as our measure of OGM (e.g., Sumner et al., 2011). We collapsed responses across positive and negative cue words given robust support for a unidimensional model of OGM (e.g., Griffith et al., 2009). The proportion of specific memories was calculated by dividing the number of specific memories by 12 (i.e., omissions were counted as nonspecific memories).
Structured Clinical Interview for DSM-IV (SCID)
Trained advanced graduate students and Bachelor’s-level research assistants administered the SCID (First, Spitzer, Gibbon, & Williams, 2002) to assign Axis I diagnoses at baseline and annual follow-up assessments. Interviewers’ assessments were supervised by doctoral-level clinical psychologists. SCID data were used to classify participants as those with or without a lifetime history of MDD at the second AMT. Kappas and adjusted kappas for clinically significant MDD diagnoses for the baseline and first four annual follow-up assessments ranged from .56–.83 and .84–.94, respectively.2
Genotyping
Participants provided saliva samples for DNA collection using Oragene® kits (DNA Genotek, Ontario, Canada) at home and then mailed the samples via the postal system to YEP study offices. Oragene® kits contain a preservative that keeps the DNA samples stable at room temperature for at least five years. DNA extraction was performed by Kbioscience (United Kingdom), and genotyping of 5-HTTLPR was conducted by the UCLA Core Genetics Lab based on a previously modified published protocol described elsewhere (Taylor et al., 2006). We coded 5-HTTLPR genotype under an additive model. We report results based on traditional 5-HTTLPR genotypes first, followed by results for reclassification of 5-HTTLPR genotype based on the single nucleotide polymorphism (SNP) rs25531 as an analysis of secondary interest. Some research suggests that this adenine to guanine (A/G) SNP may modify the function of a subset of L alleles. Specifically, the combination of the L allele of 5-HTTLPR and the G allele of rs25531 (LG) may result in a reduced level of transporter expression that is similar to that of the S allele, and the LA variant may only show greater transcriptional activity (Hu et al., 2005; Wendland, Martin, Kruse, Lesch, & Murphy, 2006). However, several published reports have not supported this notion (e.g., Martin, Cleak, Willis-Owen, Flint, & Shifman, 2007; Philibert et al., 2008). Three individuals were not successfully genotyped for rs25531 and were excluded from models using this SNP.
Procedure
At the YEP baseline assessment, a lifetime SCID was administered to assess Axis I psychopathology (additional measures not relevant to the current study were administered at baseline as well). The SCID was also administered at annual follow-up assessments to assess Axis I psychopathology since the previous interview. Starting in the sixth year of the study, participants (who had been enrolled in the study for 4 to 6 years) provided a DNA sample and completed the AMT.
Results
Descriptive Statistics for Participants
Descriptive statistics as a function of 5-HTTLPR genotype status are presented in Table 1. Genotype groupings were as follows: 19.5% S/S (n = 72), 49.7% S/L (n = 184), and 30.8% L/L (n = 114). Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium, χ2 ≤ 1.06, ns. In the full sample of participants (N = 370), the mean proportion of specific memories generated on the AMT was .71 (SD = .21, range = .08–1.00). At the time of the AMT, 36.5% of participants had a history of MDD (n = 135). As shown in Table 1, there were no significant differences between S/S, S/L, and L/L genotype groups in terms of age at the time of the AMT, gender, race/ethnicity (Caucasian vs. other), history of MDD, or OGM.
Table 1.
Descriptive Statistics as a Function of 5-HTTLPR Genotype
| 5-HTTLPR Genotype | ||||
|---|---|---|---|---|
| Variable | S/S (n = 72) | S/L (n = 184) | L/L (n = 114) | |
| Age at OGM assessment | 22.4 years (1.0) | 22.4 years (0.9) | 22.3 years (0.9) | p = .77 |
| Percentage female | 65.3% | 69.0% | 70.2% | p = .77 |
| Percentage Caucasian | 44.4% | 50.0% | 51.8% | p = .61 |
| Percentage with a history of MDD | 43.1% | 34.2% | 36.0% | p = .42 |
| Proportion of specific memories | .73 (.22) | .71 (.20) | .69 (.23) | p = .52 |
Note. Means and standard deviations in parentheses are presented for the age and OGM variables. OGM = Overgeneral autobiographical memory. MDD = Major depressive disorder.
5-HTTLPR and History of MDD as Predictors of OGM
We used hierarchical linear regression to examine whether the interaction between 5-HTTLPR genotype and MDD history significantly predicted the proportion of specific memories generated on the AMT (each predictor was added on its own step over and above the effects of participant age and gender). Neither the main effect of 5-HTTLPR genotype (number of S alleles), F change(1, 366) = 1.51, p = .22, b = 0.02, SE(b) = 0.02, β = 0.06, t = 1.23, p = .22, nor the main effect of history of MDD, F change(1, 365) = .02, p = .89, b = 0.003, SE(b) = 0.02, β = 0.01, t = 0.15, p = .89, was significant. However, the interaction of 5-HTTLPR genotype and history of MDD was significant, F change(1, 364) = 4.00, p = .047, b = −0.07, SE(b) = 0.03, β = −0.19, t = −2.00, p = .047.3
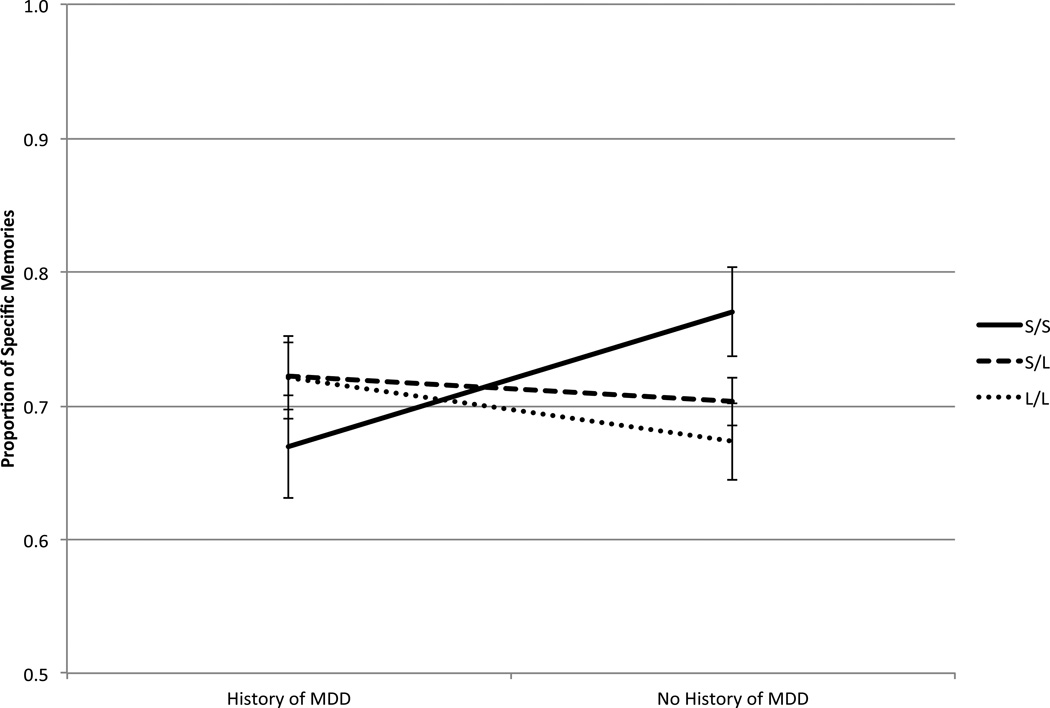
The interaction was decomposed by examining simple main effects. Among those with zero (n = 114) or one (n =184) S alleles, history of MDD was not significantly related to the proportion of specific memories, b = 0.04, SE(b) = 0.05, β = 0.09, t = 0.91, p = .36 and b = 0.02, SE(b) = 0.03, β = 0.05, t = 0.64, p = .53, respectively. In contrast, among those with two S alleles (n = 72), history of MDD was a significant predictor of the proportion of specific memories, b = −0.14, SE(b) = 0.05, β = −0.32, t = −2.74, p = .01. As shown in Figure 1, S homozygotes with a history of MDD exhibited a lower mean proportion of specific memories (M = 0.67, SD = 0.22) than S homozygotes without a history of MDD (M = 0.77, SD = 0.21). We further probed the 5-HTTLPR Genotype × History of MDD interaction by examining the relationship between the number of S alleles and the proportion of specific memories separately in those with and without a history of MDD. In those without a history of MDD (n = 235), a greater number of S alleles was significantly associated with a greater proportion of specific memories, b = 0.04, SE(b) = 0.02, β = 0.14, t = 2.12, p = .04. In those with a history of MDD (n = 135), there was an inverse association between the number of S alleles and the proportion of specific memories, although this was not significant, b = −0.03, SE(b) = 0.02, β = −0.09, t = −1.04, p = .30.
Figure 1.
Proportion of specific memories retrieved on the AMT as a function of 5-HTTLPR genotype and history of MDD. Error bars represent standard error of the mean. For clarity of presentation, the range of the proportion of specific memories extends from 0.50 to 1.00. Even though this variable ranged from 0.08 to 1.00 in the full sample, almost 87% of the sample had values greater than or equal to 0.50. Sample sizes for the subgroups are as follows: S/S genotype: history of MDD (n = 31) and no history of MDD (n = 41); S/L genotype: history of MDD (n = 63) and no history of MDD (n = 121); L/L genotype: history of MDD (n = 41) and no history of MDD (n = 73).
Given our relatively diverse sample, we addressed population stratification (i.e., the presence of systematic differences in allele frequencies as a function of subpopulations in the sample; e.g., Tang et al., 2005). Black race and Hispanic ethnicity (the two largest minority groups in our sample), as well as their two-way interactions with 5-HTTLPR genotype and history of MDD, were covaried to partial out the influence of group membership from the interaction effect. The interaction of history of MDD with 5-HTTLPR genotype had an almost identical effect size as before and approached, but fell short of, the conventional level of statistical significance in this model, b = −0.06, SE(b) = 0.03, β = −0.18, t = −1.91, p = .06. In addition, the 5-HTTLPR Genotype × History of MDD interaction had a highly similar effect size and approached, but did not reach, conventional statistical significance when including the main effect of gender and two-way interactions between gender and 5-HTTLPR genotype and history of MDD, b = −0.06, SE(b) = 0.04, β = −0.16, t = −1.78, p = .08. These findings suggested that the interaction between 5-HTTLPR genotype and MDD history was not accounted for by Black race, Hispanic ethnicity, or gender.
Effect of rs25531 Reclassification
As an analysis of secondary interest, 5-HTTLPR L alleles were reclassified based on the rs25531 A/G SNP given that some, but not all, research has found that the LG allele may function similarly to the S allele. In this regression model, 5-HTTLPR genotype was represented by the number of S/LG alleles. Reclassification by rs25531 led to 27 individuals (7.4% of participants with rs25531 genotype data) traditionally classified as having zero S alleles being considered as having one (n = 22) or two (n = 5) S/LG alleles and 31 individuals (8.4% of participants with rs25531 genotype data) traditionally classified as having one S allele being considered as having two S/LG alleles. No significant main effects or 5-HTTLPR Genotype × History of MDD interaction emerged in this model: F change(1, 363) = 0.11, p = .75, b = 0.01, SE(b) = 0.02, β = 0.02, t = 0.32, p = .75, for the main effect of 5-HTTLPR genotype based on rs25531; F change(1, 362) = 0.011, p = .94, b = 0.002, SE(b) = 0.02, β = 0.004, t = 0.07, p = .94, for the main effect of history of MDD; F change(1, 361) = 1.04, p = .31, b = −0.03, SE(b) = 0.03, β = −0.11, t = −1.02, p = .31, for the 5-HTTLPR Genotype × History of MDD interaction. In other words, no significant effects were detected with the triallelic classification of 5-HTTLPR.
Discussion
To our knowledge, this is the first study to examine associations among 5-HTTLPR genotype, a history of major depressive disorder (MDD), and overgeneral autobiographical memory (OGM). In addition, this is the largest investigation of 5-HTTLPR and OGM to date. Among individuals with the S/S 5-HTTLPR genotype, those with a history of MDD exhibited lower levels of memory specificity than those without a history of MDD. In contrast, there was no significant relationship between MDD history and OGM in 5-HTTLPR L allele carriers. Furthermore, there was evidence that a greater number of S alleles was associated with greater memory specificity in individuals without a history of MDD.
The significant association between history of MDD and OGM among S homozygotes is consistent with the notion that variation in intermediate phenotypes, such as OGM, may be more readily observed in S homozygotes (Uher & McGuffin, 2008). (Intermediate phenotypes are dependent variables or “markers” of disorder risk that may be more proximally related to biological functioning, and therefore also genetic risk, than more distal dependent variables like depression.) In addition, our finding adds to the body of literature on Genotype × Depression interactions in predicting cognitive functioning, including aspects of memory. For example, Price et al. (2013) found that among carriers of the S allele of 5-HTTLPR (and not among L homozygotes), greater depressive symptoms were associated with worse immediate memory.
Although the precise pattern of our findings was somewhat unexpected, we believe that our results are consistent with Belsky and Pluess’s (2009) differential susceptibility to environmental influence hypothesis, which posits that genetic variants that may be maladaptive in harsh environments may also be beneficial in normal or supportive environments. In other words, those carrying a particular genetic variant may function worse than those without that variant when exposed to a risky environment but they may also function better than those without that variant in the absence of the risky environment. A growing body of evidence suggests that 5-HTTLPR genotype is associated with greater sensitivity or plasticity to environmental influences, such that carriers of the S allele may exhibit either enhanced or diminished functioning depending on their experience. For example, in Caspi et al.’s (2003) seminal study on 5-HTTLPR, stressful life events, and depression, those with two S alleles reported the highest level of depressive symptoms (compared to those with one or two L alleles) in the context of four or more stressful life events but they reported the lowest level of depressive symptoms in the absence of any reported stressful life events. Several studies have replicated this pattern of results with depression and extended them to anxiety, such that those with S alleles report higher levels of depression or anxiety compared to those with L alleles within the context of a history of adversity but they report lower levels of depression or anxiety compared to those with L alleles in the absence of such adversity (see Belsky & Pluess, 2009, for a review).
Our finding that a history of MDD was associated with OGM primarily in those with two copies of the S allele can be understood within the context of this differential susceptibility framework. In this framework, depression may be a proxy, in part, for certain environmental experiences. Such a conceptualization is supported by evidence that depression predicts increased stress over and above what is predicted by stress levels at baseline (i.e., stress generation; e.g., Hammen, 1991; Liu & Alloy, 2010; Uliaszek et al., 2012). Further evidence for this notion comes from findings that depressed individuals display increased levels of maladaptive interpersonal behaviors (e.g., excessive reassurance seeking) that are thought to erode their social support networks and elicit interpersonal rejection (e.g., Joiner & Timmons, 2009). In those without a history of MDD (construed as a proxy, in part, for having normal or adaptive experiences), a greater number of S alleles was associated with greater memory specificity—a cognitive phenomenon associated with several benefits, including enhanced interpersonal problem-solving (e.g., Goddard, Dritschel, & Burton, 1996). However, in those with a history of MDD (construed as a proxy, in part, for having more maladaptive experiences), a greater number of S alleles was associated with lower memory specificity, although this relationship did not reach statistical significance. Effect sizes for genetic influences on information processing to date have been found to be small (e.g., Gibb et al., 2012), and our sample did not provide sufficient power for this follow-up test within those with a history of MDD (n = 135; power estimated to be 0.27). Nevertheless, our results suggest that the 5-HTTLPR S/S genotype may be associated with differential memory functioning depending on the nature of the emotional experience.
Although the mechanisms underlying these findings have yet to be elucidated, we suggest one potential explanation for these observed relationships. The 5-HTTLPR S allele has been associated with attention to emotional stimuli in general (Gibb et al., 2012). It is possible that this attentional bias may contribute to enhanced encoding of detail and subsequent greater specificity in memory recollection among individuals with the S/S genotype (and, at least in part, potentially explain some of the greater susceptibility to environmental influence associated with this genotype). However, among those with the S/S genotype, the experience of depression may lead to memory-related brain changes that impair memory specificity. In accord, repeated episodes of depression have been shown to contribute to loss of hippocampal volume through stress toxicity, reduced neurotrophic factors, and excess glutamatergic neurotransmission (see MacQueen & Frodl, 2011, for a review). In line with the notion that the 5-HTTLPR S allele may be associated with greater susceptibility to environmental experience, individuals with MDD with the S allele of 5-HTTLPR may be especially susceptible to hippocampal damage. Frodl et al. (2010) found that MDD patients with the S allele who reported a history of childhood stress (defined as high levels of emotional neglect) exhibited smaller hippocampal volume than patients with only one risk factor (either S carrier status or a history of emotional neglect). Although the biological underpinnings for why individuals with the S allele of 5-HTTLPR might be more susceptible to hippocampal damage have yet to be elucidated, serotonin transporter knockout rats have been found to exhibit alterations in synaptic plasticity and stress responsiveness compared to wild-type rats, which could contribute to changes in hippocampal structure (see Frodl et al., 2010). Recollection memory is highly dependent on the hippocampus (MacQueen & Frodl, 2011), and thus greater OGM may be a manifestation of changes at the neural level after depressive episodes. Nevertheless, further research is needed to replicate our findings and to directly examine potential mechanisms in order to better understand what may be underlying these associations.
Our results differ from reports of associations between 5-HTTLPR genotype and other cognitive biases (e.g., attentional bias) in healthy populations (e.g., Gibb et al., 2012). That is, we found differential levels of OGM only when both the S/S genotype and a history of MDD were taken into consideration. However, there are also some other findings that 5-HTTLPR genotype may be associated with cognitive biases primarily in those with increased vulnerability to depression, such as those with a history of maternal depression (see Gibb et al., 2012). More research comparing associations between 5-HTTLPR genotype and cognitive biases in those with and without a history of MDD is warranted in order to better understand these associations.
Furthermore, consistent with two other non-replications of the effect of rs25531 (Martin et al., 2007; Philibert et al., 2008), we found that reclassification of 5-HTTLPR genotype based on this SNP did not strengthen results and yielded somewhat smaller effect size estimates compared to the traditional 5-HTTLPR genotype. This finding may indicate that rs25531 does not modify the function of the traditional 5-HTTLPR genotype in the manner that some researchers have proposed (Hu et al., 2005; Wendland et al, 2006). A second potential explanation is that reclassification of 5-HTTLPR genotype based on rs25531 reduced power to obtain a significant interaction effect. Reclassifying traditional 5-HTTLPR genotypes resulted in a smaller number of participants in the LA/LA group (i.e., the reference group of those with no S/LG alleles; n = 86; 23.4% of those with valid genotype data based on rs25531) compared to the original L/L group (n = 114; 30.8% of those with valid genotype data for 5-HTTLPR). This change decreased the effective minor allele frequency, and lower minor allele frequencies provide reduced power to detect an interaction effect (Luan, Wong, Day, & Wareham, 2001). Until there is greater clarity on the influence of rs25531, it may be helpful for future studies of 5-HTTLPR to analyze data with and without reclassification of 5-HTTLPR by this SNP.
In spite of several strengths including clinical diagnostic interview assessments of MDD history and the largest genetically-informed sample of AMT completers to date, the current investigation has several limitations. First, as with all studies of genetic risk factors conducted on racially and ethnically heterogeneous samples, population stratification is a concern. However, this concern is partially mitigated by the fact that the 5-HTTLPR Genotype × MDD History interaction remained similar in effect size when Black race and Hispanic ethnicity (the two largest minority groups in our sample) and each of their two-way interactions with 5-HTTLPR genotype and MDD history were covaried in the model. Even though population stratification is best detected through direct modeling (e.g., by use of unlinked marker loci), research suggests that self-identified race and ethnicity correspond highly with such genetic clustering approaches for major ethnic groups in the United States (Tang et al., 2005). Second, it is possible that another functional genetic marker in linkage disequilibrium with 5-HTTLPR, rather than 5-HTTLPR itself, could be accounting for the observed relationships. Third, we only examined history of MDD, rather than vulnerability for initial MDD, so we were unable to differentiate whether the experience of MDD or factors conferring risk for MDD were responsible for the 5-HTTLPR Genotype × History of MDD interaction in predicting OGM.
Despite these limitations, we believe that our findings provide further insight into the underlying influences on OGM. Our results indicate that the 5-HTTLPR genotype and a history of MDD may interact to contribute to one aspect of memory functioning. Future research should examine the mechanisms underlying these relationships and investigate the extent to which genetic influences on serotonergic vulnerability, past depression history, and OGM all relate to risk for future depression onsets.
Acknowledgements
We would like to thank the National Institute of Mental Health (Grant# R01 MH065652 to Drs. Mineka and Zinbarg, R01 MH065651 to Dr. Craske, and F31 MH088014 to Dr. Sumner), and an Institute for Policy Research, Northwestern University, Faculty Fellowship (to Dr. Adam) for supporting our research. We also gratefully acknowledge the assistance of the many students who helped with data collection.
Footnotes
OGM has been associated with trauma-related psychopathology (see Williams et al., 2007 for a review), and thus we chose to exclude the small number of individuals who met criteria for posttraumatic stress disorder and acute stress disorder from these analyses. We also excluded those with a history of bipolar disorder, major depression due to a general medical condition, substance-induced mood disorder, and dysthymia in order to have a relatively homogenous sample of those with a history of unipolar major depression.
Reliability of categorical SCID diagnoses was assessed with both Cohen’s kappa and adjusted kappa due to uneven prevalence of counts in the categories being compared (specifically, low base rates of cases with disorders relative to cases with no disorders). This imbalance has been found to attenuate Cohen’s kappa. An adjusted kappa adjusts cell frequencies to evenly distribute the prevalence of cases across categories.
When we excluded the three participants who were in a current major depressive episode at the time of the AMT, the 5-HTTLPR Genotype × History of MDD interaction remained statistically significant with at least as large a standardized effect size, F change(1, 361) = 4.49, p = .04, b = −0.07, SE(b) = 0.03, β = −0.20, t = −2.12, p = .04. Thus, this interaction did not seem to be due to current MDD status at the AMT.
References
- Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philosophical Transactions of the Royal Society B. 2012;367:2378–2381. doi: 10.1098/rstb.2012.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Booij L, Van der Does W, Benkelfat C, Bremner D, Cowen PJ, Fava M, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion: A reanalysis. Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire (adult and junior) London: Hodder & Stoughton; 1975. [Google Scholar]
- Finkel D, McGue M. Age differences in the nature and origin of individual differences in memory: A behavior genetic analysis. International Journal of Aging and Human Development. 1998;47:217–239. doi: 10.2190/JEPX-G60A-6QK3-9DR3. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I disorders, research version, non-patient edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, Meisenzahl EM. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2012 doi: 10.1080/02699931.2012.712950. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard L, Dritschel B, Burton A. Role of autobiographical memory in social problem solving and depression. Journal of Abnormal Psychology. 1996;105:609–616. doi: 10.1037//0021-843x.105.4.609. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Sumner JA, Debeer E, Raes F, Hermans D, Mineka S, Craske MG. An item-response theory/confirmatory factor analysis of the Autobiographical Memory Test. Memory. 2009;17:609–623. doi: 10.1080/09658210902939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad ADM, Williams JMG, McTavish SFB, Harmer CJ. Low-dose tryptophan depletion in recovered depressed women induces impairments in autobiographical memory specificity. Psychopharmacology. 2009;207:499–508. doi: 10.1007/s00213-009-1693-2. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Cowen PJ, Harmer CJ. Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biological Psychiatry. 2005;57:517–524. doi: 10.1016/j.biopsych.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Timmons KA. Depression in its interpersonal context. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: Guilford Press; 2009. pp. 322–339. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Bergouignan L, Boni C, Gorwood P, Pélissolo A, Fossati P. Genetics and personality affect visual perspective in autobiographical memory. Consciousness and Cognition. 2009;18:823–830. doi: 10.1016/j.concog.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan JA, Wong MY, Day NE, Wareham NJ. Sample size determination for studies of gene-environment interaction. International Journal of Epidemiology. 2001;30:1035–1040. doi: 10.1093/ije/30.5.1035. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression Evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SAG, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Molecular Psychiatry. 2007;12:421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight KM, Allen J, Delgado PL. Tryptophan depletion and depressive vulnerability. Biological Psychiatry. 1999;46:498–505. doi: 10.1016/s0006-3223(99)00095-5. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Gunter TD, Beach SRH, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:565–570. doi: 10.1002/ajmg.b.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, Strong J, Eliassen J, McQueeny T, Miller M, Padula CB, Lisdahl K. Serotonin transporter gene moderates associations between mood, memory, and hippocampal volume. Behavioural Brain Research. 2013;242:158–165. doi: 10.1016/j.bbr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambeth A, Blokland A, Harmer CJ, Kilkens TOC, Nathan PJ, Porter RJ, Riedel WJ. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: A pooled analysis of nine studies. Neuroscience and Biobehavioral Reviews. 2007;31:516–529. doi: 10.1016/j.neubiorev.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Sumner JA. The mechanisms underlying overgeneral autobiographical memory: An evaluative review of evidence for the CaR-FA-X model. Clinical Psychology Review. 2012;32:34–48. doi: 10.1016/j.cpr.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Griffith JW, Mineka S, Rekart KN, Zinbarg RE, Craske MG. Overgeneral autobiographical memory and chronic interpersonal stress as predictors of the course of depression in adolescents. Cognition and Emotion. 2011;25:183–192. doi: 10.1080/02699931003741566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Mineka S, Zinbarg RE, Craske MG, Vrshek-Schallhorn S, Epstein A. Examining the long-term stability of overgeneral autobiographical memory. Memory. 2013 doi: 10.1080/09658211.2013.774021. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SLR, Zhu X, Brown A, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton JM, Hammen C. A longitudinal examination of stress generation in depressive and anxiety disorders. Journal of Abnormal Psychology. 2012;121:4–15. doi: 10.1037/a0025835. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch K-P, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, Hermans D, Raes F, Watkins E, Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychological Bulletin. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Broadbent K. Autobiographical memory in suicide attempters. Journal of Abnormal Psychology. 1986;95:144–149. doi: 10.1037//0021-843x.95.2.144. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton J, Rose RD, Waters AM. The Northwestern-UCLA youth emotion project: Associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]