Abstract
Background
Depression affects 18-30% of HIV-infected patients in Africa and is associated with greater stigma, lower antiretroviral adherence, and faster disease progression. However, the region's health system capacity to effectively identify and treat depression is limited. Task-shifting models may help address this large mental health treatment gap.
Methods
Measurement-Based Care (MBC) is a task-shifting model in which a Depression Care Manager (DCM) guides a non-psychiatric (e.g., HIV) provider in prescribing and managing antidepressant treatment. We adapted MBC for depressed HIV-infected patients in Cameroon and completed a pilot study to assess feasibility, safety, acceptability, and preliminary efficacy.
Results
We enrolled 55 participants; all started amitriptyline 25-50mg daily at baseline. By 12 weeks, most remained at 50mg daily (range 25-125mg). Median (interquartile range) PHQ-9 depressive severity scores declined from 13 (12-16) (baseline) to 2 (0-3) (week 12); 87% achieved depression remission (PHQ9<5) by 12 weeks. Intervention fidelity was high: HIV providers followed MBC recommendations at 96% of encounters. Most divergences reflected a failure to increase dose when indicated. No serious and few bothersome side effects were reported. Most suicidality (prevalence: 62% at baseline; 8% at 12 weeks) was either passive or low-risk. Participant satisfaction was high (100%), and most participants (89%) indicated willingness to pay for medications if MBC were implemented in routine care.
Conclusions
The adapted MBC intervention demonstrated high feasibility, safety, acceptability, and preliminary efficacy in this uncontrolled pilot study. Further research should assess whether MBC could improve adherence and HIV outcomes in this setting.
Keywords: HIV, depression, depression treatment, intervention, Measurement-Based Care, Africa
Introduction
Depression is a common comorbidity among HIV-infected individuals in sub-Saharan Africa, with important implications for the success of HIV medical care. Depression affects an estimated 18-30% of patients receiving HIV care in Africa.(1) Among HIV-infected patients in Africa, depression is associated with greater perceived stigma, lower antiretroviral medication adherence, tuberculosis comorbidity, and more advanced disease stage.(1-3) This growing literature among African populations is consistent with a much deeper literature delineating the high prevalence and negative clinical consequences of depression for HIV-infected individuals in high-income countries.(2, 4-7)
Despite the high prevalence and negative consequences of depression, the capacity of health systems in much of sub-Saharan Africa to effectively treat depressive disorders is virtually nonexistent.(8) The World Health Organization's Mental Health Gap Action Programme (mhGAP) estimates that in low-income countries, 75% of those needing mental health treatment do not have access to any such treatment.(8) Among Sub-Saharan African nations, the median number of mental health professionals is 1.7 per 100,000 population,(9) less than one-fiftieth the rate in the United States (92.7 mental health professionals per 100,000 population).(10) This shortfall in mental health treatment affects not only the general population but also HIV-infected individuals, with related consequences for the success of their HIV clinical care.
In the face of this dearth of skilled mental health professionals, the primary hope for addressing the large burden of mental health needs in low-income countries lies in task-shifting models that focus on training non-specialists, such as primary care providers and community workers, to address common mental health problems.(8) These models generally emphasize supervision of non-specialists by more skilled professionals, with a stepped-care approach that permits the majority of patients to be managed effectively by the non-specialists while complex cases are referred for more specialized treatment.
One such model, Measurement-Based Care (MBC), has a large evidence base from studies with primary care patients in the United States.(11) The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, the largest trial of depression treatment to date, demonstrated that with MBC, primary care settings can deliver depression treatment equivalent to that provided in specialty psychiatric clinics.(11) MBC was recently adapted for use with HIV-infected patients in the US,(12) with particular attention paid to antiretroviralantidepressant interactions. However, the model has not previously been used in settings with the extremely sparse mental health infrastructure that characterizes much of sub-Saharan Africa. We adapted MBC for implementation with HIV-infected patients with depression in Bamenda, Cameroon, and completed a prospective pilot study to assess the feasibility, safety, acceptability, and preliminary efficacy of MBC for HIV-infected patients in this setting.
Methods
Measurement-Based Care
Measurement-Based Care (MBC) is an evidence-based, resource-efficient depression management strategy which equips non-psychiatric medical providers to deliver best-practices medication management for depression.(12) Consistent with principles of task-shifting and chronic disease management successfully applied to a range of chronic illnesses, MBC relies on a non-physician Depression Care Manager (DCM) to regularly assess key metrics of depression treatment. The DCM then provides decision support to the treating (non-psychiatric) provider regarding antidepressant initiation, dosing, and switching. With appropriate supervision, the role of the DCM may be effectively filled by individuals with a range of training, including nurses or social workers.
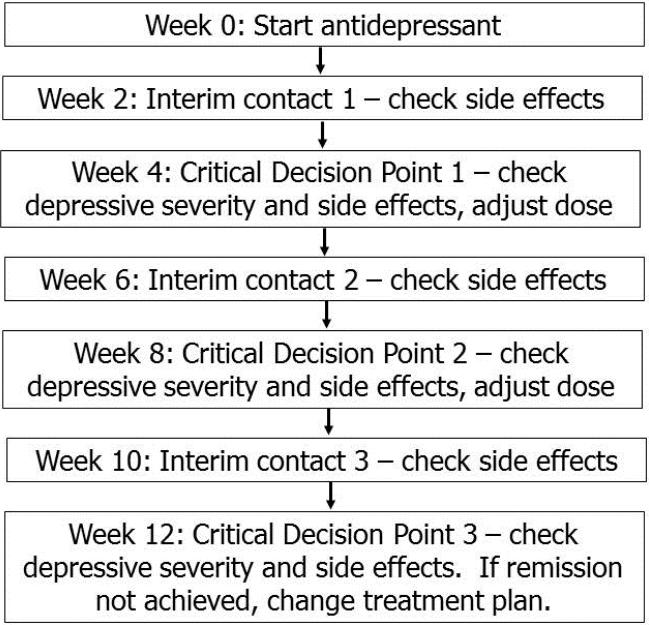
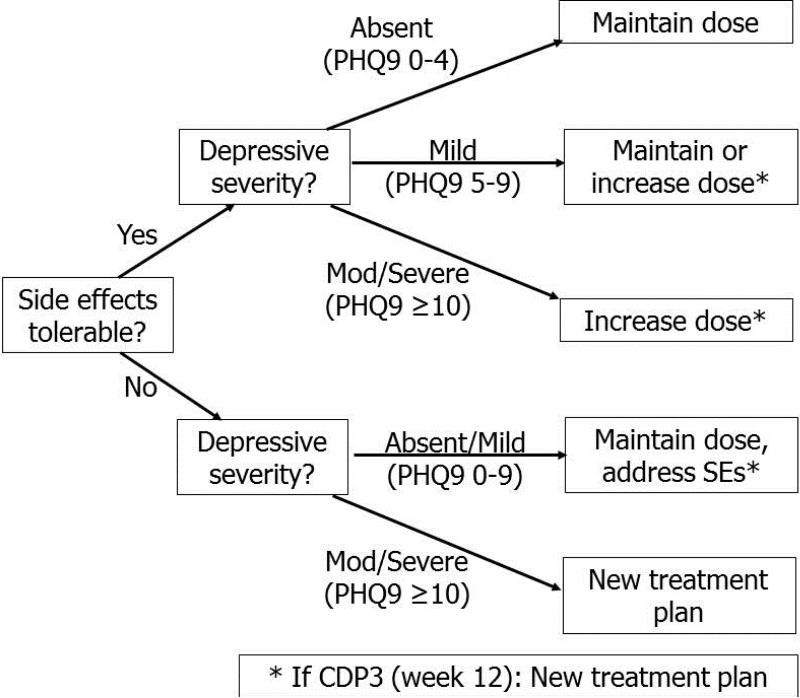
In the general MBC timeline, a patient initiates an antidepressant after confirmation of current major depression and ruling-out of a bipolar or psychotic disorder (Figure 1). Critical Decision Points (CDPs) for treatment adjustment occur every 4 weeks. At each CDP, the patient rates his or her depressive severity and the intensity and tolerability of any side effects using standardized measures. These metrics guide a recommendation to increase, maintain, or decrease the antidepressant dose or to change treatments (Figure 2). In general, if the patient is tolerating the medication but the depressive severity assessment indicates that remission (full resolution of symptoms) has not been achieved, a dose increase will be recommended and then re-evaluated at the next CDP. At CDP3 (i.e., after 12 weeks and up to 2 dose increases), the patient is determined either to have achieved remission (in which case she enters a maintenance phase of reduced contact frequency) or to have demonstrated treatment resistance to the current medication (in which case a new treatment plan is recommended). Additional interim contacts occur between CDPs, at weeks 2, 6, and 10, to monitor tolerability and address side effects if needed.
Figure 1.
General schedule of Measurement-Based Care patient contacts to monitor and adjust acute phase antidepressant treatment.
Figure 2.
Critical Decision Point decision tree for antidepressant treatment adjustment after assessment of depressive severity and tolerability of side effects.
Adaptation of MBC
As part of an NIMH-funded grant (“Adaptation of a Depression Treatment Intervention for HIV Patients in Cameroon,” R34 MH084673, 2009-2012),(13) we adapted MBC for use in Cameroon. The adaptation process consisted of the following steps: (1) focus group discussions to adapt the language of a depression screening instrument (the PHQ-9) (previously described);(14) (2) a validation study to determine the test characteristics of the PHQ-9 among HIV-infected patients in Cameroon (previously described);(14) (3) key informant interviews with a variety of health care providers to define the existing (pre-intervention) standard of care for patients presenting with mental health disorders; (4) adaptation of medication and dosing recommendations in consultation with local medical professionals to account for the antidepressants available; and (5) adaptation of staffing, supervision, and referral pathway plans to the local context in consultation with local medical professionals.
Pilot study procedures
This adaptation was followed by a pilot study to assess feasibility, acceptability, safety, and preliminary efficacy of the adapted MBC intervention. The role of Depression Care Manager was filled by a local individual with a nursing certificate (standard training for nurses) with 12 years of experience as a pharmacy attendant and nurse at the Bamenda Regional Hospital but without prior mental health training. Pre-launch training by study investigators focused on diagnosis of depression and excluding diagnoses, approaches to depression treatment with an emphasis on Measurement-Based Care, and management of suicidality and medication side effects.
After training, recruitment for the pilot study occurred from April 15-November 30, 2011., HIV-infected patients presenting for care at the Bamenda Day Hospital AIDS Treatment Center were routinely screened for depression using the PHQ-9 and invited to participate in the study if they met the following eligibility criteria: (1) PHQ-9 score ≥10; (2) current major depressive disorder confirmed through clinical assessment by the treating HIV physician; (3) 18-65 years; (4) currently prescribed combination antiretroviral therapy. To simplify issues of translation and ensure consistency of measurement for this pilot study, participants needed to be able to communicate effectively in English to participate. In the English-speaking region of Camerron where this study was conducted, all medical care is delivered in English and >90% of patients presenting for care at the Bamenda Day Hospital AIDS Treatment Center can communicate effectively in English. Potential participants were considered not eligible if they met any of the following exclusion criteria: (1) current acute, high-risk suicidality or history of bipolar or psychotic disorder as determined through clinical assessment by the treating HIV physician; (2) current substance abuse problem that in the judgment of the clinician would need to be addressed prior to depression treatment. Potentially eligible patients received information about all study activities, after which interested individuals provided written informed consent.
At the time of enrollment, the Depression Care Manager provided a recommendation about antidepressant prescription to the treating HIV physician. Consistent with the standard MBC timeline,(12) the DCM had follow-up contact with each participant every 2 weeks through week 12 post-enrollment (acute phase of treatment), with weeks 4, 8, and 12 representing Critical Decision Points. Depressive symptom severity was measured with the PHQ-9,(15) while side effects were evaluated with the Frequency, Intensity, and Burden of Side Effects Rating Scale (FIBSER).(16) At the end of the acute phase (12 weeks), participants completed a series of questions addressing their experiences with the MBC intervention. After the acute phase, the DCM and participant had maintenance-phase contacts at months 4, 8, and 12. Participants also completed research interviews with a separate interviewer (not part of the clinical care team) and provided a blood sample for CD4 and HIV RNA viral load measures at baseline and at months 4, 8, and 12. Participants received a small stipend at each CDP to reimburse them for transportation, time, and clinic costs. All study medications (first- and second-line) were provided free of charge for the duration of the study.
To ensure quality of care, the DCM had weekly supervision via teleconference with a psychiatrist, the study PI [BNG], to review all visits and clinical decisions from the past week. Supervision was guided by a simple participant tracking spreadsheet which summarized key metrics for each participant visit: depressive severity, intensity of side effects, suicidality, and treatment plan. A Data Safety and Monitoring Committee (DSMC) reviewed study protocols and data annually.
Ethical approval
All study activities were approved by the Cameroon National Ethics Committee (No. 111/CNE/SE/09), the University of North Carolina at Chapel Hill's Biomedical Institutional Review Board (IRB) (# 09-0852), and the Duke University Health System IRB (# Pro00016937). The study also received administrative approval from the Ministry of Public Health in Cameroon.
Measures
Feasibility was assessed by the ability to (1) identify appropriate and available antidepressants, (2) identify and train an appropriate Depression Care Manager, (3) identify and recruit eligible patients, (4) retain participants through a complete acute phase treatment course, (5) monitor depressive symptoms and side effects and provide algorithm-concordant recommendations to the treating HIV provider, (6) hold regular supervision to review clinical indicators and decisions for quality assurance.
Fidelity was assessed by the congruency between the MBC recommendations (based on the patient's depressive severity and medication tolerability) and the HIV provider's treatment decision, recorded along with all other key clinical indicators in a tracking database that was reviewed on a weekly basis with the supervising psychiatrist. Specific reasons for divergence between the MBC recommendation and the HIV provider's treatment plan were documented.
Safety was assessed by the occurrence of specific symptoms that could be signs of anticholinergic toxicity (delirium or confusion, emerging mania, urinary tract infections, urinary dysfunction, poor liver function, oral thrush, and insomnia), assessed both by participant self-report and clinical exam, and by the frequency and severity of suicidal ideation.
Acceptability was assessed by participant self-report of satisfaction with the intervention, perceived physical and mental health benefit of the intervention, whether they would recommend the intervention to others, whether the time cost of the intervention had been acceptable, and whether the time and financial cost of the treatment approach would be acceptable in the absence of a study.
Preliminary efficacy was assessed by the proportion of participants achieving remission of their depression (PHQ9 total score <5) at each time point.
Analysis Plan
Given the design of this pilot study, the results presented here are descriptive in nature. Sample characteristics and measures of efficacy, safety, and acceptability are summarized with medians and interquartile ranges (IQR) or frequencies and percentages. Fidelity is described by classifying clinical decisions at each CDP as either congruent (following algorithm recommendation) or divergent (not following recommendation), with reasons for divergence detailed.
Results
Adaptation Process
The adaptation process yielded minor modifications to MBC. The most substantive modification reflected the reality that the only readily available antidepressant in Cameroon was amitriptyline (AMI), an older tricyclic antidepressant (TCA) which is primarily used at lower doses to treat peripheral neuropathic pain. As part of the Cameroon National Essential Drug List, AMI is nearly universally available at a heavily subsidized price. AMI has the potential for sedation and anticholinergic side effects such as blurred vision, constipation, urinary retention, and dry mouth, especially in HIV-infected individuals.(17-19) In treating depression in high-income countries, TCAs have largely been replaced by selective serotonin reuptake inhibitors (SSRIs) or other newer agents with more favorable side effect profiles. The MBC approach already provides for gradual dose escalation with careful and proactive side effect monitoring, an approach that was deemed appropriate by the DSMC for monitoring AMI prescription and tolerability in this population.
A related modification involved the definition of second-line treatment options for participants who did not tolerate or respond to AMI treatment. For this study, the identified second-line options included referral to the region's psychiatric nurse, co-located at the hospital, and fluoxetine, available at some local private pharmacies at a higher cost than AMI.
A final modification related to safety monitoring. Particular attention was paid to monitoring conditions that could be an early indication of problematic TCA toxicity in this HIV population, including oral thrush, delirium, confusion, urinary tract infections, urinary dysfunction, emerging mania, insomnia, and liver dysfunction. Additionally, participants were strongly counseled to avoid all alcohol consumption, since alcohol would be expected to inhibit the action of AMI and lead to greater sedation and increased risk of persistent depressive symptoms.
Recruitment and retention
Between April 15 and November 30, 2011, 55 patients met eligibility criteria and enrolled. The majority of participants (80%) were female (Table I). Participants ranged in age from 24-53 (median: 40). Participants had relatively strong CD4 counts (median: 454; IQR: 242-612), but none had a suppressed viral load at baseline (median VL: 12,304 copies/mL; IQR: 7,308-21,555). Baseline PHQ-9 depressive severity scores were in the moderate (10-19) and severe (20-27) range for 87% and 13% of participants respectively. Only 4% of participants reported that this was their first depressive episode; half indicated that they had had at least three prior depressive episodes. Only one participant had ever received treatment for depression.
Table I.
Description of sample
| Characteristic | n (%) or median (IQR) |
|---|---|
| Total | 55 |
| Gender | |
| Male | 11 (20%) |
| Female | 44 (80%) |
| Age | 40 (33-44) |
| Marital status | |
| Married or partnered | 8 (15%) |
| Never married | 18 (33%) |
| Widowed or divorced | 29 (53%) |
| Educational attainment | |
| None | 1 (2%) |
| Primary | 31 (56%) |
| Secondary | 17 (31%) |
| Tertiary | 6 (11%) |
| Daily expenditures (USD) | 4.55 (2.80-7.31) |
| CD4 (cells/mm3) | 454 (242-612) |
| HIV RNA viral load (copies/mL) | 12,304 (7,308-21,555) |
| HIV RNA viral load <400 | 0 (0%) |
| Prior depressive episodes | |
| None | 2 (4%) |
| 1 | 6 (11%) |
| 2 | 19 (35%) |
| 3 or more | 27 (49%) |
| Ever treated for depression | 1 (2%) |
Retention was high in both the clinical and research components of the study. One participant died, for reasons not related to the study, after the week 4 visit and one participant was withdrawn due to problematic alcohol consumption and liver toxicity concerns after the week 8 visit. The 4-, 8-, and 12-week CDPs were completed by 100% (55/55), 93% (50/54), and 100% (53/53) of remaining participants, respectively (Table II). The 2-, 6-, and 10-week interim contacts (primarily for side effects monitoring) were completed by 87% (48/55), 57% (31/54), and 34% (18/53) of participants. According to the DCM, the lower retention at the interim contacts was primarily driven by transportation barriers (while CDPs generally coincided with medication refill visits, interim contacts did not) and low perceived need by participants not experiencing side effects. The research interviews at months 4, 8, and 12 were completed by all remaining participants (53/53, 100%).
Table II.
Implementation, Fidelity, and Preliminary Efficacy of Measurement-Based Care
| Baseline n (%) or Median (IQR) | 4 weeks n (%) or Median (IQR) | 8 weeks n (%) or Median (IQR) | 12 weeks n (%) or Median (IQR) | |
|---|---|---|---|---|
| Active participants | 55 | 55 | 54* | 53** |
| Attended visit | 55 (100%) | 55 (100%) | 50 (93%) | 53 (100%) |
| AMI dose prescribed | ||||
| 25 mg daily | 1 (2%) | 3 (5%) | 2 (4%) | 2 (4%) |
| 50 mg daily | 54 (98%) | 48 (87%) | 41 (76%) | 43 (81%) |
| 75 mg daily | 0 | 2 (4%) | 5 (9%) | 5 (9%) |
| 100 mg daily | 0 | 2 (4%) | 1 (2%) | 2 (4%) |
| 125 mg daily | 0 | 0 | 1 (2%) | 1 (2%) |
| PHQ-9 total score | 13 (12-16) | 6 (4-9) | 3 (2-6) | 2 (0-3) |
| Response (>50% decrease in PHQ-9 from baseline) | n/a | 34 (62%) | 44 (88%) | 50 (94%) |
| Remission (PHQ-9 < 5) | n/a | 19 (35%) | 35 (70%) | 46 (87%) |
| Clinical action taken | ||||
| Increase dose | n/a | 7 (13%) | 5 (10%) | 0 (0%) |
| Maintain dose | n/a | 45 (82%) | 45 (90%) | 53 (100%) |
| Decrease dose | n/a | 3 (5%) | 0 (0%) | 0 (0%) |
| Fidelity to intervention | ||||
| Action congruent with algorithm | 55 (100%) | 49 (89%) | 49 (98%) | 52 (98%) |
| Action divergent from algorithm | 0 (0%) | 2 (11%) | 1 (2%) | 1 (2%) |
1 death between weeks 4 and 8
1 participant withdrawn due to alcohol consumption between weeks 8 and 12
Intervention Implementation and Preliminary Efficacy
All participants were prescribed amitriptyline at their baseline visit at one of the two recommended starting doses of 25mg daily (n=1) or 50mg daily (n=54) (Table II). At the first CDP (week 4), all participants were tolerating the medication well and 35% of participants had achieved remission of their depression, defined as a PHQ-9 score <5. For the majority of participants (82%), the AMI dose was not changed at CDP1.
At the second and third CDPs (weeks 8 and 12), tolerability remained high and remission rates continued to increase, to 70% and then 87%. By 12 weeks, the median PHQ-9 score had decreased to 2, with a range of 0-12. Most participants (90% at CDP2, 100% at CDP3) did not change doses at these CDPs. Final amitriptyline doses at 12 weeks ranged from 25-125 mg daily, with most participants (n=43, 81%) being prescribed 50mg daily.
All participants remained on amitriptyline, the first-line treatment option; no participants stopped amitriptyline or transitioned to a second-line option over the 12-month study period. Reductions in depressive symptoms were maintained or improved through 12 months: 90%, 94%, and 100% of respondents achieved full remission at months 4, 8, and 12, respectively, no serious adverse events were reported, and problematic side effects remained rare.
Intervention fidelity
Overall, fidelity to intervention recommendations by the treating HIV provider was high, with HIV providers following recommendations in 96% of encounters. At week 4, the physician's action was congruent with the MBC recommendation in 49/55 cases (89%) (Table II). Specifically, of 19 participants who had achieved remission, the antidepressant prescription was maintained for 17 participants (congruent) and decreased for 2 participants who had self-decreased on their own due to sleep disturbance. Of 30 participants with mild ongoing depressive symptoms, the dose was increased or maintained in 29 cases (congruent) and decreased in 1 case due to tolerable but moderate side effects. Of 6 participants with ongoing moderate to severe depressive symptoms, the dose was increased in 3 cases (congruent) and maintained in 3 cases. In one of these cases the participant had been poorly adherent to the prescribed dose so the decision was to counsel greater adherence to the original dose. In a second case, even though the participant still had moderate symptoms, the participant had shown partial response to the original dose so the decision was to maintain the dose and reassess at week 8. In the third case the participant had shown partial response and was reporting some tolerable but frequent side effects so the decision was to maintain the dose and re-assess at week 8.
At CDP2 (week 8), the physician's action was congruent with the MBC recommendation in 49/50 cases (98%). Of 35 participants who had achieved remission, the dose was maintained in all cases (congruent). Of 12 participants with mild ongoing depressive symptoms, the dose was increased or maintained in all cases. Of 3 participants with ongoing moderate to severe depressive symptoms, the dose was increased in 2 cases (congruent) and maintained in 1 case.
At CDP3 (week 12), the physician's action was congruent with the MBC recommendation in 52/53 cases (98%). Of 46 participants who had achieved remission and 6 participants with mild ongoing depressive symptoms, the dose was maintained in all cases (congruent). The 1 participant with ongoing moderate to severe depressive symptoms was the same participant with elevated symptoms whose dose was maintained at week 8. The provider again elected to maintain the dose, although the dose was eventually increased to 100mg (at 4 months), after which the participant achieved remission.
Safety and suicidality
Suicidal ideation merits careful monitoring in any study of individuals with major depression. Some level of passive or active suicidal ideation was endorsed by 62% of participants at baseline, with 18% endorsing only passive suicidality (thoughts of being better off dead, but no thoughts about self-harm), 16% endorsing active suicidality assessed as low-risk, and 27% endorsing active suicidality assessed as moderate risk (Table III). The proportion of respondents reporting any suicidality dropped to 15% at 4 weeks, 10% at 8 weeks, and 8% at 12 weeks, with active suicidality reported by 4-7% of respondents at each of these time points. All participants reporting any suicidality were assessed for safety, and no participant was ever assessed to be an emergent threat to him or herself.
Table III.
Safety and side effects
| Baseline n (%) | 4 weeks n (%) | 8 weeks n (%) | 12 weeks n (%) | |
|---|---|---|---|---|
| N | 55 | 55 | 50 | 53 |
| Suicidality | ||||
| None | 21 (38%) | 47 (85%) | 45 (90%) | 49 (92%) |
| Passive | 10 (18%) | 4 (7%) | 3 (6%) | 1 (2%) |
| Active - Low risk | 9 (16%) | 1 (2%) | 1 (2%) | 2 (4%) |
| Active - Moderate risk | 15 (27%) | 3 (5%) | 1 (2%) | 1 (2%) |
| Active - Acute risk | 0 | 0 | 0 | 0 |
| Potential tricyclic side effects | ||||
| Oral thrush | n/a | 0 | 3 (6%) | 0 |
| Delirium or confusion | n/a | 0 | 0 | 0 |
| Emerging manic symptoms | n/a | 0 | 0 | 0 |
| UTI or urinary dysfunction | n/a | 0 | 0 | 0 |
| Insomnia | n/a | 2 (4%) | 1 (2%) | 2 (4%) |
| Signs of poor liver function | n/a | 0 | 0 | 0 |
| Any other concerning side effect | n/a | 0 | 0 | 0 |
| Patient self-report of AMI side effects: Frequency | ||||
| <Half the time | n/a | 36 (65%) | 43 (80%) | 49 (92%) |
| Half the time or more | n/a | 19 (35%) | 7 (14%) | 4 (8%) |
| Patient self-report of AMI side effects: Intensity | ||||
| None or mild | n/a | 41 (75%) | 44 (88%) | 49 (92%) |
| Moderate or severe | n/a | 13 (24%) | 6 (12%) | 4 (8%) |
| Intolerable | n/a | 1 (2%) | 0 | 0 |
| Patient self-report of AMI side effects: Impairment | ||||
| None or mild | n/a | 54 (98%) | 46 (92%) | 53 (100%) |
| Moderate or severe | n/a | 1 (2%) | 4 (8%) | 0 |
| Unable to function | n/a | 0 | 0 | 0 |
Over the course of the study, no participants showed any clinically concerning signs of medication toxicity such as delirium or confusion, emerging mania, urinary tract infections, urinary dysfunction, or poor liver function. Three participants had signs of oral thrush at week 8, and 4 participants had difficulties with insomnia (one participant reported insomnia at 2 visits).
Participants were asked to rate the frequency, intensity, and interference of any side effects they believed were attributable to the amitriptyline. While some side effects were reported, these were generally mild and posed little interference. The proportion reporting no or only mild side effects was 72% at 4 weeks, 88% at 8 weeks, and 92% at 12 weeks. In only one instance did a participant report intolerable side effects prior to physician assessment, and in this case during the clinical exam the side effects were rated as mild.
Acceptability
Overall, 100% of participants reported being “satisfied” with the Measurement-Based Care intervention (Table IV). Ninety-six percent said they found MBC to be beneficial to their health, and 98% said they would recommend MBC to others. Sixty percent said that MBC added time to their clinic visit, with a median (IQR) estimate of 40 (30-60) minutes, but all said the extra time was worthwhile. Ninety-four percent and 89% indicated that they were willing to pay for transportation and medications, respectively, if MBC were to be provided outside of a research study, and 70% and 68% said they would be able to afford the necessary transportation and medications.
Table IV.
Acceptability of intervention
| Indicator | n (%) or median (IQR) |
|---|---|
| Satisfied overall with MBC | 53 (100%) |
| Find MBC beneficial to health | 51 (96%) |
| Would recommend MBC to others | 52 (98%) |
| MBC makes clinic visits longer | 32 (60%) |
| Extra time due to MBC, minutes | 40 (30-60) |
| Additional time worthwhile | 32 (100%) |
| Additional visits for MBC | 32 (60%) |
| Number of additional visits | 2 (2-3) |
| Additional visits worthwhile | 32 (100%) |
| Time away from duties due to MBC, mins | 40 (30-90) |
| Physical discomfort from MBC | 3 (6%) |
| Severity - mild | 2 (100%) |
| Discomfort acceptable | 2 (100%) |
| Emotional discomfort from MBC | 1 (2%) |
| Severity – mild | NA* |
| Discomfort acceptable | NA* |
| Cost of transport to/from clinic, US$ | $0.80 ($0.60-$1.60) |
| Willing to pay for transport | 50 (94%) |
| Able to pay for transport | 37 (70%) |
| Willing to pay for meds | 47 (89%) |
| Able to pay for meds | 36 (68%) |
| Time to travel to clinic, minutes | 35 (20-60) |
The 1 participant identifying emotional discomfort did not rate the severity.
Discussion
In this single-group pilot study, the adapted Measurement-Based Care intervention demonstrated high levels of feasibility, safety, acceptability, and preliminary efficacy. In terms of feasibility, we were successful in training a non-specialist nurse as a Depression Care Manager to identify major depression and deliver measurement-based medication treatment recommendations to the managing HIV provider; the provider followed these treatment recommendation >95% of the time. We were also successful in maintaining regular supervision of the DCM by a study psychiatrist who reviewed recommendations and provided ongoing feedback and quality assurance. In terms of safety, few problematic side effects and no major safety concerns were observed over 3 months of acute-phase treatment. With respect to acceptability, all participants were satisfied with their MBC experience, with most expressing willingness to assume the additional costs of medications and clinic visits for their depression treatment if MBC were part of standard care. Although the study lacked a control group, efficacy was suggested by the strong and sustained reduction in depressive symptoms over 12 weeks of treatment, with accompanying reductions in suicidal ideation and with the vast majority of participants achieving full remission of their depressive symptoms.
This study represents one of several efforts to develop and test feasible, acceptable, and effective task-shifting models to enhance mental health treatment capacity in resource-constrained settings. In the MANAS trial in India, Patel and colleagues observed that a collaborative stepped-care intervention, administered by trained lay counselors and complemented with antidepressant medication by the primary care physician and supervision by a mental health specialist, was effective in initiating recovery from common mental disorders including depression.(20) Meanwhile, Bolton and colleagues have demonstrated the efficacy of lay counselor-led interpersonal group therapy to treat depression in rural Uganda.(21, 22) Petersen et al. additionally illustrated that group-based interpersonal therapy for depression headed by trained community health workers was feasible in South Africa.(23) However, to our knowledge this is the first published study of a medication management-focused task-shifting model for treatment of depression in a low-income country.
Both medication-based and counseling-based approaches to depression treatment (including involvement of traditional and spiritual healers) are likely to be important in efforts to expand access to mental health treatment in low-resource settings. While a formal cost-benefit analysis was beyond the scope of this project, the project did demonstrate that a single DCM without prior specialized training was easily able to manage 55 active patients, a caseload that would have been challenging had the intervention involved weekly counseling sessions. With appropriate supervision, depression management could also easily be integrated into the portfolio of existing clinicians managing other chronic conditions, such as the clinical officers or nurses who provide front-line HIV care in much of sub-Saharan Africa.
A primary limitation of this pilot study was the single-group design. In the absence of a randomized design, we cannot rule out the possibility that participants would have improved without treatment or simply with increased attention, rather than because of the MBC intervention specifically. However, nearly all participants reported having had past depressive episodes, with half reporting three or more prior episodes; such recurrent depression would be unlikely to resolve quickly on its own,(24-26) and screening and supportive care in the absence of formal evidence-based depression treatment generally does not have a strong or sustained effect on depressive symptoms as observed here. (27) In addition, the high prevalence of suicidal ideation, especially active ideation, at enrollment and the subsequent decline support the likelihood of a treatment effect.
An additional limitation of the study was the limited availability of alternative antidepressants and treatment options. Amitriptyline, a tricyclic antidepressant (TCA), was the only antidepressant available on the government essential drug list. Amitriptyline and other TCAs have potentially problematic toxicities including sedation and anticholinergic effects. In this small pilot study no one experienced problematic side effects from amitriptyline and most participants exhibited a strong response to treatment, but the identification of alternative treatment options would be important to consider in larger-scale implementation. Although the most concerning interactions of TCAs are with the protease inhibitor class of antiretrovirals,(18, 19) which are uncommonly prescribed in Cameroon, the addition of an antidepressant from the more benign SSRI class would likely be beneficial.
Although no medication-related safety concerns were observed in this study, the small size of this pilot may have prevented the identification of uncommon but important side effects that are possible with TCAs, including anticholinergic delirium, confusion, seizure, and cardiac arrhythmias.(17) Careful monitoring of side effects would be warranted in further studies, especially if such studies continue to rely on TCAs.
In summary, in this study we successfully adapted Measurement-Based Care for implementation with HIV patients in Bamenda, Cameroon, and demonstrated its feasibility, safety, acceptability, and preliminary efficacy in a small, single-group pilot study. Task-shifting models, particularly models such as Measurement-Based Care that equip non-specialist medical providers to effectively prescribe and monitor antidepressants, hold great potential to expand the currently extremely low capacity for mental health treatment that characterizes most low-income countries. Additional research should focus on whether evidence-based depression treatment will improve HIV-related outcomes such as retention in care, medication adherence, and virologic suppression in such settings in order to define the full potential benefit of investing in mental health treatment for HIV-infected individuals.
Acknowledgements and Funding
This work was made possible by our study participants and by study personnel Dr. Awasum Charles, Mr. Andrew Goodall, Mr. Fru Johnson, Dr. Charles Arrey Kefie, Mrs. Irene Numfor, Mr. Joseph Nyingcho, and Ms. Seema Parkash. This study was supported by grant R34 MH084673 of the National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA. BNG receives funding from the NC TRACS Institute, which is supported by grants UL1RR025747, KL2RR025746, and TLRR025745 from the NIH National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health. This publication was made possible with help from the Duke University Center for AIDS Research (CFAR), an NIH funded program (P30 AI064518). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the NIH. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Footnotes
Conflicts of interest: The authors state that no relevant conflicts of interest exist.
Author contributions: BWP, BNG, JA, KW, PN obtained funding and designed the study. BWP, BNG, JA, oversaw training and data collection. SA collected the data. BWP and JKO analyzed the data. BWP, BNG, DK drafted the manuscript. DK reviewed the literature. JA, JKO, KW, AKN, TM, CK, SA, PN reviewed the manuscript for important intellectual content. All authors contributed to and have approved the final manuscript.
References
- 1.Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, Alcohol Use and Adherence to Antiretroviral Therapy in Sub-Saharan Africa: A Systematic Review. AIDS and behavior. 2011 doi: 10.1007/s10461-011-0087-8. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS Treatment Nonadherence: A Review and Meta-analysis. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakimuli-Mpungu E, Musisi S, Katabira E, Nachega J, Bass J. Prevalence and factors associated with depressive disorders in an HIV+ rural patient population in southern Uganda. Journal of affective disorders. 2011;135(1-3):160–7. doi: 10.1016/j.jad.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Leserman J, Pence BW, Whetten K, Mugavero MJ, Thielman NM, Swartz MS, et al. Relation of lifetime trauma and depressive symptoms to mortality in HIV. The American journal of psychiatry. 2007;164(11):1707–13. doi: 10.1176/appi.ajp.2007.06111775. [DOI] [PubMed] [Google Scholar]
- 5.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70(5):539–45. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 6.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV- seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 7.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–66. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . mhGAP : Mental Health Gap Action Programme : scaling up care for mental, neurological and substance use disorders. World Health Organization; Geneva, Switzerland: 2008. [PubMed] [Google Scholar]
- 9.World Health Organization . Mental Health Atlas 2011. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 10.World Health Organization . Mental Health Atlas : 2005. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 11.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Balasubramani GK, McGrath PJ, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551–60. doi: 10.1007/s11606-008-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JL, Gaynes BN, McGuinness T, Modi R, Willig J, Pence BW. Treating Depression Within the HIV “Medical Home”: A Guided Algorithm for Antidepressant Management by HIV Clinicians. AIDS patient care and STDs. 2012;26(11):647–54. doi: 10.1089/apc.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaynes BN, Pence BW, Atashili J, O'Donnell J, Kats D, Ndumbe PM. Prevalence and Predictors of Major Depression in HIV-Infected Patients on Antiretroviral Therapy in Bamenda, a Semi-Urban Center in Cameroon. PLoS One. 2012;7(7):e41699. doi: 10.1371/journal.pone.0041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pence BW, Gaynes BN, Atashili J, O'Donnell JK, Tayong G, Kats D, et al. Validity of an interviewer-administered patient health questionnaire-9 to screen for depression in HIV-infected patients in Cameroon. Journal of affective disorders. 2012 doi: 10.1016/j.jad.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 16.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. Journal of psychiatric practice. 2006;12(2):71–9. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Penzak SR, Reddy YS, Grimsley SR. Depression in patients with HIV infection. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2000;57(4):376–86. doi: 10.1093/ajhp/57.4.376. quiz 87-9. [DOI] [PubMed] [Google Scholar]
- 18.de Maat MMR, Ekhart GC, Huitema ADR, Koks CHW, Mulder JW, Beijnen JH. Drug interactions between antiretroviral drugs and comedicated agents. Clinical pharmacokinetics. 2003;42(3):223–82. doi: 10.2165/00003088-200342030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Washington, DC: Mar 27, 2012. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2012 Available from: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 20.Patel V, Weiss HA, Chowdhary N, Naik S, Pednekar S, Chatterjee S, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. 2010;376(9758):2086–95. doi: 10.1016/S0140-6736(10)61508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass J, Neugebauer R, Clougherty KF, Verdeli H, Wickramaratne P, Ndogoni L, et al. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes: randomised controlled trial. Br J Psychiatry. 2006;188:567–73. doi: 10.1192/bjp.188.6.567. [DOI] [PubMed] [Google Scholar]
- 22.Bolton P, Bass J, Neugebauer R, Verdeli H, Clougherty KF, Wickramaratne P, et al. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. Jama. 2003;289(23):3117–24. doi: 10.1001/jama.289.23.3117. [DOI] [PubMed] [Google Scholar]
- 23.Petersen I, Bhana A, Baillie K, Mha PPRPC. The feasibility of adapted group- based interpersonal therapy (IPT) for the treatment of depression by community health workers within the context of task shifting in South Africa. Community mental health journal. 2012;48(3):336–41. doi: 10.1007/s10597-011-9429-2. [DOI] [PubMed] [Google Scholar]
- 24.Keller MB, Boland RJ. Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biological psychiatry. 1998;44(5):348–60. doi: 10.1016/s0006-3223(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 25.Keller MB, Lavori PW, Rice J, Coryell W, Hirschfeld RM. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. The American journal of psychiatry. 1986;143(1):24–8. doi: 10.1176/ajp.143.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):18–25. [PubMed] [Google Scholar]
- 27.O'Connor EA, Whitlock EP, Beil TL, Gaynes BN. Screening for depression in adult patients in primary care settings: a systematic evidence review. Annals of internal medicine. 2009;151(11):793–803. doi: 10.7326/0003-4819-151-11-200912010-00007. [DOI] [PubMed] [Google Scholar]