Abstract
Objectives
Exercise blood pressure is a robust predictor of cardiovascular disease risk. Endothelial dysfunction occurs early in development of cardiovascular disease and is associated with greater exercise blood pressure in adults. However, it is not yet clear whether endothelial function is associated with exercise blood pressure in youth. The purpose of this study was to examine the relationship between endothelial function, indexed by brachial artery flow-mediated dilation, and submaximal exercise blood pressure in healthy adolescents.
Design
Cross-sectional study.
Method
Adolescents (N = 45) completed a graded submaximal treadmill test. Blood pressure was measured during rest and each exercise stage. Ultrasound measurement of brachial artery flow-mediated dilation was completed on a separate visit. Pearson correlations and multiple regression were used to assess the unadjusted and multivariate adjusted associations between flow-mediated dilation and exercise blood pressure, respectively.
Results
Lower flow-mediated dilation was associated with lower diastolic blood pressure (r = 0.37, p = 0.01) and greater pulse pressure (r = −0.38, p = 0.01) during exercise. The significance did not change when adjusting for age, gender, fitness, or resting blood pressure. Exploratory analyses suggest that flow-mediated dilation was associated with exercise diastolic blood pressure primarily among adolescents with low resting diastolic blood pressure.
Conclusions
Studies in youth are important to understand the early pathogenesis of cardiovascular disease. Findings from this study suggest that endothelial function may play a role in regulating blood pressure responses during submaximal exercise in healthy adolescents.
Keywords: Exercise, Blood pressure, Endothelial function, Cardiovascular disease, Youth
1. Introduction
Altered blood pressure (BP) responses during acute exercise are associated with cardiovascular disease (CVD) morbidity and mortality in adults1 and can add valuable information to the prediction of future CVD beyond that of resting BP.2,3 Moreover, in clinical settings, exercise tests are commonly used to identify individuals with CVD. While the association between exercise BP and CVD in adults has been well-studied, less is known about the mechanisms whereby greater BP during acute exercise is associated with greater CVD risk. Additionally, little is known about the association between exercise BP and cardiovascular risk in youth. Studies investigating the early pathogenesis of CVD are of great public health importance because the initiation of the atherosclerotic process and the antecedents of CVD appear in youth.4,5
The endothelium plays an essential role in protecting against the initial stages of atherosclerotic progression.6 Normal arterial function requires a balance between vasodilation and vasoconstriction, which is achieved in part by endothelial cell secretions of vasoactive substances (e.g. nitric oxide). This balance helps regulate blood flow and vascular tone during rest and exercise.7,8 Alterations in endothelial function likely contribute to BP responses during acute exercise9 and may be a potential mediator in the relationship between exercise BP and CVD risk. In adults, endothelial dysfunction is associated with greater exercise BP.10–12 However, few studies have assessed the relationship between endothelial function and exercise BP in youth.
BP responses to maximal aerobic exercise are frequently assessed in CVD research; however, youth spend a very small percentage of time during conditions of maximal exertion in everyday life. BP responses to submaximal exercise intensities are more comparable to the intensities of activities that youth routinely experience. Examination of the association between endothelial function and submaximal exercise BP in healthy youth is important to understand how endothelial function may impact blood pressure during normal daily activities and may provide insight into the mechanisms whereby exercise BP is associated with greater CVD risk in youth.13
Flow-mediated dilation (FMD) of the brachial artery, assessed non-invasively via ultrasound, has been validated in youth as a marker of vascular endothelial function.14 Thus, the purpose of this study was to examine the relationship between endothelial function, indexed by brachial artery FMD, and submaximal exercise BP in healthy, normal weight, adolescents. We hypothesized that lower FMD (e.g. worse endothelial function) would be associated with greater exercise BP. In exploratory fashion, we also examined whether the relationship between FMD and exercise BP varied by fitness level or resting BP given that these factors are associated with greater cardiovascular disease risk and exercise BP.13,15
2. Methods
Adolescents, aged 13–16, years were recruited for study through flyers and an existing database of families who had provided consent to be contacted for research studies. Exclusion criteria included history of CVD, smoking, body mass index (BMI) > 85th percentile, currently taking medications that could influence cardiovascular or endothelial functions (e.g. BP medication, depression medication), or any medical condition that would limit the ability to complete the exercise protocol (i.e. exercise-induced asthma, musculoskeletal conditions, insulin dependent diabetes). Additionally, adolescents were excluded if they had a resting SBP > 140 or resting DBP > 90. Parents provided written informed consent for their child’s participation and the adolescent provided written assent before participating in the study. Ethical approval of the study was obtained from the Social and Behavioral Sciences Institutional Review Board at the University at Buffalo.
Adolescents were tested on 2 days: an exercise day and an ultrasound day to assess FMD. Prior to each meeting, adolescents were instructed not to eat anything at least 3 h prior to the visit and not to participate in any intense physical activity or to consume any caffeine the day of or the day before the visit. The ultrasound appointment was completed at least 24 h after the exercise appointment, so that the exercise protocol would not affect the FMD measurement.
On the exercise day, height, weight and pubertal maturation were measured at the beginning of the appointment. Body weight was measured to the nearest 0.01 kg with the subjects wearing shorts and a t-shirt. Height was measured with a stadiometer. BMI was calculated according to the following formula: (BMI = kg/m2). BMI percentile was determined according to age and sex using the Center for Disease Control and Prevention growth charts.16 To assess maturation, adolescents were given the gender-appropriate set of standardized line drawings of pubertal development17 and classified their development based upon maturation of the secondary sex characteristics.18 Adolescents were fitted with a Polar heart rate (HR) monitor (Port Washington, NY) and an appropriate size BP cuff. BP was measured with a Suntech Tango monitor (Morrisville, NC). The Suntech Tango uses the auscultatory method aided by electrocardiographic R-wave gating and an oscillometric transducer and is a valid and reliable measure of BP during rest and exercise.19 Measurement of BP followed standard guidelines regarding cuff length and width, placement of the cuff around the arm 2.5 cm above the antecubital space, and seating of the cuff on the arm by inflating and deflating the cuff before taking any measurements. Adolescents were also fitted with a face mask attached to a metabolic cart (Vmax Encore Metabolic Cart, Sensormedics) via a sampling line to measure oxygen (O2) consumption. The metabolic cart was calibrated according to manufacturer instructions prior to each subject appointment.
Adolescents rested for 10 min to collect resting HR, BP, and O2 consumption. Adolescents then completed 5, 4-min stages of increasing intensity on a treadmill. Four minute stages were used to ensure the subject reached a steady state. The exercise protocol was used to determine submaximal exercise BP’s and to determine the O2 consumption, or physical working capacity (PWC), at a HR of 170 bpm as a measure of aerobic fitness. PWC measures are used widely to assess aerobic fitness and are validated for use in youth.20 The first stage of the exercise protocol was 2.7 METS. The velocity and % grade of the treadmill was progressively increased (approximately 1 MET increase each stage) until a HR of 170 bpm was attained or the subject completed 5 stages. Before beginning the protocol, subjects were given time to learn to walk comfortably on the treadmill. HR and O2 consumption were measured continuously during baseline and each exercise stage. BP was measured twice during the last 2 min of baseline and twice during the last 2 min of each exercise stage. After the test, adolescents were provided with rest and water.
FMD measures were performed by sonographers who were trained and certified at Wake Forest University School of Medicine. Brachial artery ultrasound scans were performed using a high resolution B-mode ultrasound imaging machine (Biosound Esaote, Inc., Indianapolis, IN) with a 10-MHz transducer. Prior to the test adolescents rested in the supine position for 15 min in a quiet temperature-controlled room with minimal lighting. A BP cuff was placed on the upper left arm to record BP at baseline and at the end of the test. The adolescent’s right arm was extended at the level of the heart with a beanbag placed in the right hand to prevent movement. Another BP cuff (pediatric size) was applied to the right forearm, 2 in. below the antecubital fossa. The sonographer positioned the transducer to longitudinally visualize both the near and far walls of the brachial artery. A continuous scanning procedure was used which maintained the transducer at this same location and angle of interrogation throughout the entire 8 min scan (1 min baseline, 4 min occlusion, 3 min deflation). During the occlusion period, the forearm BP cuff was inflated to 50 mmHg above the baseline SBP (but did not exceed 200 mmHg). De-identified images of the brachial artery were recorded on videotape using a super VHS recorder. Ultrasound scans were analyzed on-site using ImagePro Plus software (Media Cybernetics Inc., Bethesda, MD) by sonographers blinded to the adolescent’s exercise test results. FMD was calculated as the maximal diameter measured during the 3 min deflation period and is reported as a percent change from baseline diameter. Detailed descriptions of the image acquisition/analysis methods and reproducibility of the FMD protocol have been reported elsewhere.21 Notably, this protocol has been used to assess FMD in numerous studies including the Cardiovascular Health Study.22
Variables were examined for normality. FMD was square-root transformed for analyses in order to normalize the distribution. The average BP obtained during the last exercise stage (6.5 MET intensity) was used in all analyses to represent exercise BP. Although not presented here, analyses including the other exercise stages showed similar results. Pulse pressure (PP) was calculated by subtracting DBP from SBP. Sex differences in participant characteristics were assessed via independent t-tests. Unadjusted correlations between FMD, exercise BP, and covariates were examined with Pearson correlations.
Multiple linear regression was used to determine if FMD was associated with exercise BP (SBP, DBP, PP) after multivariate adjustment. We tested the association of FMD and exercise BP when adjusting for age, gender, fitness, and resting BP. We also considered the influence of baseline brachial artery diameter in these models. Separate models were constructed for exercise SBP, DBP, and PP as the outcome variables. Maturation was considered as a covariate in all analyses, but was not associated with the outcomes and did not alter any of the regression models. Therefore, maturation was not included as a covariate in the final analyses. BMI percentile was also considered as a potential covariate but was not included in the final analyses because it was highly correlated with fitness and because our sample was restricted to normal weight youth. Interactions between FMD and fitness, as well as interactions between FMD and resting BP, were assessed with cross-product terms in multiple linear regression models adjusting for age and sex. An alpha level of 0.05 for was used for all analyses.
3. Results
Fifty-three adolescents met the eligibility criteria and completed the study. FMD scans were unreadable for 5 subjects (2 boys, 3 girls). Additionally, BP readings were not recorded for 3 subjects during exercise. Thus, the final sample included for analyses consisted of 45 adolescents (23 boys, 22 girls). Adolescents excluded from analyses did not differ from those included on any key study variables including age, maturation, BMI percentile, fitness, or resting BP.
Participant characteristics of the adolescents included in the analyses are shown in Table 1. Boys had significantly greater (p < 0.05) fitness than the girls. There were no other differences between the boys and girls for any other characteristics, including FMD. Additionally, FMD was not associated with any demographic or physical characteristics and was not significantly associated with fitness or resting BP (data not shown). The mean change in BP during exercise (exercise BP – baseline BP) was 41.10 ± 16.14 mmHg for SBP and −12.52 ± 12.02 mmHg for DBP, which is consistent with previously reported normative data in healthy youth.23
Table 1.
Characteristics of the participants (N = 45).
| Age, mean (SD), y | 14.4 (1.1) |
| Boy, n (%) | 23 (51.1) |
| BMI %, mean (SD) | 47.6 (23.6) |
| Caucasian, n (%) | 38 (84.4) |
| Pubertal maturation, n (%) | |
| Tanner Stage II | 2 (6.7) |
| Tanner Stage III | 15 (33.3) |
| Tanner Stage IV | 22 (48.9) |
| Tanner Stage V | 5 (11.1) |
| PWC170, mean (SD), ml/kg/min | 36.8 (9.0) |
| Rest SBP, mean (SD), mmHg | 106.6 (7.2) |
| Rest DBP, mean (SD), mmHg | 63.2 (7.7) |
| Exercise SBP, mean (SD), mmHg | 147.7 (15.8) |
| Exercise DBP, mean (SD), mmHg | 50.7 (9.7) |
| FMD, mean (SD), % | 7.4 (2.9) |
BMI % = BMI percentile calculated based on the Center for Disease Control and Prevention age/sex growth charts; BMI % ≥ 85% is considered overweight.
DBP = diastolic blood pressure.
FMD = flow mediated dilation; the maximal percent change from baseline of brachial artery diameter.
PWC170 = O2 consumption at a heart rate of 170; used as a measure of aerobic fitness.
SBP = systolic blood pressure.
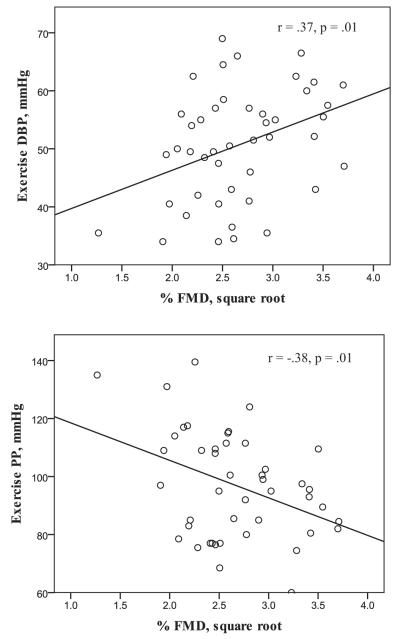
When examining the relationship between FMD and exercise BP, lower FMD was significantly associated with a lower DBP (r = 0.37, p = 0.01) and higher PP (r = −0.38, p = 0.01) in unadjusted models (Fig. 1). FMD remained significantly associated with both DBP (B = 6.5, SE = 2.6, p = 0.02) and PP (B = −12.4, SE = 4.9, p = 0.02) when adjusting for age, gender, fitness, and resting BP. However, the relationship between FMD and DBP was slightly attenuated (B = 6.0, SE = 3.2, p = 0.07) when adjusting for baseline brachial artery diameter in addition to adjustment for age, gender, fitness, and resting DBP. Adjusting for baseline brachial diameter did not affect the significance of the FMD-PP relationship (B = −15.5, SE = 6.0, p = 0.01). FMD was not associated (p ≥ 0.05) with SBP in any models.
Fig. 1.
The unadjusted relationship between flow-mediated dilation (FMD) and diastolic blood pressure (DBP; top panel) and pulse pressure (PP; bottom panel) during acute submaximal exercise.
The association between FMD and exercise DBP varied by resting DBP (interaction p = 0.03). FMD was significantly associated with exercise DBP primarily among adolescents with lower resting DBP (Table 2). Adjusting for baseline brachial artery diameter did not change the significance of the interaction or the significance of the stratified models. The association between FMD and exercise SBP and the association between FMD and exercise PP did not vary significantly by resting SBP or PP, respectively. Similarly, associations between FMD and any exercise BP measures did not differ by fitness level.
Table 2.
Associations between flow-mediated dilation and exercise DBP by median split of rest DBP.
| N | B | SE | p | |
|---|---|---|---|---|
| Low rest DBP (<63 mmHg) | 21 | 9.29 | 3.01 | 0.007 |
| High rest DBP (≥63 mmHg) | 24 | −0.84 | 4.91 | 0.87 |
DBP = diastolic blood pressure.
Models are adjusted for age and sex.
4. Discussion
In this sample of healthy, normal weight, adolescents lower FMD was associated with lower DBP and higher PP during acute submaximal exercise. The association between FMD and exercise DBP was observed primarily among adolescents with low resting DBP. Studies in youth are important to understand how early alterations in endothelial function may impact blood pressure during physical activity.
The endothelium plays a critical role in regulating arterial compliance and resistance. Endothelial cells release numerous vasodilator and vasoconstrictor substances that help regulate blood flow and vascular tone during rest and exercise.8 We hypothesized that there would be an inverse relationship between FMD and exercise BP due to poorer endothelial function resulting in greater BP’s during physical exertion. Our hypothesis was based on previous studies in adults10–12 and one study in children24 demonstrating that poorer endothelial function was associated with greater exercise BP (SBP, PP). In the present study, FMD was inversely associated with PP during exercise as we hypothesized. However, contrary to our hypotheses, FMD was not associated with exercise SBP and was positively related with exercise DBP.
PP has gained attention as an independent risk factor for CVD morbidity and mortality in adults.25 Notably, in the Framingham Heart Study, resting PP was a stronger predictor of incident coronary heart disease than either resting SBP or DBP.26 Much less work has been completed in youth, but Raitakari et al.27 found that resting PP in youth was prospectively associated with greater carotid artery intima-media thickness in adulthood. While resting PP has been associated with greater CVD risk in a number of studies, fewer studies have examined the relationship between PP during exercise and CVD risk. Of these studies, most have been in adults and only a few have examined the relationship between FMD and exercise PP. Stewart et al.10 reported a significant inverse association between FMD and PP during maximal exercise in adults with untreated high BP. Similar to these results in adults, lower FMD was associated with greater PP in our sample of healthy adolescents.
In addition to the limited number of studies examining exercise PP in youth, very few studies have assessed the association between exercise DBP and CVD risk in youth with hardly any of these studies including measures of endothelial function. Our results suggest that poorer endothelial function may be associated with lower DBP in healthy, normal weight adolescents. This finding was unexpected given that greater exercise DBP is generally thought to be associated with greater CVD risk.28 However, exploratory analyses indicated that the positive relationship between FMD and exercise DBP was due primarily to adolescents with lower resting DBP. Although there is limited literature in youth to compare our findings, Stewart et al.10 reported a marginally significant positive relationship between FMD and exercise DBP (r = 0.29, p < 0.06) among women in their study. Notably, resting DBP was significantly lower among the women compared to the men in this study. It is presently unclear why poorer endothelial function would be associated with lower exercise DBP among adolescents with low resting DBP. In healthy individuals, DBP during aerobic exercise changes very little, with small increases or decreases considered normal.23 In the present sample, DBP during exercise was unchanged or decreased from resting levels in >80% of adolescents. Perhaps adolescents with lower resting DBP require different regulatory mechanisms to keep their BP from dropping too low during exercise. Given that we are among the few studies to examine the association between endothelial function and exercise BP in youth, and the first to report an association between FMD and exercise DBP in healthy adolescents, future work is needed to replicate our findings.
This study had several limitations. Adolescents were healthy, normal weight and mostly Caucasian. The results may not generalize youth with known diseases, overweight youth, or minority youth. FMD is used extensively in research; however, FMD is only a marker and not a direct measure of endothelial function. We did not assess endothelium-independent vasodilation with nitroglycerin. Thus, we cannot be certain that a 4-min period of forearm occlusion produced an endothelium-dependent nitric oxide derived dilation. Furthermore, although great efforts were made to standardize protocols, it is possible that a variety of factors (technical, environmental, physiological) could have influenced measurement of FMD. Our small sample size potentially limited power to detect differences, especially when assessing interactions. We did not assess BP recovery after exercise. The relationship between BP recovery and endothelial function in youth is an important area for future investigation given that impaired BP recovery is associated with greater CVD risk in adults.29 The cross sectional study design does not allow us to determine causality. Thus, we are unable to determine whether endothelial function affects the release of vasoactive substances which affects BP responses during exercise or whether changes in BP during exercise impact the endothelium. Future work is needed to clarify the precise mechanisms underlying the association between FMD and exercise BP.
5. Conclusion
Studies in youth are important to understand the early pathogenesis of CVD. Endothelial dysfunction occurs early in the development of CVD and is associated with future cardiovascular events. Findings from this study suggest that poorer endothelial function is associated with alterations in exercise BP among healthy, normal weight adolescents. Thus, submaximal exercise testing in youth may be a useful tool to identify early alterations in endothelial function.
Practical implications.
Exercise testing, even at the submaximal level, may be useful to identify youth with early alterations in endothelial function.
Clinicians and researchers should pay greater attention to exercise diastolic blood pressure and pulse pressure, in addition to the more commonly analyzed systolic blood pressure.
Indentifying risk factors for cardiovascular disease in youth is important for early intervention.
Acknowledgements
We thank Vivian Boyd and Debbie Saltino for their technical assistance in completing the ultrasound measurements. Thank you to the Mark Diamond Research Fund for their financial support which helped to reimburse participants.
References
- 1.Weiss SA, Blumenthal RS, Sharrett AR, et al. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121(19):2109–2116. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundal R, Kjeldsen SE, Sandvik L, et al. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24(1):56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Singh JP, Larson MG, Manolio TA, et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99(14):1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 5.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 6.Mehta JL. Endothelium: coronary vasodilation, and organic nitrates. Am Heart J. 1995;129(2):382–391. doi: 10.1016/0002-8703(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 7.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 8.Maiorana A, O’Driscoll G, Taylor R, et al. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33(14):1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tzemos N, Lim PO, MacDonald TM. Exercise blood pressure and endothelial dysfunction in hypertension. Int J Clin Pract. 2009;63(2):202–206. doi: 10.1111/j.1742-1241.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 10.Stewart KJ, Sung J, Silber HA, et al. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17(4):314–320. doi: 10.1016/S0895-7061(03)01003-3. [DOI] [PubMed] [Google Scholar]
- 11.Olson KM, Augeri AL, Seip RL, et al. Correlates of endothelial function and the peak systolic blood pressure response to a graded maximal exercise test. Atherosclerosis. 2012;222(1):202–207. doi: 10.1016/j.atherosclerosis.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Thanassoulis G, Lyass A, Benjamin EJ, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125(23):2836–2843. doi: 10.1161/CIRCULATIONAHA.111.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schieken RM, Clarke WR, Lauer RM. The cardiovascular responses to exercise in children across the blood pressure distribution. The Muscatine study. Hypertension. 1983;5(1):71–78. doi: 10.1161/01.hyp.5.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues AN, Perez AJ, Carletti L, et al. The association between cardiorespiratory fitness and cardiovascular risk in adolescents. J Pediatr (RioJ) 2007;83(5):429–435. doi: 10.2223/JPED.1696. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 17.Morris N, Undry J. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 18.Tanner JM. Growth at Adolescence. 2nd ed Blackwell Scientific; Oxford, England: 1962. [Google Scholar]
- 19.Taylor RS, Gallen I. Evaluation of SunTech 4240 during rest and during exercise: a novel automated blood pressure device. J Cardiopulm Rehabil. 1994;14:330–334. [Google Scholar]
- 20.DuRant RH, Dover EV, Alpert BS. An evaluation of five indices of physical working capacity in children. Med Sci Sports Exerc. 1983;15(1):83–87. doi: 10.1249/00005768-198315010-00015. [DOI] [PubMed] [Google Scholar]
- 21.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8(5):319–328. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 22.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 23.Becker Mde M, Barbosa e Silva O, Moreira IE, et al. Arterial blood pressure in adolescents during exercise stress testing. Arq Bras Cardiol. 2007;88(3):329–333. doi: 10.1590/s0066-782x2007000300012. [DOI] [PubMed] [Google Scholar]
- 24.Treiber F, Papavassiliou D, Gutin B, et al. Determinants of endothelium-dependent femoral artery vasodilation in youth. Psychosom Med. 1997;59(4):376–381. doi: 10.1097/00006842-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Domanski M, Norman J, Wolz M, et al. Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I) Hypertension. 2001;38(4):793–797. doi: 10.1161/hy1001.092966. [DOI] [PubMed] [Google Scholar]
- 26.Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100(4):354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 27.Raitakari OT, Juonala M, Taittonen L, et al. Pulse pressure in youth and carotid intima-media thickness in adulthood: the cardiovascular risk in young Finns study. Stroke. 2009;40(4):1519–1521. doi: 10.1161/STROKEAHA.108.525253. [DOI] [PubMed] [Google Scholar]
- 28.Akhras F, Upward J, Jackson G. Increased diastolic blood pressure response to exercise testing when coronary artery disease is suspected. An indication of severity. Br Heart J. 1985;53(6):598–602. doi: 10.1136/hrt.53.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McHam SA, Marwick TH, Pashkow FJ, et al. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34(3):754–759. doi: 10.1016/s0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]