Abstract
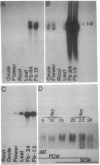
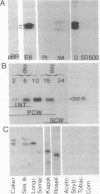
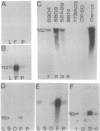
Cotton, an important natural fiber, is a differentiated epidermal cell. The number of genes that are active in fiber cells is similar to those in leaf, ovule, or root tissues. Through differential screening of a fiber cDNA library, we isolated five cDNA clones that are preferentially expressed in fiber. One of the cDNA clones, pCKE6, corresponded to an abundant mRNA in fiber. Transcripts for E6 were detected throughout the development of the fiber. Immunoprecipitation of in vitro translation products and Western blot analysis of fiber proteins showed two polypeptides in the range of 30-32 kDa as the products of E6 mRNA. Sequence analysis and hybrid-selected RNA translation also suggest that E6 mRNAs encode two polypeptides. Concentrations of E6 mRNA and protein are highest during the late primary cell wall and early secondary cell wall synthesis stages. Sequence comparison of E6 with other known eukaryotic and prokaryotic genes reveals no significant homology (GenBank; December 1991). E6 or a homologous gene(s) is conserved in several members of Malvaceae as well as in one other fiber-producing plant, kapok, but is not found in several other plants examined or in Acetobacter xylinum. A genomic clone corresponding to pCKE6 was isolated, and the promoter element of the E6 gene was shown to direct the expression of a carrot extensin mRNA in a tissue-specific and developmentally regulated fashion in transgenic cotton plants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M. E., John M. C., Ashley P., MacDonald R. J., Simpson E. R., Waterman M. R. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5628–5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M. C., Delmer D. P. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977 Jun;59(6):1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Willing R. P., Mascarenhas J. P. Analysis of the Complexity and Diversity of mRNAs from Pollen and Shoots of Tradescantia. Plant Physiol. 1984 Jul;75(3):865–868. doi: 10.1104/pp.75.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Fear A. L., Calhoon R. D., Eichinger G. H., Mayer R., Amikam D., Benziman M., Gelfand D. H., Meade J. H., Emerick A. W. Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8130–8134. doi: 10.1073/pnas.87.20.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]