Abstract
Multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system (CNS) involving demyelinating and neurodegenerative processes. Several of the major pathological CNS alterations and behavioral deficits of MS are recapitulated in the experimental autoimmune encephalitis (EAE) mouse model in which the disease process is induced by administration of myelin peptides. Development of EAE requires infiltration of inflammatory cytokine-generating monocytes and macrophages, and auto-reactive T cells, into the CNS. Very late antigen-4 (VLA-4, α4β1) is an integrin molecule that plays a role in inflammatory responses by facilitating the migration of leukocytes across the blood-brain barrier during inflammatory disease, and antibodies against VLA-4 exhibit therapeutic efficacy in mouse and monkey MS models. Here we report that the tellurium compound AS101 (ammonium trichloro (dioxoethylene-o,o’) tellurate) ameliorates EAE by inhibiting monocyte ant T-cell infiltration into the CNS. CD49d is an alpha subunit of the VLA-4 (α4β1) integrin. During the peak stage of EAE, AS101 treatment effectively ameliorated the disease process by reducing the number of CD49d+ inflammatory monocyte/macrophage cells in the spinal cord. AS101 treatment markedly reduced the pro-inflammatory cytokine levels, while increasing anti-inflammatory cytokine levels. In contrast, AS101 treatment did not affect the peripheral populations of CD11b+ monocytes and macrophages. AS101 treatment reduced the infiltration of CD4+ and CD49+/VLA4 T cells. In addition, treatment of T cells from MS patients with AS101 resulted in apoptosis, while such treatment did not affect T cells from healthy donors. These results suggest that AS101 reduces accumulation of leukocytes in the CNS by inhibiting the activity of the VLA-4 integrin, and provide a rationale for the potential use of Tellurium IV compounds for the treatment of MS.
Keywords: inflammation, integrin, macrophages, multiple sclerosis, spinal cord, VLA-4
Introduction
Multiple sclerosis (MS) is a debilitating autoimmune disorder in which the myelinating cells (oligodendrocytes) and neurons are damaged be aberrant reactivity of lymphocytes to myelin-associated proteins (Frohman et al., 2006). The overall prevalence of MS is approximately 0.1%, but is at least three times more common in women and varies geographically (Noonan et al., 2010). The clinical manifestations of MS include sensory and motor disturbances, cognitive impairment and mood disturbances. The regions of white matter pathology in MS are characterized by an inflammatory infiltrate consisting mainly of lymphocytes and mononuclear phagocytes (Prat and Antel, 2005; Okun et al., 2010). The exact cause of MS is unknown, although it is believed to be caused by interactions between as yet unidentified environmental factors and susceptibility genes. There is as yet no cure for MS, and currently available therapies, including interferon-β, glatiramer and VLA-4 monoclonal antibodies are aimed at suppressing the immune response to relieve symptoms (Jones and Coles, 2010; Bar-Or et al., 2011; Meuth et al., 2012).
In MS, chronic activation of monocytes and macrophages adversely affects myelin and axons by producing pro-inflammatory cytokines (TNF, IL-1β and IL-6), chemokines (SDF-1, CXCL-1 and PSGL-1) and reactive oxygen species (superoxide and nitric oxide) (Hendriks et al., 2005; Huitinga et al., 1990; Dhib-Jalbut, 2007; King et al., 2007; Holman et al., 2011). Macrophages and monocytes also serve as antigen-presenting cells for the reactivation of infiltrating myelin-reactive CD4+ T cells (Greter et al., 2005). Therefore, the interruption of the process of infiltration and migration of monocytes and auto-reactive T cells across the blood-brain barrier (BBB) is one approach for treating MS. Although mechanisms of monocyte and T cell infiltration into the CNS remain to be established, considerable evidence suggests a key role for the integrins VLA-4/VCAM-1 and LFA-1/CR3/ICAM-1 (Hendriks et al., 2005; Floris et al., 2002). VLA-4 (very late antigen-4; CD49d/CD29) is expressed by most mononuclear leukocytes but it is observed on neutrophils only under special conditions (Wayner et al., 1989). For monocytes, VLA-4 is implicated in monocyte transmigration across the vascular endothelium (Huo et al., 2000). In 2004, it was reported that Natalizumab, an antibody against VLA-4 can effectively reduce the progression of MS and relapse (Dalton et al., 2004). However, serious side effects of Natalizumab treatment have been reported including progressive multifocal leukoencephalopathy (Bloomgren et al., 2012).
The ammonium trichloro (dioxoethylene-o,o’) tellurate compound is a non-toxic immunomodulator that has demonstrated therapeutic efficacy in preclinical studies of cancer (Sredni et al., 1987, 1996, 2004a), hair loss (Sredni et al., 2004b), human papillomavirus (Friedman et al., 2009), ischemic stroke (Okun et al., 2007) and Parkinson’s disease (Sredni et al., 2007). The mechanism(s) of action of AS101 is not fully established, but it does inhibit the production of IL-1β and IL-10, and can directly inhibit caspases 1, 3 and 8 by interacting with thiol groups (Sredni et al., 2004a; Strassmann et al., 1997; Kalechman et al., 2007; Brodisky et al., 2007, 2010). These anti-inflammatory and anti-apoptotic effects of AS101 can protect neurons against degeneration in animal models of stroke and Parkinson’s disease (Okun et al., 2007; Sredni et al., 2007). In addition, it was recently reported that AS101 can inhibit the ligand binding activity of integrins/VLA-4 (Indenbaum et al., 2012). Because VLA-4 is believed to play a pivotal role in the migration of T cells, monocytes and macrophages into the CNS in MS (Yusuf-Makagiansar et al., 2002; Sheremata et al., 2005; Steinman, 2005), we evaluated the potential therapeutic efficacy of AS101 treatment in a mouse EAE MS model. Our findings suggest that AS101 can ameliorate MS-like disease processes by a mechanism involving suppression of VLA-4 mediated accumulation of inflammatory monocytes, macrophages and auto-reactive T cells in the CNS.
Methods
Animals
Female C57BL/6 mice (2–3 months old) were purchased from the Jackson Laboratories (Bar Harbor, ME) and were kept in the National Institute on Aging mouse barrier facility with access to standard food and water ad libitum. All procedures were approved by the Animal Care and Use Committee of the National Institute on Aging.
Induction and clinical assessment of EAE
Mice were immunized subcutaneously with 200 µg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK; purchased from AnaSpec) emulsified in CFA (Difco) containing 4 mg/ml of Mycobacterium tuberculosis. Mice were injected intraperitoneally with 200 ng of pertussis toxin (Sigma) at the time of immunization and 48 hr after immunization. AS101 was administered intraperitoneally once daily at a dose of 1 mg/kg beginning on the day of immunization. EAE symptoms were evaluated daily using the following scale: 0, no symptoms; 1, flaccid tail; 2, mild hind limb paralysis; 3, both hind legs paralyzed with residual mobility; 4, both hind legs completely paralyzed; 5, unable to feed or drink and euthanized or found moribund. The motor performance of EAE mice was assessed using an accelerating rotarod (Med Associates, Inc.) by placing the mouse on the rotating drum and measuring the time period that the mouse was able to maintain its balance on the rod (latency to fall in seconds) as the rotation speed accelerated from 4 to 40 rpm over a 5 minute period.
Histology
Mice were euthanized using CO2 gas and perfused transcardially with 10% formaldehyde in PBS. The spinal cords were removed and post-fixed in 10% formaldehyde. Tissues were embedded in paraffin and sectioned on a microtome (5 µm). Tissues were stained with Luxol Fast Blue (LFB) to assess demyelination or immunostained with a rat anti-F4/80 monoclonal antibody (AbD Serotec), a goat anti-Iba-1 polyclonal antibody (Abcam) or a CD4 antibody (Abcam) after antigen retrieval. After washing the sections were incubated in the presence of biotinylated-conjugated anti-rat or anti- goat antibodies, horseradish peroxidase-conjugated streptavidin, followed by incubation in the presence of diaminobenzidine substrate (Vector Laboratories). Slides were counterstained with hematoxylin and images were acquired using a Zeiss Axioplan fluorescence microscope with the AxioCam HRc. All data were analyzed using MCID core 7.0 (InterFocus Imaging Ltd) and the images were processed using Photoshop CS2 (Adobe).
Isolation of mononuclear cells and flow cytometry
Beginning 1 day after the immune challenge with MOG to induce EAE, mice were treated daily with 1 mg/kg AS101 or PBS and subgroups of mice (n = 5/group) were euthanized on post immunization days 7, 14 or 21. The mice were euthanized by CO2 inhalation, and perfused transcardially with cold PBS. Brains and spinal cords were removed and minced into small tissue pieces which were then incubated in digestion buffer (2.5 mg/ml collagenase D and 1 mg/ml DNase I in DMEM) at 37°C. Cells were filtered through a 70 µm nylon cell strainer (BD Falcon) and the resulting suspensions were layered on a 30%–70% discontinuous Percoll gradient and centrifuged at 390 g for 20 minutes. The cells collected from the interface between 70% and 30% were washed and counted. All staining was performed in cold HBSS with 1% BSA and 0.1% sodium azide. Non-specific binding to Fc receptors was blocked using an antibody to CD16/32 (BD Biosciences). The cells were stained with antibodies to CD11b-APC, F4/80- eFluor 450, Ly6C- PerCP-Cy5.5, Ly6G-APC-Cy7, and CD49d-PE (BD Biosciences or eBioscience) for 30 minutes on ice. After two washes with the HBSS, the cells were fixed in PBS containing 1% paraformaldehyde. The stained cells were run on a FACS Canto II flow cytometer (BD Biosciences) and the data was analyzed using Flow Jo software (Treestar). CD11b and F4/80 expression in macrophages was measured as the mean fluorescence intensity (MFI).
Reverse transcription and real-time PCR
Total RNA was extracted from spinal cord and spleen using Trizol reagent (Invitrogen), and single stranded cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using Maxima SYBR Green (Thermo) following the instruction of manufacturer. A 2 µl aliquot from each cDNA synthesis and primers at a final concentration of 0.3 µM were combined in a 25 µl reaction volume (primer sequences provided in Supplemental Table S1). Reactions were run in duplicate on the Applied Biosystems 7300 Real-Time PCR system, and SYBR green dye intensity was analyzed using 7300 System SDS software (Life Technologies). Following an initial denaturation at 95°C for 10 minutes, the samples were subjected to 40 amplification cycles as follows: 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. The values were normalized to 18S RNA levels.
Generation of MBP- and MOG-reactive T cell lines
A T-cell vaccination (TCV) study, involving immunization of relapsing remitting MS patients with attenuated autologous CD4+ T-cell lines, generated from peripheral blood mononuclear cells (PBMC) in response to encephalitogenic epitopes of myelin basic protein (MBP), MOG and proteolipid protein (PLP), was approved by the Institutional Review Board of Sheba Medical Center. All patients signed informed consent to the study. PBMC from MS patients or healthy individuals were separated on Ficoll-Hypaque and plated in 96-well U-bottom microplates (Costar, Cambridge, MA) in the presence of synthetic peptides bearing sequences of encephalitogenic epitopes of MBP, MOG and PLP peptide. Peptide antigens corresponding to amino acid residues; 82–99, 87–110 and 151– 170 of MBP; 34–56 and 64–82 of MOG and 41–58 of PLP were used at a concentration of 15 µg/ml for cell stimulation. Selection of wells for expansion of patients lines was performed by 3H-thymidine incorporation into subdivided primary cultures grown with the peptides for 72 hours and yielding a stimulation index >3 relative to control cells, grown without the peptide. These wells were subcultured on freshly obtained antigen presenting cells (irradiated PBMC) and expanded with 5 U/ml of recombinant IL-2 in flasks. The cell lines were maintained in RPMI 1640 medium supplemented with 10% cosmic calf serum (HyClone), 2 mM L-glutamine, penicillin (100 U/ml), streptomycin (100 µg/ml) (Life Technologies, Grand Island, NY). MS T cell lines were characterized by CD4+ content, TNF-α secretion, IFNγ production following rhIL-2 washout and propagation with antigen for 72 hours, high IL-17 mRNA expression levels and CD45RO memory cell determinant expression by >90% of cells. Cultures with CD4 content >60% were used for the experiments, since in the majority of cases it is CD4+ cells that are thought to cause the pathology. The study was approved by the local ethics committee and the research followed the tenets of the Declaration of Helsinki. Whole cell extracts were prepared from the T cells using the disassociation buffer [20 mM Tris pH 7.8, 1% NP-40, 100 mM NaCl, 50 mM NaF, 0.1% SDS, 2 mM EDTA, 10% Glycerol, 1 mM DTT] and complete Protease Inhibitor mixture, 40 µg/ml (Boehringer, Mannheim, Germany), containing 0.6% NP40. The protein content was calibrated using the BCA protein assay reagent kit (Pierce, Rockford, IL). Samples were separated on 8–10% SDS-PAGE and transblotted onto nitrocellulose filters (Schleicher & Schuell, Dassel, Germany). The membranes were probed with the relevant primary antibodies, with peroxidase-tagged second antibodies and developed with enhanced chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate; Pierce). Light emission was detected by exposure to Agfa Scientific Imaging Film.
Human naive T cells
PBMC from healthy human donors after informed consent was obtained were separated on Ficoll-Hypaque. To remove monocytes and dendritic cells, PBMC were incubated in a humidified atmosphere (2 hours, 37° C, 5% CO2) on 10 mm tissue culture plates in RPMI 1640 supplemented with 10% autologous donor serum. Non-adherent cells were then collected, and exclusion of adherent cells was repeated. After the second incubation, non-adherent cells were collected and CD3+ expression was detected in > 85% of the cells.
T cell apoptosis
After treatment with various concentrations of AS101 for 24, 48 or 72 hours, 106 cells were washed twice in Ca2+- and Mg2+- free PBS and resuspended in 85 µL of Annexin-V binding buffer (Annexin V-FITC/PI kit, MBL). Samples were then labeled with FITC-conjugated human Annexin-V and propidium iodide (15 minutes at room temperature in the dark). The percentage of cells undergoing apoptosis was analyzed by FACScan (Becton Dickinson, Mountain View, CA), using CellQuest Software. The Annexin-V+ propidium iodide− cells corresponded to the early apoptotic cells, whereas the Annexin-V+ propidium iodide+ were cells in the late apoptosis state.
Measurement of mitochondrial transmembrane potential (Δψm)
Rhodamine123 (10 µM) was added to ~106 T cells suspended in 1 ml complete tissue culture medium (with 10% serum), and incubated for 20 minutes at 37°C in the dark. The cells were then centrifuged twice with cold PBS and finally suspended in 300 µl PBS. Cell fluorescence was analyzed by flow cytometry (Becton Dickinson, Mountain View, CA). Excitation fluorescence was produced with 488 nm light and green emission was collected at 530 nm. Data were analyzed using Cellquest software.
Statistical analysis
The data were analyzed by analysis of variance and an appropriate post-hoc test for pairwise comparisons (Systat Software). Values are presented as mean ± S.E.M. P < 0.05 was considered statistically significant.
Results
AS101 treatment ameliorates EAE symptoms
We induced EAE in wild-type C57BL6 mice by immunization with MOG peptide, and then treated the mice with 1 mg/kg of AS101 in 100 µl PBS once daily for 21 days post-immunization. A control group of mice was administered 100 µl PBS once daily. AS101 treatment significantly suppressed the development of EAE symptoms compared to the PBS-treated control mice (Fig. 1A). Two mice in the PBS EAE group reached stage 5 and had to be euthanized, whereas no mice in the AS101 EAE group reached stage 5. Mice in both groups lost weight coincident with the development of EAE symptoms (Fig. 1B). Rotarod testing demonstrated a highly significant preservation of sensory – motor function in the AS101-treated mice compared to the PBS-treated control mice (Fig. 1C).
Figure 1.
AS101 treatment delays the onset and attenuates the progression of EAE in mice. C57BL/6 mice were immunized with MOG35-55 mixed with CFA (5 sham mice, 15 PBS-treated EAE mice and 15 AS101-treated EAE mice). Pertussis toxin was injected on days 0 and 2. AS101 (1 mg/kg) was administered once daily beginning on day 0 (MOG immunization). (A) Values for clinical scores measured daily. The values for AS101 versus PBS in EAE mice were significant on days 12 – 21 (**p<0.01). (B) Body weights were measured once per week. (C) Rotarod test results. The values for AS101 versus PBS in EAE mice were significant at the 2 and 3 week time points (*p<0.05; **p<0.01). (D) Representative examples of spinal cord sections from the indicated groups stained with Luxol Fast Blue (14 days post-immunization). (E) Results of measurements of lesion areas determined from Luxol Fast Blue-stained spinal cord sections of mice in the indicated groups at the indicated post-immunization time points. **p<0.01 compared to the corresponding value for the AS101-treated group.
Demyelination is a hallmark of MS, and occurs in the spinal cord in the EAE model of MS (Swanborg, 1999) Data obtained from evaluation of spinal cord sections stained with Luxol Fast Blue (Smyser, 1973) demonstrated a significant attenuation of white matter damage caused by MOG immunization in AS101-treated mice compared to PBS-treated control mice (Fig. 1D, E).
AS101 treatment inhibits infiltration of monocytes and macrophages into the spinal cord
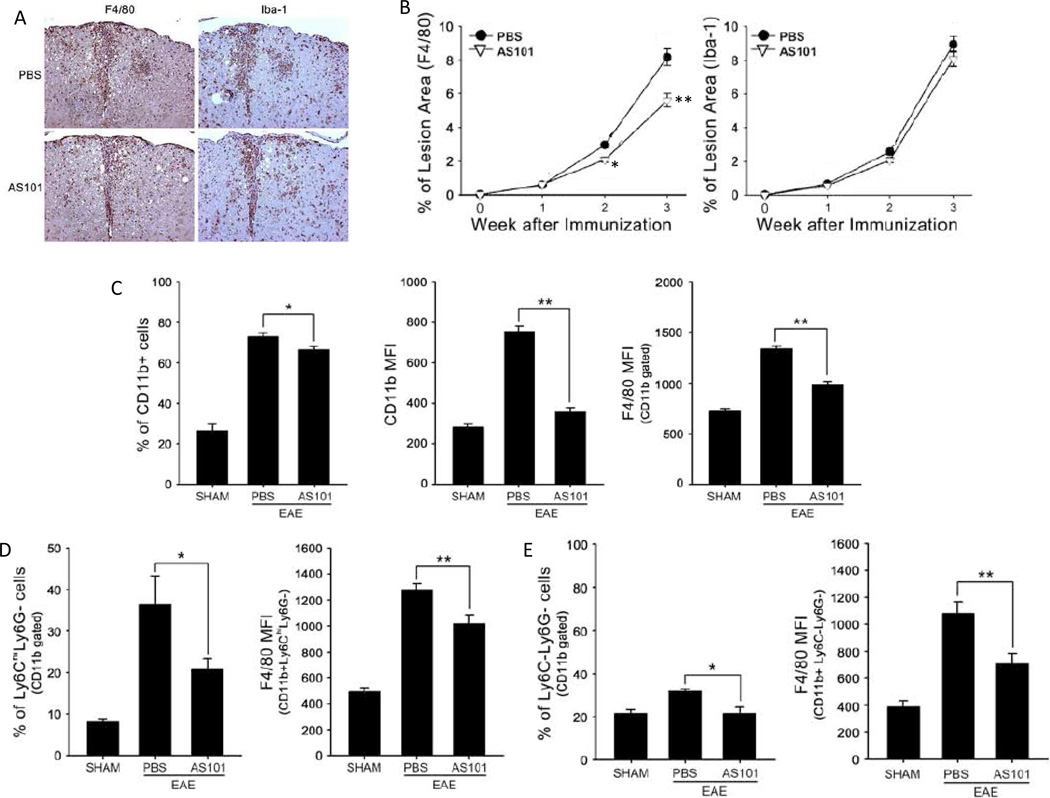
Circulating macrophages, and microglia residing in the CNS, are important effector cells in the pathogenesis of demyelination in MS (Jack et al., 2005; Lassmann, 2010). We evaluated the distribution of macrophages in the spinal cords of AS101-treated and control mice during EAE by immunohistochemical staining using antibodies against macrophage- and microglia-specific antigens (antibodies F4/80 and Iba-1, respectively). AS101-treated mice exhibited reduced numbers of F4/80-positive cells in spinal cord sections compared to PBS-treated mice (Fig. 2A, B).
Figure 2.
AS101 inhibits infiltration of pro-inflammatory monocytes/macrophages into the spinal cord in EAE mice. Spinal cords (n = 5–10 per group) were removed on days 7, 14 and 21 post immunization. (A) Representative spinal cord sections from EAE mice treated with PBS oor AS101 immunostained with F4/80 and Iba-1 antibodies (day 14 post immunization). (B) Results of quantification of % of lesion area occupied by F4/80 or Iba-1 immunoreactive cells (n=5 per group). *p<0.05, **p<0.01 compared to corresponding value for PBS-treated mice. (C – E) Mononuclear cells were isolated from spinal cords of mice in the sham, EAE PBS, and EAE AS101 groups (n = 5–10 mice per group). Cells were stained with CD11b, F4/80, Ly6C and Ly6G, then analyzed using flow cytometry. F4/80 expression in the cells is presented as mean fluorescence intensity (MFI). AS101 treatment reduced: the percentage of CD11b+ cells and the number of CD11b-expressing F4/80-positive cells (C), the proportion of inflammatory monocytes and the number of F4/80 expressing cells (D); and the proportion of CD11b+ Ly6C-Ly6G (resident monocyte) (D and E). *p<0.05, **p<0.01.
In mice, ‘inflammatory’ and ‘resident’ monocytes have been identified based primarily on the amount of time they spend in the blood before migrating into tissues (Mosser and Edwards, 2008; Geissmann et al., 2010). CD11b+Ly6ChighLy6G cells (inflammatory monocytes) are increased in the CNS during EAE induction (Zhu et al., 2007; King et al., 2009). CD11b+Ly6ChighLy6G cells are involved innate and adaptive immune responses in the CNS in MS patients, and in EAE animal models wherein they function as antigen presenting cells (APCs). We found that AS101 treatment reduced the percentage of CD11b+ cells and the number of CD11b-expressing F4/80-positive cells (Fig. 2C). The proportion of inflammatory monocytes also decreased in the AS101-treated EAE mice, as did the number of F4/80 expressing cells (Fig. 2D). Additionally, the proportion of CD11b+Ly6C-Ly6G (resident monocyte) cells was significantly reduced in AS101-treated mice compared to PBS-treated mice Fig. 2D, E). These results suggest that AS101 can prevent infiltration of CD11b+ monocytes and macrophages from peripheral blood into the CNS.
We next isolated splenocytes from EAE mice treated with AS101 or PBS for 14 days. Compared to unimmunized mice, the EAE mice exhibited a large reduction in splenocyte populations, with the extent of the splenocyte depletion not significantly different in AS101-treated and PBS-treated mice (Fig. 3A). EAE induced significant increases in the percentage of CD11b+ cells and F4/80-expressing cells in the spleens of both control and AS101-treated mice with no significant difference between these two groups (Fig. 3B). Similarly, the numbers of inflammatory monocytes (CD11b+Ly6ChighLy6G−) in the spleens of AS101-treated mice were not significantly different compared to PBS-treated mice (Fig. 3C).
Figure 3.
AS101 does not significantly affect numbers of pro-inflammatory monocytes and macrophages in the spleen. (A – C) Splenic mononuclear cells were isolated from sham mice, EAE PBS-treated mice and EAE AS101-treated mice on day 14 post immunization (n = 5–10 mice per group). Cells were stained with CD11b, F4/80, Ly6C and Ly6G, and analyzed using flow cytometry. F4/80 expression in the cells was presented as mean fluorescence intensity (MFI), *p<0.05, **p<0.01 compared to the SHAM group value.
AS101 treatment suppresses inflammatory cytokine expression in the spinal cord of EAE mice
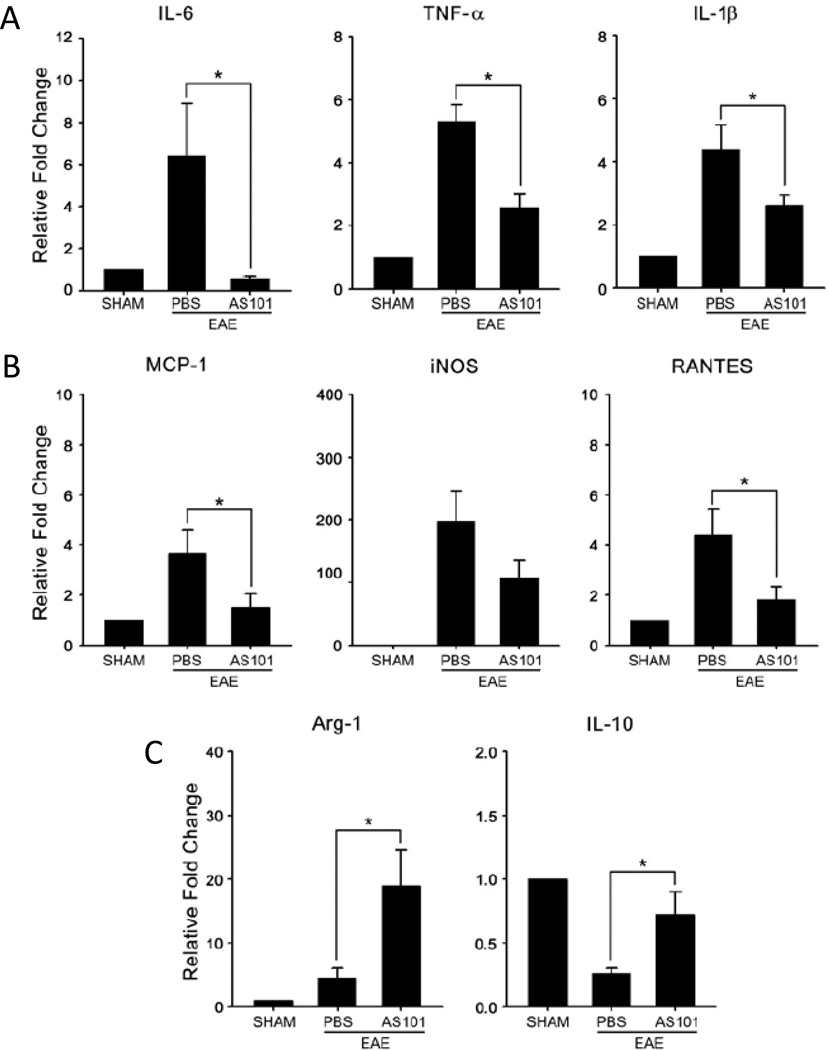
Activated macrophages express high levels of the pro-inflammatory cytokines TNF-α, IL- 1β, IL-6 and nitric oxide synthase (iNOS) (Mosser and Edwards, 2008). We measured pro-inflammatory cytokine mRNA levels during the early symptomatic period of EAE (14 days after immunization). AS101 treatment significantly attenuated the elevation of IL-6, TNF-α, IL-1β, MCP-1, iNOS and RANTES mRNAs in the spinal cord compared to PBS-treated control mice (Fig. 4A). On the other hand, the levels of IL-10 and Arginase-1, which exhibit anti-inflammatory actions, were elevated in AS101-treated mice (Fig. 4B).
Figure 4.
AS101 suppresses pro-inflammatory cytokine expression and elevates anti-inflammatory cytokine expression in the spinal cords during EAE. (A – C) Relative mRNA expression of: the pro-inflammatory cytokines IL-6, TNF-α and IL1-β (A): the chemokines MCP-1, iNOS and RANTES (B); and the anti-inflammatory cytokines Arginase-1 and IL-10 (C) in the spinal cord tissue of mice in the indicated groups at 14 days after immunization (6 mice per group). *p<0.01.
To determine whether suppression of pro-inflammatory cytokine expression by AS101 was specific to the CNS, we measured levels of cytokine mRNAs in spleen tissue samples from PBS-treated and AS101-treated EAE mice, and unimmunized (sham) mice. AS101 did not significantly affect the expression of IL-6, TNFα, iNOS or MCP-1, but did significantly attenuate the elevation of IL-1β mRNA levels (Fig. 5). AS101 had no significant effect on the expression levels of IL-10 or Arginase-1 in the spleens of EAE mice (Fig 5).
Figure 5.
AS101 has little or no effect on the expression of cytokines in the spleen. (A – C) Relative mRNA expression of: the pro-inflammatory cytokines IL-6, TNF-α and IL1-β (A); the chemokines MCP-1 and iNOS (B); and the anti-inflammatory cytokines Arginase-1 and IL-10 (C) in spleen tissue of mice in the indicated groups at 14 days after immunization (6 mice per group). *p<0.05, *p<0.01.
AS101 inhibits infiltration of VLA-4 expressing monocytes/macrophages
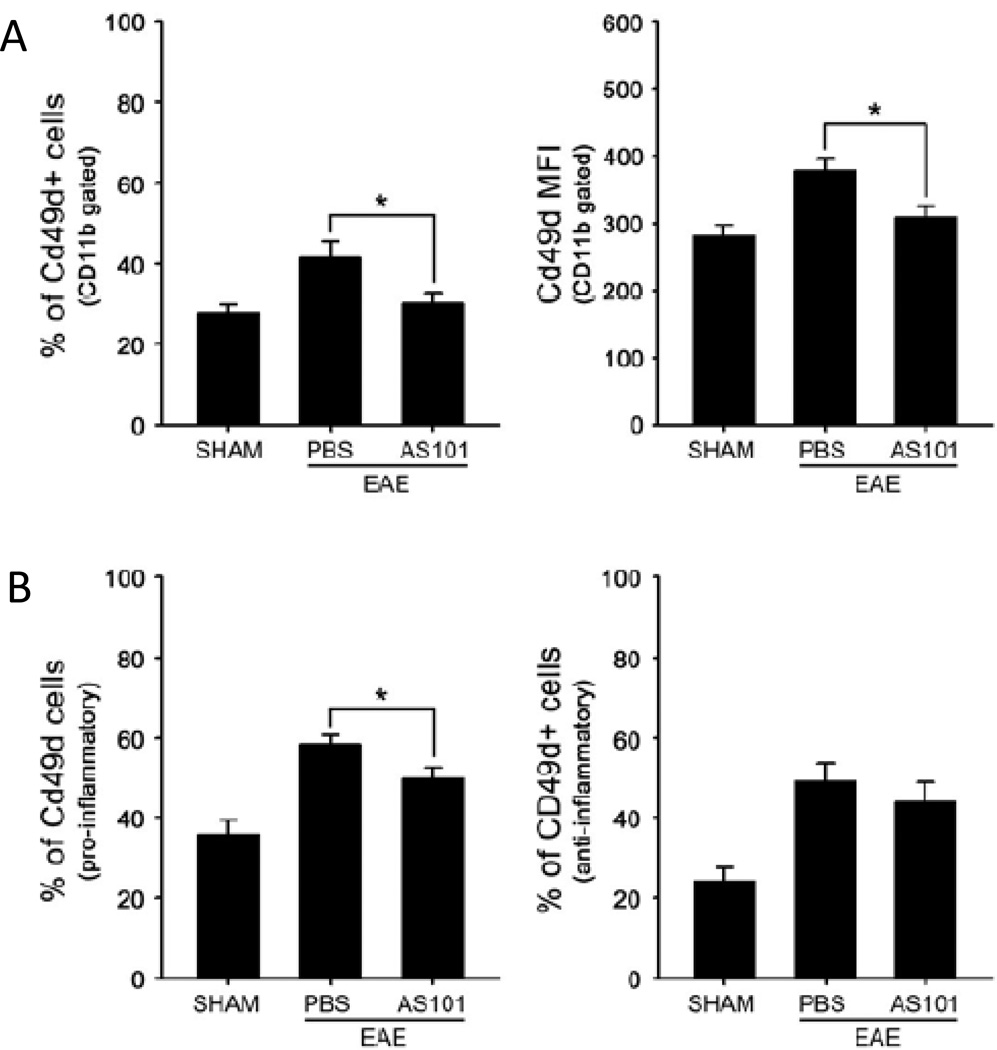
VLA-4 is an integrin dimer, which is composed of CD49d (α4) and CD29 (β1). It is expressed in most leukocytes (except neutrophils) and, by binding to fibronectin and the immunoglobulin superfamily member VCAM-1, VLA-4 plays a key role in the migration of immune cells to sites of tissue infection, injury and disease (Wayner et al., 1989). To determine whether AS101 inhibits the infiltration of VLA-4 positive monocytes into the spinal cord in EAE mice, we isolated CD11b+ cells from spinal cord after the onset of EAE symptoms (14 days after MOG immunization). EAE mice treated with PBS exhibited a significant elevation of CD49d+ cells in the spinal cord, whereas AS101-treated mice exhibited no elevation of CD49d+ cells compared to unimmunized sham control mice (Fig. 6A). AS101 treatment also suppressed the elevation of the proportion of CD49d+ cells in the pro-inflammatory monocyte population in EAE mice (Fig. 6B). These results suggest that AS101 inhibits the VLA-4 mediated infiltration of monocytes into the spinal cord in an animal model of MS.
Figure 6.
AS101 suppresses the migration of VLA-4 positive cells into the spinal cord in EAE mice. Quantification of brain infiltrates by flow cytometry. Spinal cord mononuclear cells from SHAM, EAE PBS-treated mice and EAE AS101-treated mice (n = 5––10 per group) were isolated on day 14 post immunization and subjected to flow cytometric analysis for VLA-4 in CD11b+ (A) and CD11b+Ly6C+Ly6G cells (B). *p<0.05.
AS101 inhibits the infiltration of reactive lymphocytes into the CNS of mice with EAE
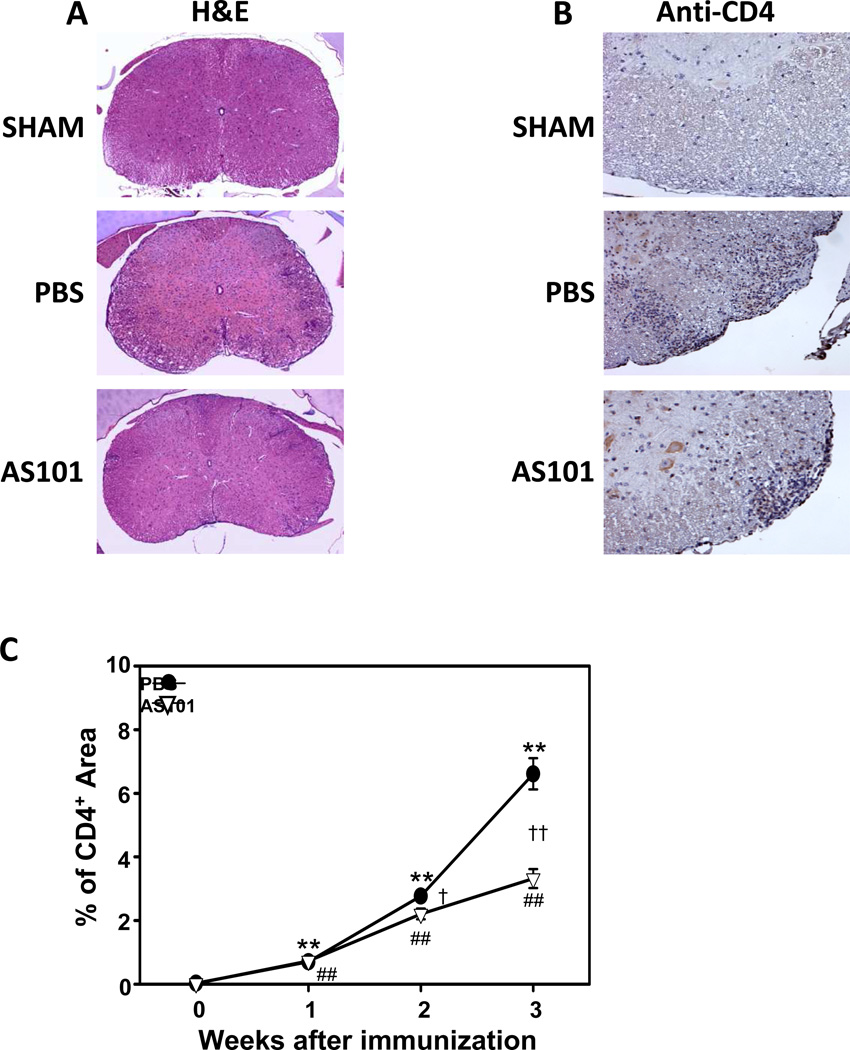
Macrophages and lymphocytes (mostly T cells) localize to the CNS, initiate the inflammation and lead to the MS symptoms and to the disease progression (Rezai-Zadeh et al., 2009). Preclinical studies in which treatments resulted in improved functional outcome have generally demonstrated significant reductions of cellular infiltration into the CNS (Prendergast and Anderton, 2009). We evaluated inflammatory cell penetration through the blood brain barrier (BBB) by examining spinal cord sections stained with H&E (Fig. 7A) or immunostained with CD4 antibody (Fig. 7B) from mice in the three groups. As expected, few or no leukocytes and CD4+ cells were observed in the white matter of spinal cords from sham (non-immunized) mice. In contrast, there was a progressive accumulation of leukocytes and CD4+ cells in the white matter of PBS-treated EAE mice (Fig. 7A-C). Counts of CD4+ cells demonstrated a significant attenuation of the accumulation of CD4+ cells in the white matter of AS101-treated EAE mice compared to PBS-treated EAE mice (Fig. 7C). These data suggest that AS101 reduces the extent of CD4+ T cell penetration through the BBB.
Figure 7.
AS101 prevents CD4+ cell migration in the EAE model. Histopathological assessment of EAE mice. Spinal cords (n = 5–10 per group) were removed on days 7, 14 and 21 post immunization. (A) The spinal cord sections were removed on day 14 post immunization from sham, PBS and AS101 groups and were stained with Hematoxylin and Eosin. Bar = 500 µm. (B) Representative images showing CD4 immunoreactivity in spinal cord sections from mice in the indicated groups (post-immunization day 14). Bar = 100 µm. (C) Results of quantitative analysis of CD4 cell staining (percentage of total spinal cord section area that exhibited CD4 immunoreactivity). **p<0.01 versus sham; ##p < 0.01 versus sham; †p < 0.05, ††p < 0.01 versus PBS value at the same time point).
AS101 prevents cell migration via VLA-4 (CD49) inhibition in the EAE model
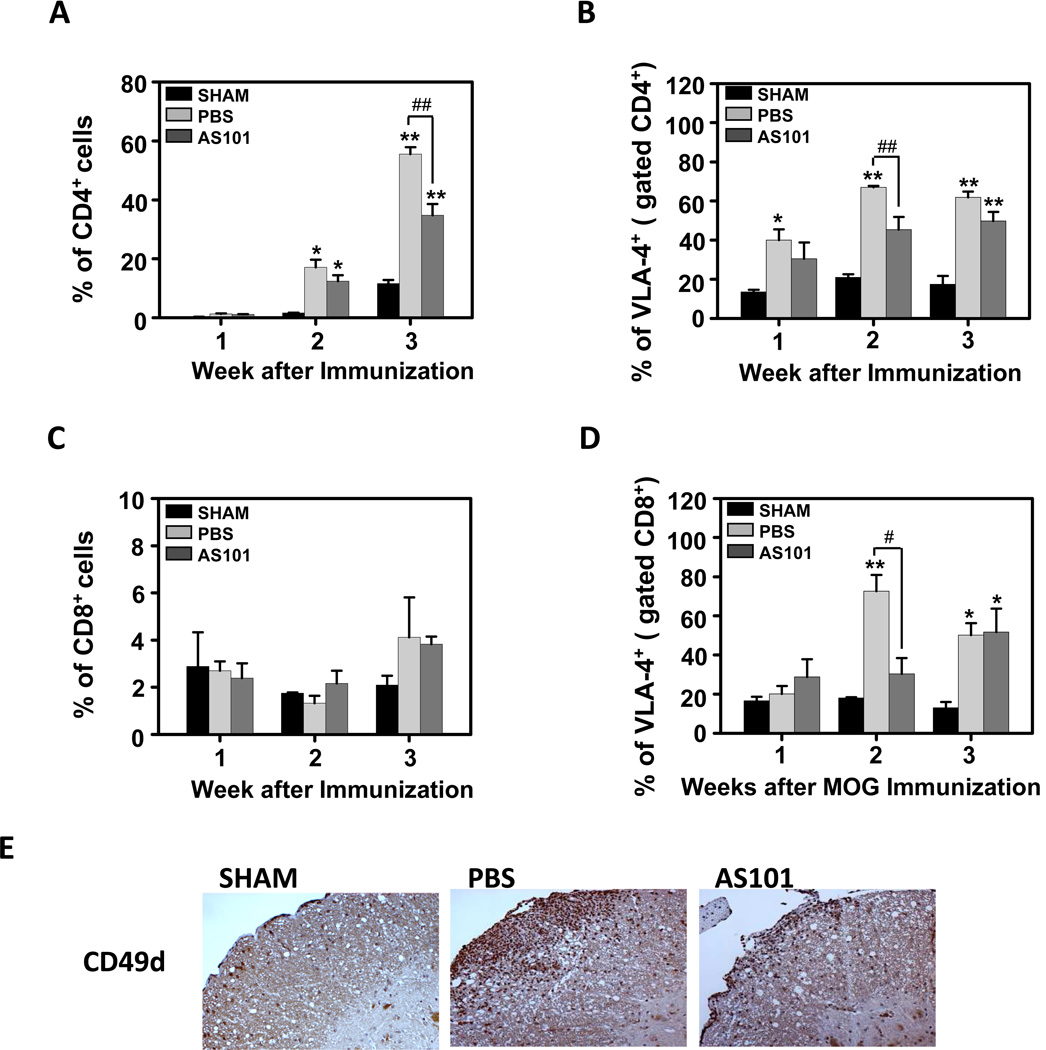
The VLA-4 (α4β1) integrin, also known as CD49, has been shown to play a key role, treatment with anti VLA-4 prevented accumulation of leukocytes in the CNS and the development of EAE model and anti VLA-4 inhibited transmigration of MS T cells specifically in patients (Yednock et al., 1992; Steinman, 2009). Hence, therapies designed to interfere with VLA-4 were found useful in treating inflammatory diseases including MS and inflammatory bowel disease. Previous studies in our laboratory showed that AS101 is able to oxidize and inhibit the free thiols on the alpha 4 chain of the VLA-4 integrin. Therefore, we hypothesized that AS101 might exert the aforementioned beneficial effects in the EAE model due to such integrin inhibition. To test this hypothesis, we evaluated the effects of AS101 on VLA-4 positive cells in the CNS of EAE mice. Mononuclear cells were isolated from the brain on post-immunization days 7, 14 and 21, and the cells were stained using a VLA-4 antibody for flow cytometry analysis. Most of the cells that penetrated through the BBB were CD4 positive cells, which are known to affect the inflammatory process (Holman et al., 2011; Kivisakk et al., 2009). The infiltration of CD4+ cells into the brain of PBS-treated mice was not evident at 1 week post-immunization, was moderate at 2 weeks, and accelerated between 2 and 3 weeks (Fig. 8A). AS101 significantly inhibited T helper (CD4+) cell migration into the brain of EAE mice compared to the PBS-treated EAE mice (Fig. 8A). Further analysis showed specific reduction of CD4/VLA-4 positive cells in the brains of AS101-treated EAE mice compared to PBS-treated EAE mice (Fig. 8B). The extent of cytotoxic T cell (CD8) migration into the brain was less than that of the CD4+ T cells (Fig. 8A, C). AS101 treatment did not significantly affect the total number of CD8+ cells that entered the brain in EAE mice (Fig. 8C). However, treatment with AS101 significantly decreased the number of CD8+ cells that expressed VLA-4 in the brain 2 weeks post-immunization (Fig. 8D). The role of AS101 in the reduction of the VLA-4+ cell infiltration into the CNS was also determined by VLA-4 immunostaining of spinal cord sections (Fig. 8E). Collectively, these findings suggest that treatment with AS101 decreases VLA-4 activity on immune cells, preventing them from migrating into the CNS, thereby inhibiting the inflammatory process, resulting in the preservation of myelin sheath viability.
Figure 8.
AS101 prevents VLA-4 positive cell migration to the central nervous system. (A-D) Quantification of brain infiltrates by flow cytometry. Whole brain mononuclear cells from sham (no disease), PBS (treatment control) and AS101 treated mice (n=5–10 per group) were removed on day 7, 14 and 21 post immunization and subjected to flow cytometry analysis for CD4(a), CD8(b) and VLA-4 (c,d). *p<0.05 versus Sham; **p<0.01 versus Sham; ##p < 0.01. (E) Representative spinal cords (n=5 mice per group) on day 14 post-immunization were stained with VLA-4 antibody. Bar = 500 µm.
AS101 induces apoptosis in MS patient-derived T-cell lines
An additional effect of VLA-4 blockade is to increase T cell apoptosis in experimental autoimmune neuritis (EAN) (Leussink et al., 2002). Here, we examined whether the effects of AS101 can be further extended to autoimmune T cell lineages responsive to encephalitogenic epitopes from MS patients. Patients-derived myelin antigen-reactive T cell lines were treated with different concentrations of AS101 for 72 hours, and the percentage of cells in early and late apoptosis was determined. Figure 9A shows that AS101 treatment increased the percentage of cells in early and late apoptosis, in a dose dependent manner. Thus, AS101 can induce apoptosis in autoimmune T cells from MS patients.
Figure 9.
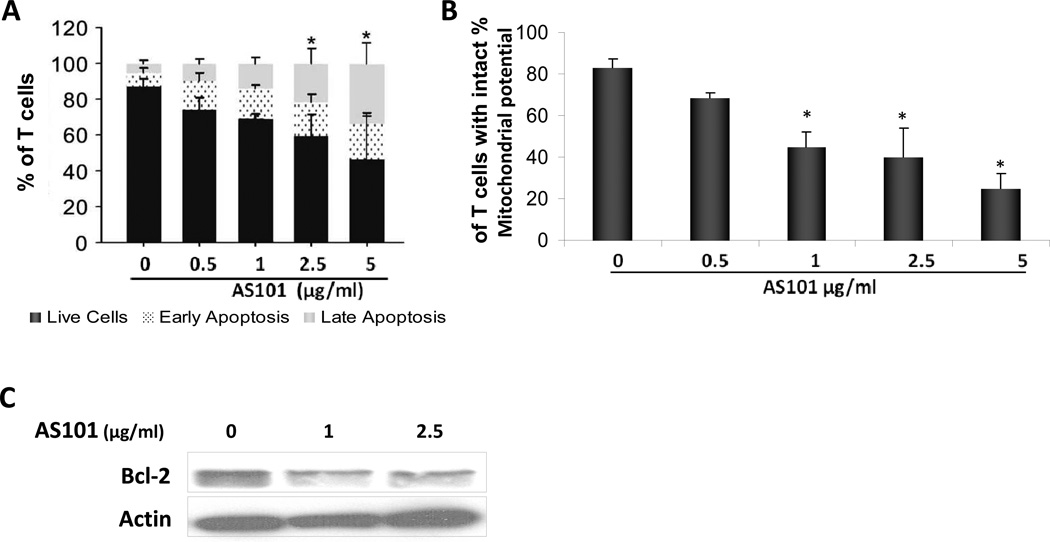
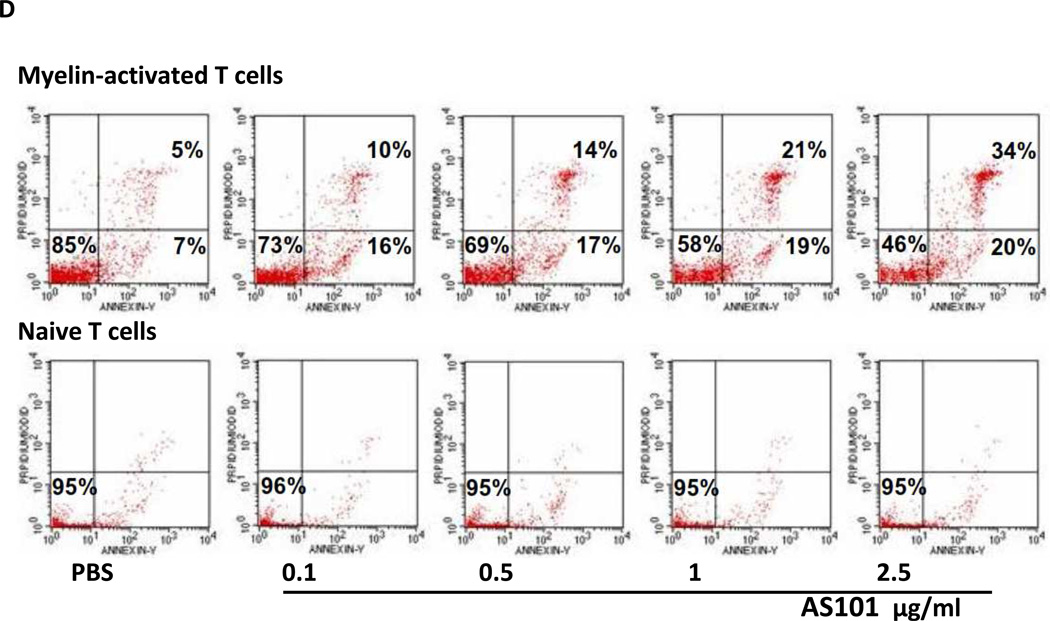
AS101 induces apoptosis in MS patient-derived T-cell lines. (A) Patient-derived T cell lines were incubated with AS101 at the indicated concentrations. After 72 hours, cells were stained with Annexin V and PI, and the percentage of live cells (Annexin V−, PI−), cells in early apoptosis (Annexin V+, PI−) and in late apoptosis (Annexin V+, PI+) were determined by FACS. The means ± SD of three independent experiments are shown. (*P<0.05) (black- live cells, dotted-early apoptosis, gray- late apoptosis). (B) The effect of AS101 on mitochondrial transmembrane potential of myelin antigen-reactive T cells. T cells were treated with AS101 at the indicated concentrations for 72 hr. The cells were incubated with rhodamine 123, and cell fluorescence reflecting the membrane potential, was analyzed by FACScan. (C) Activated T cells were incubated with AS101 (1 µg/ml and 2.5 µg/ml). After 48 hours, the cells were lysed and the levels of Bcl-2 were determined by western blot analysis. (D) Comparison of the effect of AS101 on patient-derived myelin antigen-reactive T-cells vs. naive T cells from normal donors. AS101 was administered at the indicated concentrations, to both activated and naive T cells. The percentage of live cells, cells in early apoptosis, and cells in late apoptosis were analyzed by FACScan. Myelin-activated T cells (upper panel) and naive T cells (lower panel) following treatment with AS101 alone for 72 hours. Results shown are representative from at least three different patients in each group.
Mitochondria play critical roles in signaling pathways that lead to apoptotic cell death (Susin et al., 1998). A decrease in mitochondrial transmembrane potential is one of the first irreversible steps of programmed cell death in many cell types, and is triggered by several distinct signals within the cell. To determine the role of apoptosis in the effects of AS101, the mitochondrial transmembrane potential of myelin antigen-reactive T cell cultures was examined following treatment with AS101 for 72 hours; AS101 treatment reduced the mitochondrial transmembrane potential (Fig. 9B).
Bcl-2 is an anti-apoptotic protein that stabilizes the mitochondrial membrane and blocks cytochrome c release from the mitochondrial intermembrane space and therefore prevents apoptosis (Martinou and Youle, 2011). We next tested the possibility that the apoptosis-inducing effect of AS101, which involves reduction in mitochondrial transmembrane potential, also involves changes in Bcl-2 levels in the cells. Activated T cells were treated with AS101 (1 µg/ml or 2.5 µg/ml) for 48 hours, and the levels of Bcl-2 were determined by immunoblot analysis. AS101 caused a reduction in Bcl-2 expression in myelin antigen-reactive T cells (Fig. 9C). These results suggest that the decreased Bcl-2 expression induced by AS101 may lead to mitochondrial membrane depolarization, and thus facilitate cytochrome c release, causing the cells to undergo apoptosis.
In order to compare the effects of AS101 on activated versus inactive T cells, T cells were isolated from healthy donors and incubated with the donor’s autologous serum in order to prevent their activation by exposure to foreign antigens. Resting and myelin antigen-reactive T cells were treated with AS101 alone for 72 hours and analyzed for the percentage of cells that underwent apoptosis. The results show that while AS101 induced apoptosis at levels of up to 54% in myelin-activated T cells when applied at the highest concentration, the resting T cells were not affected by AS101 (Fig. 9D).
Discussion
While interferon-β, glatiramer and monoclonal antibodies against VLA-4 exhibit a therapeutic benefit in some MS patients, they do not prevent or reverse the disease process, and can also result in serious side-effects (Jones and Coles, 2010; Bar-Or et al., 2011; Meuth et al., 2012). Our findings advance an understanding of the mechanisms of MS-like CNS damage and clinical manifestations, and demonstrate a therapeutic benefit of AS101, a drug with an outstanding safety profile (Shani et al., 1990). We found that the infiltration of monocytes and macrophages into the spinal cord of EAE mice is associated with heightened expression of pro-inflammatory cytokines (TNFα, IL-1β, IL-6) and iNOS, and reduced expression of anti-inflammatory cytokines (IL-10 and Arginase 1). Moreover, the infiltrating cell population was enriched with CD49d positive cells, suggesting an important role for VLA-4 in the recruitment of immune cells to the site of myelin damage. Clinical recovery in response to AS101 was accompanied by improvements at the histological level, with sharply curtailed T cell trafficking into the CNS as well as reductions in VLA-4+ cells present in the brain and spinal cord. Our findings suggesting a role for VLA-4 mediated monocyte infiltration in EAE pathogenesis are consistent with previous studies showing that VLA-4 antibodies can inhibit infiltration of monocytes into the CNS in EAE MS models (Yednock et al., 1992; Theien et al., 2003; van der Laan et al., 2002), with evidence that natalizumab can reduce the migratory capacity of monocytes from MS patients (Niino et al., 2006), and with the evidence that VLA-4 antibody treatment can reduce clinical deficits in MS patients (Steinman, 2005) .
AS101 is an organotellurium compound that has been demonstrated to have immunomodulatory activity at low doses of 3 – 10 mg in human subjects (Sredni et al., 1995, 1996) and at 0.1 – 1 mg/kg in rodents (Sredni et al., 2004a, 2004b, 2007; Okun et al., 2007). We found that in an EAE mouse model the infiltration into the spinal cord of pro-inflammatory monocytes characterized by CD11b+Ly6ChighLy6G− expression was decreased by AS101. Additionally, the infiltration of cells expressing F4/80, a mouse macrophage marker, was also inhibited by AS101. These effects of AS101 were specific to monocyte, macrophage and T cell movement into the CNS because AS101 did not significantly affect the proportion of pro-inflammatory monocytes elevated in the spleen in response to MOG immunization. Similarly, whereas AS101 did not affect levels of most of the cytokines measured in the spleen, it suppressed pro-inflammatory cytokine expression and elevated expression of anti-inflammatory factors. Collectively, our results therefore suggest that the therapeutic benefit of AS101 in the EAE model involves the inhibition of monocyte, macrophage and T cell migration into the CNS, and an associated inhibition of cytokine production by these cells within the CNS. However, a cause and effect relationship between monocyte and T cell infiltration and the clinical benefit in the EAE model remains to be established.
VLA-4 is considered critical for inflammatory cell migration and, indeed, we found decreased infiltration of VLA+ CD4+ T cells into the CNS of AS101-treated EAE mice compared to PBS-treated animals with similar initial disease severity. In addition to inhibition of T cell migration and pro-inflammatory cytokine expression, suppression of VLA-4 activity by AS101 also induced apoptosis in auto-reactive T cells from MS patients, but not in T cells from healthy donors. The latter results are consistent with a previous study showing that, in an adoptive transfer model of experimental autoimmune neuritis, treatment of animals with anti-VLA-4 monoclonal antibody led to a rapid and significant substantial increase of apoptotic T cells in the sciatic nerve, with a maximum effect preceding the significant decrease of T cell infiltration (Leussink et al., 2002).
In addition to suppressing chronic inflammation, AS101 has neuroprotective actions in animal models of focal ischemic stroke (Okun et al., 2007) and Parkinson’s disease (Sredni et al., 2007). The latter studies provided evidence that AS101 protects neurons against oxidative and metabolic insults in vivo by suppressing oxidative stress, inhibiting caspases, and inducing the expression of neurotrophic factors and Bcl-2 Moreover, AS101 protected cultured neurons from being damaged under conditions of energy deprivation, oxidative stress and overactivation of glutamate receptors (excitotoxicity) (Okun et al., 2007). The latter findings suggest the possibility that, in addition to inhibiting leukocyte infiltration into the CNS, AS101 might also have a therapeutic benefit in the EAE model by acting directly on neurons. Studies of MS patient CNS tissue samples and EAE animal models have demonstrated that axons degenerate and neurons die in MS, and have provided evidence for the involvement of oxidative stress, excitotoxicity and apoptotic mechanisms in the neurodegenerative process (Ahmed et al., 2002; Geurts and Barkhof, 2008; Gonsette, 2008; Haider et al., 2011).
In conclusion, our studies show that the AS101 has a significant suppressive effect on EAE onset and progression. AS101 treatment inhibits the migration of inflammatory monocytes and auto-reactive T cells that express VLA-4 integrin. In contrast to natalizumab, which increases the incidence of progressive multifocal leukoencephalopathy (McCormack, 2013), no discernible side effects of AS101 have thus far been reported from human trials of AS101 in cancer patients (Sredni, 2012). Therefore, the use of this nontoxic tellurium compound merits consideration as a promising agent for the treatment of MS.
Supplementary Material
Acknowledgement
This research was supported in part by the Intramural Research Program of the National Institute on Aging.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- Ahmed Z, Doward AI, Pryce G, Taylor DL, Pocock JM, Leonard JP, Baker D, Cuzner ML. A role for caspase-1 and-3 in the pathology of experimental allergic encephalomyelitis : inflammation versus degeneration. Am J Pathol. 2002;161:1577–1586. doi: 10.1016/S0002-9440(10)64436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, Rieckmann P, Traboulsee A, Yong VW. Targeting progressive neuroaxonal injury: lessons from multiple sclerosis. CNS Drugs. 2011;25:783–799. doi: 10.2165/11587820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. The New England Journal of Medicine. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Yosef S, Galit R, Albeck M, Longo DL, Albeck A, Sredni B. The synthetic tellurium compound, AS101, is a novel inhibitor of IL-1beta converting enzyme. J. Interferon Cytokine Res. 2007;27:453–462. doi: 10.1089/jir.2007.0168. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Halpert G, Albeck M, Sredni B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFkappaB signaling pathway and nitric oxide induction in macrophages. J Inflamm (Lond) 2010;7:3. doi: 10.1186/1476-9255-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton CM, Miszkiel KA, Barker GJ, MacManus DG, Pepple TI, Panzara M, Yang M, Hulme A, O'Connor P, Miller DH. Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. Journal of Neurology. 2004;251:407–413. doi: 10.1007/s00415-004-0332-4. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology. 2007;68:S13–S21. doi: 10.1212/01.wnl.0000275228.13012.7b. [DOI] [PubMed] [Google Scholar]
- Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Döpp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. Journal of Neuroimmunology. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- Friedman M, Bayer I, Letko I, Duvdevani R, Zavaro-Levy O, Ron B, Albeck M, Sredni B. Topical treatment for human papillomavirus-associated genital warts in humans with the novel tellurium immunomodulator AS101: assessment of its safety and efficacy. Br J Dermatol. 2009;160:403–408. doi: 10.1111/j.1365-2133.2008.08853.x. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7:841–851. doi: 10.1016/S1474-4422(08)70191-1. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. Oxidative stress and excitotoxicity: a therapeutic issue in multiple sclerosis? Mult Scler. 2008;14:22–34. doi: 10.1177/1352458507080111. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JJA, Teunissen CE, Vries HEd, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812:220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I, Rooijen Nv, Groot CJAd, Uitdehaag BMJ, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- Indenbaum V, Bin H, Makarovsky D, Weil M, Shulman LM, Albeck M, Sredni B, Mendelson E. In vitro and in vivo activity of AS101 against West Nile virus (WNV) Virus Res. 2012;166:68–76. doi: 10.1016/j.virusres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- Jones JL, Coles AJ. New treatment strategies in multiple sclerosis. Exp Neurol. 2010;225:34–39. doi: 10.1016/j.expneurol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Kalechman Y, Sredni B, Weinstein T, Freidkin I, Tobar A, Albeck M, Gafter U. Production of the novel mesangial autocrine growth factors GDNF and IL-10 is regulated by the immunomodulator AS101. J Am Soc Nephrol. 2003;14:620–630. doi: 10.1097/01.asn.0000053415.29636.4f. [DOI] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178:6695–6699. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol. 2010;225:2–8. doi: 10.1016/j.expneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Leussink VI, Zettl UK, Jander S, Pepinsky RB, Lobb RR, Stoll G, Toyka KV, Gold R. Blockade of signaling via the very late antigen (VLA-4) and its counterligand vascular cell adhesion molecule-1 (VCAM-1) causes increased T cell apoptosis in experimental autoimmune neuritis. Acta Neuropathol. 2002;103:131–136. doi: 10.1007/s004010100444. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack PL. Natalizumab: a review of its use in the management of relapsingremitting multiple sclerosis. Drugs. 2013;73:1463–1481. doi: 10.1007/s40265-013-0102-7. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Göbel K, Wiendl H. Immune therapy of multiple sclerosis--future strategies. Curr Pharm Des. 2012;18:4489–4497. doi: 10.2174/138161212802502198. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino M, Bodner C, Simard ML, Alatab S, Gano D, Kim HJ, Trigueiro M, Racicot D, Guérette C, Antel JP, Fournier A, Grand'Maison F, Bar-Or A. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59:748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- Noonan CW, Williamson DM, Henry JP, Indian R, Lynch SG, Neuberger JS, Schiffer R, Trottier J, Wagner L, Marrie RA. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 2010;7:A12. [PMC free article] [PubMed] [Google Scholar]
- Okun E, Arumugam TV, Tang SC, Gleichmann M, Albeck M, Sredni B, Mattson MP. The organotellurium compound ammonium trichloro(dioxoethylene-0,0') tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102:1232–1241. doi: 10.1111/j.1471-4159.2007.04615.x. [DOI] [PubMed] [Google Scholar]
- Okun E, Mattson MP, Arumugam TV. Involvement of Fc receptors in disorders of the central nervous system. Neuromolecular Med. 2010;12:164–178. doi: 10.1007/s12017-009-8099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A, Antel J. Pathogenesis of multiple sclerosis. Curr Opin Neurol. 2005;18:225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- Prendergast CT, Anderton SM. Immune cell entry to central nervous system--current understanding and prospective therapeutic targets. Endocr Metab Immune Disord Drug Targets. 2009;9:315–327. doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4:462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani A, Tichler T, Catane R, Gurwith M, Rozenszajn LA, Gezin A, Levi E, Schlesinger M, Kalechman Y, Michlin H. Immunologic effects of AS101 in the treatment of cancer patients. Nat Immun Cell Growth Regul. 1990;9:182–190. [PubMed] [Google Scholar]
- Sheremata WA, Minagar A, Alexander JS, Vollmer T. The role of alpha-4 integrin in the aetiology of multiple sclerosis: current knowledge and therapeutic implications. CNS Drugs. 2005;19:909–922. doi: 10.2165/00023210-200519110-00002. [DOI] [PubMed] [Google Scholar]
- Smyser GS. Counterstaining Golgi-Cox impregnations with luxol fast blue as a myelin stain. Stain Technol. 1973;48:53–57. doi: 10.3109/10520297309116581. [DOI] [PubMed] [Google Scholar]
- Sredni B, Caspi RR, Klein A, Kalechman Y, Danziger Y, Ben Ya'akov M, Tamari T, Shalit F, Albeck M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature. 1987;330:173–176. doi: 10.1038/330173a0. [DOI] [PubMed] [Google Scholar]
- Sredni B, Albeck M, Tichler T, Shani A, Shapira J, Bruderman I, Catane R, Kaufman B, Kalechman Y. Bone marrow-sparing and prevention of alopecia by AS101 in non-smallcell lung cancer patients treated with carboplatin and etoposide. J. Clin. Oncol. 1995;13:2342–2353. doi: 10.1200/JCO.1995.13.9.2342. [DOI] [PubMed] [Google Scholar]
- Sredni B, Tichler T, Shani A, Catane R, Kaufman B, Strassmann G, Albeck M, Kalechman Y. Predominance of TH1 response in tumor-bearing mice and cancer patients treated with AS101. J. Nat. Cancer Inst. 1996;88:1276–1284. doi: 10.1093/jnci/88.18.1276. [DOI] [PubMed] [Google Scholar]
- Sredni B, Weil M, Khomenok G, Lebenthal I, Teitz S, Mardor Y, Ram Z, Orenstein A, Kershenovich A, Michowiz S, Cohen YI, Rappaport ZH, Freidkin I, Albeck M, Longo DL, Kalechman Y. Ammonium trichloro(dioxoethylene-o,o')tellurate (AS101) sensitizes tumors to chemotherapy by inhibiting the tumor interleukin 10 autocrine loop. Cancer Res. 2004a;64:1843–1852. doi: 10.1158/0008-5472.can-03-3179. [DOI] [PubMed] [Google Scholar]
- Sredni B, Gal R, Cohen IJ, Dazard J-E, Givol D, Gafter U, Motro B, Eliyahu S, Albeck M, Lander HM, Kalechman Y. Hair growth induction by the Tellurium immunomodulator AS101: association with delayed terminal differentiation of follicular keratinocytes and rasdependent up-regulation of KGF expression. FASEB J. 2004b;18:400–402. doi: 10.1096/fj.03-0552fje. [DOI] [PubMed] [Google Scholar]
- Sredni B, Geffen-Aricha R, Duan W, Albeck M, Shalit F, Lander HM, Kinor N, Sagi O, Albeck A, Yosef S, Brodsky M, Sredni-Kenigsbuch D, Sonino T, Longo DL, Mattson MP, Yadid G. Multifunctional tellurium molecule protects and restores dopaminergic neurons in Parkinson's disease models. FASEB J. 2007;21:1870–1883. doi: 10.1096/fj.06-7500com. [DOI] [PubMed] [Google Scholar]
- Sredni B. Immunomodulating tellurium compounds as anti-cancer agents. Semin Cancer Biol. 2012;22:60–69. doi: 10.1016/j.semcancer.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat Rev Drug Discov. 2005;4:510–518. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- Strassmann G, Kambayashi T, Jacob CO, Sredni D. The immunomodulator AS-101 inhibits IL-10 release and augments TNF alpha and IL-1 alpha release by mouse and human mononuclear phagocytes. Cell Immunol. 1997;176:180–185. doi: 10.1006/cimm.1997.1087. [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Theien BE, Vanderlugt CL, Nickerson-Nutter C, Cornebise M, Scott DM, Perper SJ, Whalley ET, Miller SD. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood. 2003;102:4464–4471. doi: 10.1182/blood-2003-03-0974. [DOI] [PubMed] [Google Scholar]
- van der Laan LJ, van der Goes A, Wauben MH, Ruuls SR, Döpp EA, De Groot CJ, Kuijpers TW, Elices MJ, Dijkstra CD. Beneficial effect of modified peptide inhibitor of alpha4 integrins on experimental allergic encephalomyelitis in Lewis rats. J Neurosci Res. 2002;67:191–199. doi: 10.1002/jnr.10095. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. The Journal of cell biology. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.