Abstract
Aberrant changes to the insulin-like growth factor (IGF) axis promote prostate cancer development and progression, adaptation for growth and survival in a castrate environment, and invasive metastasis. Natural and synthetic compounds that target the IGF axis to prevent or reverse theses abnormalities may be extremely useful in the chemoprevention and chemotherapy of prostate cancer. Apigenin, a naturally-occurring flavone found in many fruits and vegetables, is one such compound that can correctively modulate the IGF axis to induce growth arrest and apoptosis in many pre-clinical in vitro and in vivo models of prostate cancer. Because of its known mechanism of action, low toxicity, and effectiveness at physiologically relevant levels in animal models of prostate cancer, apigenin is an excellent candidate for a pilot study to determine the effect of apigenin supplementation on prostate cancer development and progression in humans.
Keywords: apigenin, insulin-like growth factor-1 (IGF-1), insulin-like growth factor-1 receptor (IGF-1R), Insulin-like growth factor binding protein-3 (IGFBP-3), prostate cancer
Introduction
Although the death rate from prostate cancer has been steadily decreasing since the mid-1990s, prostate cancer remains one of the most prevalent cancers diagnosed in North American men and the second-leading cause of male cancer-related death behind lung cancer [1]. According to the most recent American Cancer Society estimates, approximately 241,740 new cases of prostate cancer will be diagnosed in the United States in 2012, plus an additional estimated 28,170 disease-related deaths [1]. Clinical and pre-clinical evidence strongly implicates the insulin-like growth factor (IGF) axis in the neoplastic growth and tumorigenesis of the prostate gland [2–4 and references within]. Highly-conserved among mammals due to its importance in normal growth and development, the IGF axis is altered in prostate cancer cells to promote uncontrolled tumor cell growth, survival under adverse conditions, such as androgen-deprivation, and malignant transformation [2–5]. IGF axis-targeting chemical compounds and antibodies shown to reverse or inhibit IGF-mediated signaling aberrations in prostate cancer could be successfully developed as preventive or therapeutic agents. Apigenin, a natural flavone found abundantly in many fruits and vegetables, is one anticancer compound which has shown promising results in the inhibition of IGF-signaling in prostate cancer cells [6]. This review will provide a brief overview of the IGF axis in normal physiology and growth, discuss alteration of the IGF axis necessary for the promotion of prostate cancer development and progression, and describe modulation of the IGF axis by apigenin, which causes growth arrest and apoptosis in prostate cancer cells with minimal intrinsic toxicity to normal cells.
The Insulin-like Growth Factor Axis
The insulin-like growth factor (IGF) signaling axis is an important modulator of normal growth, development, differentiation, and organogenesis [2]. This axis consists of two ligands (IGF-1 and IGF-2), six high-affinity binding proteins (IGFBP-1 through -6), at least nine low-affinity binding proteins, multiple proteases, two main receptors (IGF-1R and IGF-2R) and insulin/IGF hybrid receptors, and proteins involved in the intracellular signaling downstream of these receptors. Growth hormone is also often considered part of the IGF axis. As the IGF axis is highly-conserved in mammals, it likely arose early in evolution, possibly as a regulator of cell proliferation relative to nutrient availability [2]. Additionally, the IGF axis plays an important role in tumor development, as imbalance of this growth axis preferentially favors uncontrolled cell proliferation, survival, and malignant transformation [2].
The IGF-1 and IGF-2 Ligands
Insulin-like growth factor type I (IGF-1) and type II (IGF-2), the two ligands of the IGF axis, are single-chain polypeptide hormones sharing approximately 50 percent homology to insulin [5]. In healthy adults, both IGF-1 and IGF-2 are synthesized primarily in the liver [2]. The transcription of IGF-1 is regulated by growth hormone-releasing hormone secretion by the hypothalamus, which acts on the anterior pituitary gland causing it to synthesize and secrete stored growth hormone into the circulatory system; this polypeptide then binds to growth hormone receptors on hepatic cells, triggering a signal transduction which increases IGF-1 expression [2, 5]. Unlike IGF-1, growth hormone does not regulate IGF-2 transcription. Instead, IGF-2 transcriptional regulation appears to be much more complex, involving a non-coding anti-sense RNA and trans regulated genomic imprinting, the mechanism of which is still being fully deciphered [7]. The human body does not store IGF-1 and -II [2]; rather, the liver releases the proteins as endocrine hormones into the bloodstream, where they act as a circulating pool of both free and complexed IGF ligands [8]. Within the adult human population, significant variation in the circulating levels of IGF ligands exists, and a normal reference range still remains poorly defined. The reference range of serum IGF-1 for adults over 50 years of age, as defined by Quest Diagnostics from a large patient sample in 2012, is 50–320 ng/ml (however, growth hormone status of the individuals used to define this adult reference range remains unknown) [9]; serum IGF-2 levels are approximately double the level of serum IGF-1 [10]. In humans, circulating levels of IGF-1 gradually increase after birth, peak throughout puberty and early adulthood, and then gradually decline thereafter [11]; however, compared to other growth factors, levels of circulating IGFs remain relatively constant throughout one’s lifespan [11]. Circulating IGF-1 is able to exert a negative feedback on both growth hormone-releasing hormone production in the hypothalamus and growth hormone production and release by the anterior pituitary gland [5].
The IGF Receptors and Downstream Signaling
Free IGF ligands are bioavailable and can interact with type-I and type-II IGF receptors, the insulin receptor (IR), and insulin-IGF receptor hybrids located on the plasma membrane of cells within target organs [2,5]. The type-I IGF receptor (IGF-1R) is a transmembrane receptor tyrosine kinase (RTK) that shares approximately 70 percent homologous structure with the insulin receptor [3]. IGF-1R is initially translated as a single chain peptide precursor that is disulfide-bonded and cleaved within the Golgi complex to yield a heterodimer comprised of two subunits—α and β; a pair of heterodimers then combine to form the complete heterotetramer receptor [5]. IGF-1R binds preferentially to IGF-1, but it will also bind IGF-2 and insulin; albeit with 2- to 15-fold and 1000-fold lower affinity than IGF-1, respectively [5, 12]. Binding of IGF ligand to the α-subunits of IGF-1R induces a conformational change, activating the intrinsic tyrosine kinase activity of the β-subunits [13]. This leads to autophosphorylation of Tyr-1131, Tyr-1135, and Tyr-1136 (in humans), triggering downstream signaling [13].
The two major signaling pathways downstream of IGF-1R are the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB/Akt) pathway and the Ras/mitogen activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway [3]. The insulin receptor substrate protein (IRS-1/2) is one immediate downstream target of IGF-1R [2, 14]; IRS-1 serves as a scaffold protein with 18 tyrosine phosphorylation sites to which downstream effectors are recruited and bind via their SH2 (Src homology 2) or PTB (phosphotyrosine-binding) domains [14]. One such downstream effector is PI3K [2]; IRS-1 recruits, phosphorylates, and activates PI3K, catalyzing formation of lipid second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) from phosphatidylinositol-4,5-bisphosphate (PIP2) at the plasma membrane [6]. Akt is then recruited to the plasma membrane by interaction of its pleckstrin homology domain with PIP3; this interaction results in a structural conformational change within Akt, exposing Thr-308 and Ser-473 [6]. Upon exposure, Thr-308 is phosphorylated by phosphoinositide-dependent kinase-1 (PDK1), a protein also recruited to the plasma membrane by PIP3 through its pleckstrin homology domain and then directly phosphorylated and activated by IGF-1R [6]. Ser-473 is then phosphorylated by phosphoinositide-dependent kinase-2 (PDK2); this stabilizes the active conformation of the Akt protein kinase and is necessary for full activation [6]. PKB/Akt then phosphorylates a number of downstream proteins to promote cell proliferation, inhibit cell differentiation, increase metabolic actions, and inhibit apoptosis [6].
A second immediate downstream target of IGF-1R is the Src homologous and collagen containing (Shc1) signaling protein, which, following phosphorylation by IGF-1R, is responsible for activation of the Ras protein [2, 3]. Ras then triggers the MAPK pathway, a cascade of serine/threonine-selective protein kinases, resulting in phosphorylation and nuclear translocation of ERK1/2 [2, 3]. This leads to transcription factor recruitment and stimulation of cell proliferation [3]. The comprehensive effect of an active IGF-1R is the stimulation of signaling leading to cellular proliferation, anabolic activity, and survival [5].
The type-II IGF receptor (IGF-2R) binds IGF-2 with 500-fold greater affinity than IGF-1, and does not bind insulin [5]; yet, despite ligand binding, possesses no known intrinsic tyrosine kinase activity and does not appear to transduce a signal [2,3]. Although no signaling function has yet been discovered, IGF-2R is known to function as a scavenger receptor, sequestering, internalizing, and degrading IGF-2 (and IGF-1) to regulate circulating levels of IGF-2 [2,5,15]. In this manner, IGF-2R may possess anti-proliferative and pro-apoptotic character [2], and may even be considered a tumor-suppressor gene, as loss of IGF-2R expression is associated with increased IGF-2-initiated IGF-1R activation and increased cell proliferation [16].
The IGF Binding Proteins
The insulin-like growth factor-binding protein (IGFBP) super-family is comprised of at least fifteen members divided into two sub-families based on their IGF binding affinities: high-affinity IGFBPs and low-affinity IGFBPs. The high-affinity IGFBP family is made up of six well-characterized IGFBPs, designated IGFBP-1 through -6, and the low-affinity IGFBP family consists of nine IGFBP-related proteins (IGFBP-rPs) and proteolysed IGFBP fragments [17]. [Low-affinity IGFBPs will not be addressed in detail in this review; however, for more information on this topic, please refer to Ref. 17.] Although differing in molecular weight, hormonal regulation, and IGF ligand affinity, all members of the IGFBP superfamily are produced and secreted by the liver, found circulating in serum and biological fluids, and share a conserved N-terminal cysteine-rich domain [5, 17].
Over 99 percent of IGF ligands circulate in association with a high-affinity IGFBP [3, 5]. At present, six mammalian high-affinity IGFBPs (IGFBP-1 through -6) with unglycosylated molecular masses ranging from 22–31 kDa are known [5, 17, 18]. These IGFBPs are also secreted by the liver and are arranged into a sub-family based on sufficient evidence that they i) are cysteine-rich proteins (16–20 cysteines in the pre-peptides), ii) share extensive similarity in their primary amino acid sequences, and iii) bind IGFs with very high affinity, but do not bind insulin [15,17]. IGFBP-1 through -6 demonstrates binding affinities of kD ≈ 300–700 pM for IGF-1 and -II [4].
High-affinity IGFBPs are composed of three distinct domains of roughly equivalent sizes, including highly conserved N-terminal and C-terminal domains and a variable central domain [17]. Overall amino acid sequence similarity between IGFBPs ranges from 45–60 percent with the majority of the conserved residues present within the N- and C-terminal domains [17]. Each of the IGFBPs contain 16–18 conserved cysteine residues also clustered in the conserved N- and C-terminal domains, forming five (in the case of IGFBP-6) or six (in IGFBP-1 through -5) intradomain disulfide bonds in the N-terminal domain and three C-terminal intradomain disulfide bonds [15]. Due to significant disulfide bonding, both N- and C-terminal domains retain rigid, ladder-like structures [5, 15]. Each of the three domains possesses critical sub-domains which are important for the numerous functions of the IGFBPs. The folding of IGFBPs forms a crescent-shaped IGF-binding pocket where residues from both N- and C-terminal domains are involved in IGF ligand binding, masking amino acid residues on the ligand responsible for IGF and receptor interaction [5, 15, 19]. Distinctive motifs for acid labile subunit binding, integrin recognition (IGFBP-1 and -2), nuclear localization (IGFBP-3 and -5), and heparin binding (IGFBP-3, -5, and -6) have been discovered in the C-terminal domain [15]. The central domain is highly variable and unique to each IGFBP, with a shared similarity of less than 15 percent [5, 17]. The central domain does not participate in disulfide bonding, but possesses sites for post-translational modification by glycosylation and phosphorylation (IGFBP-3 and -5) and proteolytic cleavage sites, both of which are not found in either N- or C-terminal domains [15]. Central domain post-translational modification can modulate affinity for IGFs, cell surface interactions, and susceptibility to proteases, whereas proteolysis favors greatly reduced affinity for IGF-1 and -II [8]. Some IGFBPs also contain distinctive sequences necessary for acid labile subunit, heparin, and cell surface protein binding within their central domains [8, 15].
High-affinity IGFBPs have a wide range of IGF/IGF-1R-dependent and -independent effects. Depending on perspective, binding of IGFBPs to IGF-1 and -II can act to enhance or inhibit IGF action. IGFBPs bind to and transport circulating IGF ligands to receptors on their target tissues in either a binary complex of 40–50 kDa, consisting of an IGFBP and an IGF ligand, or in a ternary complex of approximately 150 kDa, consisting of an IGFBP, an IGF ligand, and an acid labile subunit (ALS), a leucine-rich glycoprotein which acts to further stabilize the IGF-1GFBP complex [8, 20]. Interaction with IGFBPs prolongs the half-life of the IGF ligand [2]. The half-life of free IGF-1 or -II is approximately ten minutes; however, when in a binary complex with an IGFBP, the half-life of the IGF ligand is increased to 30–90 minutes [21]. Unlike free IGF, free IGFBP, and binary complexes, which are able to rapidly exit the circulatory system to interact with target tissues, ternary complexes are confined to the vascular system [15, 20]. As a ternary complex, the ALS further stabilizes the interaction between IGF ligand and IGFBP and prevents IGF ligand passage into the extracellular compartment, extending IGF-1 or -II half-life to 12–15 hours [20, 21]. By sequestering IGF-1 and -II, IGFBPs prevent down-regulation of IGF receptors on target issues due to ligand over-exposure [3]. In contrast, since high-affinity IGFBPs have greater affinities for IGF ligands (kD ≈ 10−9 to 10−11) than do IGF-1Rs (kD ≈ 10−8 to 10−9), they compete with receptors for binding of IGF-1 and -II, modulating the bioavailability of IGF ligand, and reducing IGF-1R activation and signaling [2, 8, 17]. Therefore, the bioavailability of IGF-1 and -II accessible to induce IGF-1R signaling is heavily influenced by the concentration of IGFBPs in the circulation and in extracellular fluid.
Proteases released by target tissues, such as serine proteases (including prostate specific antigen, PSA), cysteine proteases, matrix metalloproteinases (MMPs), cathepsins, plasmin, and thrombin, digest and cleave the IGFBP central domain [2, 3, 5, 15]. Both the N- and C-terminal domains of IGFBPs are required for high-affinity ligand binding; proteolytic cleavage produces N-terminal and C-terminal fragments with greatly reduced affinity for IGF-1 and IGF-2 and leads to release of free ligand at the target tissues [3,15]. Free IGF ligand is then available to bind cell surface receptors. Interestingly, proteolytic cleavage of IGFBPs not only decreases their binding affinity for IGF-1 and IGF-2, but also increases their binding affinity for insulin [8].
In addition to modulating IGF bioavailability and half-life, IGFBPs are also capable of important IGF/IGF-1R-independent biological actions; IGF-independent effects are defined as bioactivity in the absence of IGF ligands, and IGF-1R-independent effects are defined as bioactivity in the presence of IGF ligands without triggering IGF-1R signaling [15]. IGF/IGF-1R-independent actions are mediated by either intact or proteolytic fragments of IGFBPs and involve structural domains of the IGFBP distinct from IGF-binding determinants [15]. For instance, IGFBPs possess domains necessary for binding extracellular matrix (ECM) proteins and cell surface proteins and glycoproteins [8, 15]. Glycosylation of the central domain of IGFBPs also improves interaction with both the ECM and the cellular plasma membrane [15]. Some IGFBPs, including IGFBP-1, -2, -3, and -5 (potentially all), bind plasma membrane receptors distinct from standard IGF and insulin receptors, triggering their own signaling cascades which act to either modulate the effects of IGF-1R/IR signaling or activate a signaling pathway completely independent of IGF or insulin signaling [8,15]. IGFBP-3 and -5 can undergo endocytosis via interactions with proteins present in caveolae, and can associate with several cytoplasmic proteins [8]; in addition, IGFBP-3 and -5 contain C-terminal nuclear localization signals and can also interact with proteins within the nucleus, inducing gene transcription and nuclear protein translocation [8,17]. Some IGFBPs can bind nuclear receptors; for example, IGFBP-3 and -5 can bind the retinoid X receptor (RXRα) and the retinoic acid receptor (RARα), and IGFBP-6 can bind the Vitamin D receptor (VDR) [8]. Additionally, some IGFBPs can bring about apoptosis by activation of the extrinsic death receptor pathway and/or the intrinsic mitochondrial pathway [15]. The investigation of the IGF/IGF-1R-independent actions of IGFBPs is an emerging field, and much remains unknown.
IGFBP-3
The most abundant circulating IGFBP is IGFBP-3, which carries 75–90 percent of all serum IGF ligand in binary or ternary complexes [5, 15]. IGFBP-3 preferentially binds to IGF-1, but will also bind IGF-2 with comparatively high affinity [15]. Approximately 90 percent of IGFBP-3 circulates bound to IGF ligand; the remainder circulates as free IGFBP-3 [15]. Mature, secreted IGFBP-3 is a protein of 264 amino acids. It retains a structure similar to other high-affinity IGFBPs, with conserved N-terminal (residues 1–87) and C-terminal (residues 184–264) domains forming a hydrophobic pocket important for IGF ligand binding and a unique central domain (residues 88–183) containing sites for proteolytic cleavage and numerous post-translational modifications [5,8,22,23]. IGFBP-3 exhibits closest sequence similarity with IGFBP-5 [8].
Out of all high-affinity IGFBPs, IGFBP-3 has the most IGF-independent actions described and most binding partners characterized; this is reflected in the numerous sub-domains identified insofar within the three main N-terminal, central, and C-terminal domains of IGFBP-3. As mentioned previously, in addition to IGF ligand binding, the N-terminal domain of IGFBP-3 also contains sites for binding nuclear receptors RXRα and RARα [24]. The central domain contains a heparin-binding sub-domain [25] and is susceptible to proteolytic cleavage [8]. Central domain residues Ser-111 and Ser-113, when unphosphorylated, enhance binding of IGFBP-3 to ALS and to cellular surfaces [26]. In addition, three potential N-glycosylation sites also exist within the central domain of IGFBP-3; Asn-89 and Asn-109 are always glycosylated, whereas Asn-172 is variably glycosylated [5, 17, 27] (accounting for the characteristic IGFBP-3 protein doublet at molecular masses 42 and 45 kDa as seen in SDS-PAGE) [5, 8, 28]. Glycosylation at these residues confers resistance from protease digestion and modulates cell surface interaction [29]. The C-terminal domain of IGFBP-3 contains by far the most sub-domains for protein partner binding; IGF ligand binding aside, the C-terminal domain also contains sub-domains necessary for ALS, RXRα, RARα, Nur77, heparin, humanin, fibrinogen, fibrin, fibronectin, plasmin, plasminogen, transferrin, type 1α collagen, caveolin-1, transferrin receptor, low density lipoprotein-related protein (LRP-1) receptor/transforming growth factor-β (TGFβ) type V receptor, latent TGFβ binding protein (LTBP-1), glycosaminoglycan, and dermatan sulfate binding, as well as a nuclear localization sequence (NLS) and a metal binding domain (MBD) [5, 8].
IGFBP-3 has been well-documented as a negative regulator of cell growth and proliferation, and evidence exists that IGFBP-3 can trigger apoptosis through the intrinsic mitochondrial pathway and the extrinsic death receptor pathway [5,30]; the mechanisms by which IGFBP-3 induces apoptosis are still being elucidated, but are believed to be IGF-independent. IGFBP-3 has been shown to bind many extracellular matrix and cell surface proteins [8]. Two putative plasma membrane receptors have been recognized: i) the LRP-1 receptor/TGFβ type V receptor, which is known to bind IGFBP-3, but the exact signaling mechanism downstream of this receptor remains elusive [5, 31] and ii) an unidentified cell surface receptor that binds the central domain of IGFBP-3, causing a rapid and transient increase in intracellular free Ca2+ concentration mediated through a Gi protein-dependent pathway [32]. Caveolin-1 within caveolae, transferrin, and the transferrin receptor function collectively to mediate the endocytosis of IGFBP-3 [33]; this endocytosis is also mediated by metal ion stimulation of the C-terminal MBD, which can bind nickel, iron, and zinc, and physically interacts with both caveolin-1 and the transferrin receptor [34]. The NLS of the IGFBP-3 C-terminal domain is responsible for translocation into the nucleus mediated by the nuclear transport factor importin β [5, 35]. IGFBP-3 central domain phosphorylation is a critical regulator of nuclear import and binding affinity to nuclear components [36]; phosphorylation at Ser-156, Ser-165, and Thr-170 by casein kinase (CK) [26], cAMP-dependent protein kinase (PKA), or double-stranded DNA-dependent protein kinase (DNA-PK) [37] reduces affinity of IGFBP-3 for IGF ligand and increases nuclear import rate of IGFBP-3 [5,36]. These three residues are highly-conserved among mammals [36]. In fact, phosphorylation at Ser-156 appears to be particularly important for both nuclear localization and apoptosis-promoting actions of IGFBP-3, as inhibition of Ser-156 phosphorylation reduces nuclear accumulation of IGFBP-3 and inhibits induction of apoptosis [5, 38]. Within the nucleus, IGFBP-3 associates with RNA polymerase II binding subunit (Rpb3), which recruits the RNA polymerase II complex [5, 39]; this may be a mechanism by which IGFBP-3 can modulate gene transcription. As mentioned previously, IGFBP-3 can also interact with RXRα within the nucleus [5, 40], leading to RXRα heterodimerization with orphan nuclear receptor Nur77 and translocation of the complex from the nucleus to the mitochondria [5, 41, 42]. In the nucleus, Nur77 functions as a transcription factor to mediate cell proliferation; in contrast, Nur77 localized within the mitochondria induces cytochrome c release and induction of apoptosis [5, 41, 42]. IGFBP-3 may also be able to interact with Nur77 in the cytoplasm, inducing intrinsic apoptosis without the need for RXRα-IGFBP-3 interaction [5, 42].
The IGF Axis in Prostate Cancer Development and Progression
The growth factors of the IGF axis can exert effects on the prostate gland by direct action of i) circulating hepatic IGF ligand and/or ii) IGF ligand produced locally by the prostate tissue (growth hormone may also directly affect prostate tissue, although this has only been confirmed in cell culture and murine models) [43]. Normally, in the prostate gland, stromal cells produce and secrete IGF-1 which acts in a paracrine manner upon epithelial cells expressing IGF-1R [44]. Prostate epithelial cells in vitro are highly-sensitive to exogenous IGF-1, with a peak effect at 10 ng/ml [3]. Normal prostate epithelial cells also express and secrete many IGFBPs, including IGFBP-2, IGFBP-3, IGFBP-4, and IGFBP-5 [44]. IGF-1 is a potent mitogen; high levels of circulating IGF-1, whether produced in the liver, the stromal cells of the prostate, or other extra-hepatic sites, are associated with increased rate of DNA synthesis and cell proliferation by promoting advancement into the S-phase of the cell cycle and increased activation of survival pathways, making apoptotic death of any damaged cells less probable [3]. In this manner, IGF-1 may act as an oncogene, turning the cellular environment of target organs into a high risk environment [2]. Sustained elevated levels of IGF-1 may promote stepwise accumulation of genetic damage and cellular transformation necessary for carcinogenesis [2].
As prostate epithelial cells become neoplastic and progress to androgen-responsive, organ-confined prostate cancer, they develop the ability to express and secrete IGF-1 themselves [2, 44, 45]. The neoplastic epithelial cells also stimulate prostatic stromal cells adjacent to the tumor to secrete higher levels of IGF-1 [2, 45]. These aberrations further elevate IGF-1 levels within the tumor microenvironment, which acts in an autocrine and paracrine manner on the prostate cancer epithelial cells. Simultaneously, neoplastic progression to androgen-responsive prostate cancer is associated with increased expression of IGF-1R on prostate cancer epithelial cells [2]. IGF-1R over-expression has been clinically confirmed in surgically-resected, organ-confined prostate tumors, and human androgen-responsive prostate cancer cultured cells express IGF-1R at similar or higher concentrations to noncancerous prostate epithelial cell lines [2]. IGF-1R is over-expressed in androgen-dependent prostate cancer cells because it is an androgen receptor (AR) responsive gene; increased AR activity in androgen-dependent prostate cancer cells leads to increased AR-responsive gene expression [2]. Interestingly, androgen response elements (ARE) have also been identified in the distal region of the IGFBP-3 promoter [5]. Many tumor suppressor properties of IGFBP-3 have been demonstrated, including the sequestration of IGF ligands, induction of cancer cell apoptosis, association with anti-growth and anti-angiogenic signaling and senescence, inhibition of tumor cell adhesion to extracellular matrix components in both the presence and absence of IGFs, and suppression of prostate cancer cell metastasis in vivo [46]. In androgen-responsive prostate cancer cultured cell lines (and in some castration-resistant prostate cancer cell lines), androgens, such as dihydrotestosterone and testosterone, suppress IGFBP-3 mRNA and protein expression levels in a dose- and time-dependent manner [47]. Additionally, many proteases, including PSA (an AR-responsive gene and serine protease secreted in increased amounts from prostate cancer cells) and MMP-7 (a protease synthesized and secreted exclusively by tumor cells implicated in invasion, morphogenesis, and angiogenesis), are present in significantly elevated concentrations within the prostate tumor microenvironment [2,5]; these proteases digest IGFBPs, such as IGFBP-3. Decreased mRNA and protein expression of IGFBP-3 and increased protease secretion may be responsible for the greatly diminished levels of IGFBP-3 within the prostate cancer microenvironment, leading to increased proportion of free IGF-1 and IGF-2 surrounding the prostate tumor and increased IGF-1R activation and signaling [2, 5, 48]. Androgens also promote progression through the cell cycle, decreasing the fraction of cells in G0-phase of the cell cycle and advancing the cells into G1-phase; in G1, cells become even more responsive to the proliferative effects of IGF-1 [3]. IGF-1 in combination with dihydrotestosterone stimulates DNA synthesis in LNCaP cells significantly more so than stimulation with IGF-1 alone [49].
In prostate cancer cells, signals from the IGF-1R and other growth factor receptors can become exaggerated or inappropriate due to molecular pathology of proteins involved in or controlling signaling downstream of the receptor. For example, seen frequently in prostate cancer is loss of function of phosphatase and tension homologue (PTEN), a phosphatase that negatively regulates intracellular levels of PIP3 and functions as a tumor suppressor by negatively regulating the PKB/Akt signaling pathway, one of the two major signaling pathways downstream of IGF-1R discussed previously in this review [2]. In many human prostate cancer cells, mitogenic and cell survival signaling from the IGF-1/IGF-1R pathway is constitutively active, due to constitutively active downstream signaling elements, such as Akt and Ras (due to mutation of kinase itself or loss of regulating phosphatase) [50]. Additionally, in many cancers, including prostate cancer, aberrant DNA methylation and histone deacetylation may silence IGFBP-3 expression [5]. Tumor suppressor p53 is an inducer of IGFBP-3 mRNA expression; therefore, prostate cancer cell lines and tumors with mutant p53 express low levels of IGFBP-3 [46]. This may be one reason why IGFBP-3 was identified by microarray analysis as the most reduced transcript in comparison of normal and prostate cancer tissues [5]. Overall, aberrations in the IGF-1/IGF-1R signaling pathway combine to give the prostate cancer cells a growth and survival advantage.
Androgen-withdrawal treatment is initially associated with an increase in AR mRNA and protein expression; however, due to lack of ligand, expression of AR-target genes, including IGF-1R, decreases [3, 51]. Concurrently, IGFBP-3 transcription is upregulated in the prostate in response to androgen-deprivation therapy, and is in part responsible for the induction of apoptosis seen in the prostate tumor post-androgen withdrawal [2, 3]. Alterations in the IGF axis important for survival signaling in an androgen-deprived environment are associated with the progression of organ-confined, androgen-dependent prostate cancer to an androgen-resistant or -independent state. Prostate cancer progression toward castration-resistance and/or metastases is associated with increased expression and secretion of autocrine IGF-1 and increased expression of IGF-1R [45, 52, 53]. Specifically, in castration-resistant and androgen-independent primary tumors and lymph node metastases, IGF-1R is highly over-expressed by tumor epithelial cells, adjacent phenotypically-normal epithelial cells, and adjacent stromal cells; IGF-1R is expressed in androgen-resistant or -independent tumors at a much greater intensity than in androgen-responsive primary tumors, and with a much different expression pattern than normal prostate epithelium [45]. Krueckl et al. found that cell surface expression of IGF-1R is androgen-dependent in both androgen-responsive LNCaP and androgen-resistant C4-2 cell lines, but that basal expression of IGF-1R in C4-2 cells and in human metastatic androgen-independent prostate cancer specimens from bone, lymph nodes, and liver was significantly higher than in LNCaP cells and in human androgen-dependent primary tumor specimens [52]. This group also determined that signaling through IGF-1R was not androgen-dependent in androgen-resistant prostate cancer cells [52]. Therefore, under androgen-deprived conditions, castration-resistant and androgen-independent cells may use IGF-1 as an anti-apoptotic growth factor, which activates the over-expressed IGF-1R and signals through PI3K/Akt, but not MAPK, to mediate growth, survival, and migratory response to IGF-1 [52, 54]. Additionally, IGF-1R signaling through Akt may promote non-ligand activation of the AR, allowing continued proliferation of androgen-independent cells in an androgen-deprived environment [3]. To further complicate matters, nutritional influences on IGF-1 circulating levels during the malnourishment and wasting associated with advanced metastatic prostate cancer, or cachexia, often lowers circulating IGF-1 levels [2]. Therefore, in summary, maintaining a certain level of IGF-1/IGF-1R signaling is necessary for normal prostate epithelial tissue growth and differentiation; however, increased, aberrant IGF-1/IGF-1R signaling may promote prostate epithelial transformation, neoplasia, and androgen-dependent prostate cancer, and may be absolutely required for survival and growth in an androgen-deprived environment, for progression to castration-resistant, and for migration necessary for metastatic development.
The importance of IGF-1 and IGF signaling in the development and progression of prostate cancer has been established using pre-clinical mouse models. For example, DiGiovanni et al. demonstrated that the over-expression of human IGF-1 specifically in the basal epithelial cells of the mouse prostate could induce a chronic activation of IGF-1R in epithelial cells of the prostate, leading to the spontaneous, progressive development of hyperplasia, prostatic intraepithelial neoplasia, and well-differentiated adenocarcinoma in the prostate tissue of this mouse model [55]. This study demonstrated that constitutive signaling through the IGF-1R alone was sufficient to induce tumorigenesis in the mouse prostate. A second murine model was developed by crossing the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model (which simulates the prostate tissue transformation as occurs in human prostate cancer progression under physiological serum androgen concentrations) with mice homozygous for the missense lit/lit mutation (which inactivates the growth hormone-releasing hormone receptor in the anterior pituitary gland and reduces circulating levels of growth hormone and, subsequently, IGF-1). These lit/lit TRAMP mice demonstrated a significant reduction in the percentage of mice whose prostate glands displayed neoplastic changes and improved survival compared to TRAMP mice without the lit/lit mutation, an effect that was reversed with exogenous IGF-1 [56]. Furthermore, to assess how IGF-1 influences androgen-responsive, castration-resistant, and androgen-independent growth of human prostate cancer, Takahara et al. developed a mouse model to monitor xenograft growth of androgen-responsive human prostate cancer LNCaP cells or androgen-independent human prostate cancer PC-3 cells in intact and castrate Nod/SCID mice either homozygous for the missense lit/lit mutation or heterozygous (lit/+) [57]. Androgen-responsive and castration-resistant growth of LNCaP and androgen-independent growth of PC-3 was found to be significantly repressed in lit/lit mice compared to lit/+ littermates [57]. Likewise, LNCaP and PC-3 cells grown in vitro with serum from lit/lit mice showed decreased proliferation compared to cells grown in serum from lit/+ mice; this suppressed growth of the prostate cancer cells in lit/lit serum was rescued by extraneous IGF-1 and, to a lesser extent, growth hormone [57]. This study gives evidence that circulating IGF-1 significantly contributes to androgen-responsive, castration-resistant, and androgen-independent growth of human prostate cancer cells in a xenograft model. Circulating concentrations of IGFBP-3 may also be a critical component in the development of prostate cancer, as up-regulation of IGFBP-3 expression in pre-clinical models leads to increased apoptosis in the prostate [42].
Case-control studies and meta-analyses performed in the late-1990s to mid-2000s analyzed circulating IGF-1 and IGFBP-3 levels in men with prostate cancer as compared to healthy controls in an effort to determine whether IGF-1 and/or IGFBP-3 could be used as biomarkers for the detection of prostate cancer. The majority of these studies noted a significant positive correlation between IGF-1 and prostate cancer incidence [58–62]; only one case-control study by Chen et al. found that serum IGF-1 concentration was not associated with incidence of prostate cancer [63]. Of note, in a case-control study nested within the α-Tocopherol, β-Carotene (ATBC) Cancer Prevention Study, Woodson and colleagues monitored both prostate cancer disease progression and changes in serum IGF-1 levels in 100 subjects diagnosed with prostate cancer (and n = 400 control subjects) for at least five years after baseline blood draw [62]. These researchers found that serum IGF-1 increased significantly (18 percent increase) over both time and prostate cancer progression in cases compared to matched controls (4 percent decrease, p = 0.02) [62]. Potential association between IGFBP-3 and prostate cancer is more uncertain as investigations have produced inconsistent data. Two case-control studies [60, 62] and one meta-analysis [61] found that levels of IGFBP-3 were significantly higher in prostate cancer patients compared to controls; whereas, another case-control study reported that circulating IGFBP-3 concentration was lower in prostate cancer patients than in healthy controls, especially in aggressive prostate cancer disease, where IGFBP-3 levels were 76 percent lower in patients with advanced prostate cancer compared to control subjects [63]. Therefore, epidemiologic studies tentatively point to circulating IGF-1, but not circulating IGFBP-3, as a potential biomarker for detection of prostate cancer. Additionally, Chokkalingam et al. and Nam et al. studied serum IGF-1 and IGFBP-3 levels in patients presenting with benign prostatic hyperplasia (BPH) and high-grade prostatic intraepithelial neoplasia (HGPIN), respectively [64, 65]. In a case-control study of 206 BPH cases and 306 control subjects in Shanghai, China, elevated levels of IGF-1 were significantly associated with a 2.8-fold elevated risk of BPH (when comparing the highest and lowest tertile); whereas, elevated levels of IGFBP-3 were significantly associated with a 60 percent reduced risk of BPH [64]. The Nam et al. case-control study of 103 men with HGPIN and 205 men with normal prostate histology demonstrated that mean serum IGF-1 concentration in men with HGPIN was significantly higher than controls with an odds ratio of 1.94; mean IGFBP-3 was also elevated in men with HGPIN, perhaps secondary to increasing IGF-1 levels, although this trend was not statistically significant [65]. When comparing serum IGF-1 and IGFBP-3 levels in 45 benign prostatic hyperplasia (BPH), 24 localized prostate cancer, and 19 metastatic prostate cancer cases, Aksoy et al. observed no significant differences in serum IGF-1 levels between the three experimental groups, but did detect significantly decreased IGFBP-3 levels in localized and metastatic prostate cancer cases compared to BPH cases, which the authors attributed to increased proteolysis by elevated PSA [66]. Because of inconsistent results and limited literature, additional studies are needed to determine if IGF-1 and IGFBP-3 could also serve as potential biomarkers for BPH and HGPIN.
More recently, investigators have studied use of serum or plasma IGF-1 and IGFBP-3 as predictors of future development of prostate cancer in men; however, results from these studies have been highly inconsistent. Although two meta-analyses [67,68] and two case-control studies [69,70] established a positive association between high levels of circulating IGF-1 and the development of prostate cancer, far more (seven case-control) studies demonstrated no association between elevated IGF-1 and future prostate cancer diagnosis [62,71–76]. A meta-analysis performed by Roddam et al. revealed that increased serum or plasma IGF-1 concentration may be more positively associated with low Gleason score (≤ 6) prostate cancer than high Gleason score prostate cancer, although not all patient records contained disease stage or grade [67]. Elevated pre-diagnosis circulating levels of IGFBP-3 was found to be associated with increased risk for developing prostate cancer in one meta-analysis [67] and three case-control studies [70–72], but was associated with decreased risk of prostate cancer in one meta-analysis [68], and was found to have no association with prostate cancer development in six case-control studies [62, 69, 73–76]. The IGF-1-to-IGFBP-3 molar ratio was also analyzed as a predictor in several studies and was consistently found to have no association with future diagnosis of prostate cancer [68, 72, 74, 76]. However, a high IGF-1-to-IGFBP-3 molar ratio was significantly associated with a 2.34-fold increased risk of developing prostate cancer in obese men (BMI > 30), especially for advanced, aggressive disease (OR = 2.80, 95% CI = 1.11–7.08) [74]. In summary, cumulative data suggests that neither circulating IGF-1 or IGFBP-3 concentrations nor the IGF-1-to-IGFBP-3 molar ratio are likely to have value as predicators of whether a man will develop prostate cancer. Nonetheless, IGF-1 levels do seem to correlate with the presence of an already established diagnosis of prostate cancer, and, therefore, may have more value as an additional screening measure alongside PSA.
Use of compounds targeting the IGF signaling axis could be successful in treatment of a variety of prostate tumors, including androgen-responsive (adjuvant to androgen-deprivation therapy) or androgen-resistant, organ-confined or metastatic. The majority of the IGF axis modulators currently being investigated for cancer chemotherapy target the IGF-1R receptor; therefore, any prostate cancer over-expressing IGF-1R would make a great target for these modulators. Current IGF-1R modulators under investigation include i) antisense or siRNA strategies to reduce IGF-1R expression, ii) introduction of dominant-negative IGF-1R to interfere with receptor action, iii) the administration of small molecule inhibitors that interfere with IGF ligand-IGF-1R binding, and iv) small molecule RTK inhibitors [2, 4, 53], although each of these IGF-1R-targeting strategies has limitations. For example, although successful in the inhibition of cell proliferation and apoptosis induction in cancer cells in vitro, RTK inhibitors, such as imatinib, demonstrate strict specificity for IGF-1R only at specific concentrations under carefully-controlled conditions; therefore, RTK inhibitors would be problematic for use in in vivo treatment as tissue concentrations of these RTK inhibitors would vary greatly throughout the body (Orally-consumed RTK inhibitor concentrations would be highest in the liver and intestine, posing the greatest risk for complications through unintentional inhibition of liver and intestine RTKs) [77]. Additionally, any chemotherapeutic targeting of the IGF-1R would have to be sufficiently specific to avoid co-targeting the IR, which is 70 percent homologous to the IGF-1R [4]. An additional quandary exists, as in the selection pressure to survive, tumor cells may compensate for IGF-1R blockade by increasing alternate growth factor receptor signaling, such as through the epithelial growth factor receptor (EGFR) [2]. The PI3K/Akt signaling pathway downstream of the IGF-1R, which is often constitutively active in prostate cancer, can also be targeted simultaneously with small molecule inhibitors, although this would likely give rise to many side effects in vivo. In summary, drug-related toxicities and side effects prevent the use of many IGF axis-targeting agents as in vivo anticancer agents. Identification of agents with low intrinsic toxicity in pre-clinical models that specifically target the IGF axis for selective elimination of prostate cancer cells without adversely affecting normal prostate cells would be beneficial in the design and development of new anticancer strategies.
Apigenin as a Modulator of IGF Axis in Prostate Cancer
Prospective studies have demonstrated that frequent consumption of a diet rich in fruits and vegetables is protective against many human cancers [6], and Asian men who consume a plant-based diet rich in flavonoids display the lowest risk for prostate cancer among the world population [78]. Apigenin, or 4′,5,7-trihydroxyflavone, is a flavone found in many fruits and vegetables, including oranges, grapefruits, parsley, celery, onions, wheat sprouts, cereals of millet and wheat, and in some seasonings, such as coriander, licorice, marjoram, oregano, rosemary, and tarragon [6]. It is also present in chamomile tea prepared from the dried flowers of Matricaria chamomilla, in red wine, and in beer brewed from natural ingredients [6]. Apigenin is most often found naturally as apigenin-7-O-glucoside or an acylated derivative, but can also be present in foods as the dimer biapigenin [79]. Apigenin has been shown to inhibit cellular proliferation, suppress tumorigenesis and angiogenesis, and induce apoptosis in a variety of human cancers, including leukemia and carcinomas of the lung, thyroid, skin, colon, breast and prostate, without affecting normal, noncancerous cells [6]. Apigenin inhibits proliferative signaling by decreasing phosphorylation and activity of many protein tyrosine kinases and serine/threonine kinases, including receptor tyrosine kinases EGFR and IGF-1R, Src tyrosine kinase, protein kinase C, MAPKs, PI3K, and Akt [6, 50, 80–82]. Apigenin can induce a reversible cell cycle arrest in either G0/G1 phase in p53/p21-proficient cancer cells by increasing p53 protein stability and inducing p21 protein expression or in G2/M phase in p53/p21-deficient cancer cells by inhibiting cyclin-dependent kinase 1 (cdk1/cdc2) kinase activity [6]. Apigenin alters the Bax/Bcl-2 ratio in favor of apoptosis, and causes mitochondrial release of cytochrome c, induction of apoptotic protease activating factor 1 (Apaf-1), and formation of the apoptosome, leading to caspase activation and poly (ADP-ribose) polymerase (PARP) cleavage in cancer cells [6]. Apigenin has been shown to inhibit tumor cell invasiveness and metastatic properties by suppressing the expression of both tumor necrosis factor alpha (TNFα)-induced intracellular adhesion molecule-1 and vascular endothelial growth factor (VEGF) [6]. Apigenin inhibits aromatase and 17β-hydroxysteroid dehydrogenase activities and decreases intracellular and secreted levels of PSA in androgen-responsive LNCaP prostate cancer cells [6]. In addition, apigenin is a highly effective modulator of the IGF-1 signaling axis [50, 83–86].
Shukla et al. first demonstrated the corrective modulation of the IGF axis in prostate cancer by apigenin at physiologically attainable doses. Using androgen-refractory 22Rv1 tumors subcutaneously injected into the flanks of nude mice, apigenin treatment with 20 or 50μg/mouse/day for eight weeks, beginning either two weeks after tumor implantation (chemotherapeutic regimen) or two weeks prior to tumor inoculation (chemopreventative regimen), led to both a decrease in tumor volume and an increase in apoptosis of tumor cells [84]. 20 and 50μg/mouse/day apigenin dosage was chosen to mimic a physiologically attainable dose of 40–120 mg per day in humans [84], and resulted in a serum apigenin concentration of approximately 0.8 to 1.2 μM [84]. Oral intake of apigenin at these doses did not cause any apparent toxic effect on the mice as determined by chow intake; total body weight was decreased in association with apigenin treatment, especially at 50μg/mouse/day, however, the authors attributed this to tumor weight reduction observed in apigenin-treated mice [84]. Oral intake of apigenin increased IGFBP-3 levels and reduced IGF-1 levels in both serum and within the tumor xenograft in a statistically significant, dose-dependent manner [84]. Serum and 22Rv1 tumor xenograft apigenin concentrations also increased in a dose-dependent manner, and positively correlated with inhibition of xenograft growth, induction of apoptosis within the 22Rv1 xenograft, and increased protein expression of IGFBP-3 and decreased protein expression of IGF-1 within both the xenograft and circulating throughout the serum [84].
The effects of apigenin observed in the mouse xenograft model were confirmed in vitro, and a potential mechanism involving the IGF axis was investigated. In human 22Rv1 prostate cancer cell culture, treatment with 20 and 40μM apigenin increased IGFBP-3 and decreased IGF-1 protein expression and secretion, inhibited cell growth, and induced apoptosis [84]. Additionally, apigenin inhibited IGF-1-stimulated cell cycle progression in serum-starved 22Rv1 cells; addition of 100ng/ml IGF-1 significantly increased the proportion of serum-starved 22Rv1 cells advancing from G0/G1 to S-phase in the cell cycle, whereas, simultaneous treatment of serum-starved 22Rv1 cells with both 100ng/ml IGF-1 and 40μM apigenin led to arrest in G0/G1 and increased the percentage of cells in sub-G1-phase, indicative of apigenin-induced loss of DNA during apoptosis [84]. Prolonged exposure to apigenin also inhibited IGF-induced IRS-1 scaffold protein tyrosine phosphorylation in 22Rv1 cell culture [84]. In an effort to determine whether the effects of apigenin were at least partially mediated an increase in the levels of IGFBP-3 secreted from 22Rv1 cells, IGFBP-3 expression and secretion was inhibited using an antisense oligonucleotide; this oligonucleotide significantly decreased IGFBP-3 expression and secretion, and reduced the apoptosis induction and cell growth inhibition effect of apigenin treatment on 22Rv1 cells [84]. Therefore, the effect of apigenin on androgen-refractory 22Rv1 prostate cancer cells and tumor xenografts is at least partially due to modulations in the IGF axis, such as increased IGFBP-3 expression and secretion [84]. Strong induction of IGFBP-3 and inhibited expression and secretion of IGF-1 leading to inhibition of IGF-1R activation and signaling, as demonstrated by decreased IRS-1 tyrosine phosphorylation, may be one mechanism of apigenin-induced tumor growth inhibition and apoptosis [84]. Increased IGFBP-3 secretion may have also led to apoptosis in 22Rv1 cell culture and in vivo models through IGF-1R-independent pathways, although this was not examined by Shukla et al.
Like 22Rv1 cells, androgen-independent DU145 cells display low basal levels of phosphorylated Akt. This is because both cell lines still possess a functional PTEN, a phosphatase that reverses the formation of PIP3, preventing Akt recruitment to the plasma membrane and subsequent activation. When exposed to 25ng/ml IGF-1, IGF-1Rs on the plasma membrane of DU145 cells were activated and autophosphorylated, leading to subsequent serine phosphorylation of IRS-1 and activation of Akt [83]. In their 2009 manuscript, Shukla and Gupta demonstrated that apigenin has the ability to significantly inhibit IGF-1-induced phosphorylation of IGF-1Rs, preventing the activation of downstream kinase Akt, and leading to a dose-dependent decrease in glycogen synthase kinase 3β (GSK-3β) and cyclin D1 phosphorylation and increase in protein levels of the cyclin-dependent kinase inhibitor p27/kip1, causing dose-dependent cell cycle arrest in G0/G1 phase, inhibition of progression into S-phase, and apoptosis [83]. Unlike 22Rv1 and DU145, the androgen-responsive LNCaP prostate cancer cell line and androgen-independent PC-3 prostate cancer cell line possess high basal levels of phosphorylated Akt due to mutation and loss of PTEN function. In these cancer cells, Akt is considered constitutively active, and further activation by growth factors is not necessary for downstream signaling leading to growth and proliferation. Despite this, apigenin was also able to cause a dose-dependent decrease in phosphorylation of IGF-1R and Akt in both LNCaP and PC-3 cell lines, inhibiting downstream signaling and inducing cell cycle arrest and apoptosis [83]. Therefore, apigenin is capable of suppressing both IGF-1-stimulated and constitutively active phosphorylation along the IGF-1R/Akt signaling pathway in androgen-dependent, androgen-resistant, and androgen-independent prostate cancer cell lines, leading to blockage of cell cycle progression at the G1 → S transition phase, growth arrest, and apoptosis.
In this same manuscript, to demonstrate the effects of apigenin on tumor growth and IGF-1R signaling in an in vivo xenograft model of androgen-independent prostate cancer, Shukla and Gupta injected PC-3 cells subcutaneously into the flanks of nude mice and then treated the mice with either 50μg/mouse/day apigenin or control vehicle by oral gavage as a chemotherapeutic regimen for 8 weeks [83]. Compared to vehicle-treated control mice, the growth of PC-3 tumor xenografts in apigenin-administered nude mice was significantly inhibited; tumor volume was reduced by 51 percent and tumor weight was decreased by 40 percent [83]. Consistent with earlier findings in cultured cells, there was a marked 2.86-fold increase in induction of apoptosis in the apigenin-treated PC-3 tumor xenografts compared to control xenografts [83]. Lysates generated from apigenin-treated PC-3 xenografts also displayed marked decrease in protein expression of IGF-1R, phosphorylated IGF-1R, Ser-473 phosphorylation of Akt, phosphorylated GSK-3β, and protein expression of cyclin D1, with a concomitant increase in p27/kip1 protein expression compared to control tumor xenograft lysates [83]. These results demonstrated that apigenin inhibits IGF-1R signaling (even when signaling downstream of the IGF-1R is constitutively active), leading to suppression of tumor cell proliferation and tumor growth, and induction of apoptosis within the PC-3 tumor xenograft.
Most recently, Shukla et al. examined the chemopreventive effect of apigenin on prostate cancer development and progression in the TRAMP autochthonous mouse prostate cancer model [86]. As disease progressed, serum IGF-1 concentration remained significantly higher and serum IGFBP-3 concentration was significantly reduced in TRAMP transgene heterozygous animals as compared to non-transgenic animals [86]. Additionally, throughout prostate cancer progression, phosphorylation of Akt on Ser-473 and phosphorylation of Erk (Erk1/2—Thr202/185 and Tyr204/187) was elevated within the tissue of the dorsolateral prostate in TRAMP transgene heterozygous verses non-transgenic animals [86]. Because of these results, Shukla et al. hypothesized that IGF-1 signaling could be a major target in the prevention of prostate cancer development [86]. To test this hypothesis, TRAMP mice were force-fed either 20 or 50μg/mouse/day apigenin in methyl cellulose vehicle or vehicle alone (as positive control) by oral gavage for 20 weeks; these mice were also compared to non-transgenic animals (as negative controls) [86]. After 20 weeks of treatment, incidence of palpable tumor was significantly reduced in heterozygous mice receiving apigenin in a dose-dependent fashion; 100 percent of positive control transgenic mice receiving only vehicle had a palpable tumor, whereas 56 percent of mice orally-gavaged with 20μg/day apigenin and only 38 percent of mice receiving 50μg/day apigenin developed a palpable prostate gland tumor [86]. Similarly, all positive control transgenic mice developed metastases, most commonly in the lymph nodes, but also in the lungs and/or liver; whereas, none of the TRAMP transgene heterozygous mice receiving 20 or 50μg/mouse/day apigenin developed metastases [86]. The dorsolateral prostates of apigenin-treated mice showed a greater proportion of non-neoplastic tissue and presented with a smaller percentage of the dorsolateral prostate lobe involved in adenocarcinoma; overall, adenocarcinoma found in apigenin-treated animals was well-differentiated, verses the higher proportion of moderately-or poorly-differentiated tumors seen in the positive control TRAMP transgenic animals [86]. Apigenin also significantly reduced cellular proliferation in the dorsolateral prostate, lowered serum and dorsolateral prostate IGF-1 concentration, restored serum and dorsolateral prostate IGFBP-3 levels to approximately normal (as compared to non-transgenic littermates), and significantly inhibited the phosphorylation of Akt and Erk in the dorsolateral prostate all in a dose-dependent manner [86]. Therefore, this study demonstrated that apigenin is capable of suppressing prostate carcinogenesis at physiologically-achievable concentrations in part through reduction in free IGF-1 and inhibition of IGF-1R signaling.
Oral consumption of apigenin can induce several alterations in the IGF axis that may be beneficial for chemoprevention and chemotherapy of prostate cancer, including inhibition of IGF-1R signaling and induction of prostate cancer cell cycle arrest and apoptosis. Intake of apigenin causes a significant decrease in both circulating IGF-1 levels and in the amount of IGF-1 secreted from the prostate tumor [84, 86], and also causes a simultaneous, significant increase in both circulating IGFBP-3 concentration and in the quantity of IGFBP-3 secreted from and present within the prostate tumor cells [84, 86]. The resultant decrease in IGF-1 levels, compounded with increased concentration of IGFBP-3 that can bind and sequester IGF-1, and apigenin-induced reduction in PSA secreted from prostate cancer cells yield a combined effect of significantly curtailing the amount of bioavailable IGF-1 present in the microenvironment of the prostate tumor [84, 86, 87]. Reduced free IGF-1 results in less IGF-1 binding to and activation of IGF-1Rs present on the cell surface of prostate cancer cells, leading to significantly decreased IGF-1R tyrosine phosphorylation and inhibited downstream signaling [50, 83, 86]. Apigenin inhibits the Ser-473 phosphorylation and activation of Akt, curtailing one of the main pathways downstream of IGF-1R [50, 83, 86], and leading to decreased phosphorylation of GSK-3β, diminished activity of cyclins and cyclin-dependent kinases necessary for G1 → S phase transition, and increased protein expression of cyclin-dependent kinase inhibitors, for the combined effect of inducing cell cycle arrest of the prostate cancer cells at G1-phase [83, 85]. Inhibition of Akt also led to decreased Ser-136 phosphorylation of Bad and reduced Bad-14-3-3β association [50], allowing Bad to translocate to the outer mitochondrial membrane where it binds anti-apoptotic Bcl-2 family proteins, releasing pro-apoptotic Bax and inducing apoptosis [50]. Apigenin also inhibits of ERK1/2, which can also induce apoptosis [86]. Additionally, an elevated concentration of free IGFBP-3 present within the prostate tumor microenvironment can lead to increased IGF-1/IGF-1R-independent signaling by the binding of free IGFBP-3 to prostate cancer cell surface receptors. This excess of unbound IGFBP-3 can also be brought into the prostate cancer cell by endocytosis mediated by caveolae, causing nuclear translocation of IGFBP-3 that can induce intrinsic apoptosis of the tumor cell.
Conclusions
The IGF axis plays a major role in prostate cancer development and progression. As neoplastic prostate epithelial cells progress to an organ-confined, androgen-dependent prostate adenocarcinoma, IGF-1 secretion from prostate stromal cells increase, and epithelial cells begin to express and secrete IGF-1; additionally, IGF-1R becomes over-expressed on the plasma membrane of the prostate cancer cells and on stromal and epithelial cells adjacent to the tumor [2]. Due to increased secretion of proteases from the prostate tumor, IGFBP-3 protein levels within the tumor microenvironment decrease significantly [2, 5, 48]. These alterations result in increased IGF-1R signaling essential in androgen-dependent prostate cancer for cell proliferation and tumor growth, enhanced metabolic activity, and apoptosis avoidance [2, 5, 48]. The IGF axis is also critically important in the transition of organ-confined, androgen-dependent prostate cancer to more aggressive forms, such as metastatic and androgen-independent prostate cancer [45, 52–54]. In androgen-resistant and metastatic prostate cancer, the IGF-1R is even more over-expressed and signals in an androgen-independent manner through the PI3K/Akt signaling pathway to promote growth, proliferation, and migration [45, 52–54]. Additionally, the IGF-1 ligand and its downstream effector, Akt, appear to be important in the promotion of AR activation in the absence of androgens, allowing tumor survival and proliferation after androgen withdrawal [3]. Apigenin, a natural anticancer agent, has been shown to target the IGF axis and its intracellular signaling in androgen-responsive, androgen-resistant, and androgen-independent prostate cancer cells [6, 83–86; Figure 1]. Oral consumption of apigenin has been found to increase circulating and prostate tumor IGFBP-3 levels [84, 86], decrease circulating and prostate tumor levels of IGF-1 [84, 86], inhibit IGF-1R activation and downstream signaling in prostate cancer tumors [50, 83, 86], and induce cell cycle and growth arrest and apoptosis of prostate cancer cells and tumor xenografts without harming normal tissues or causing toxicity in pre-clinical in vivo TRAMP and xenograft mouse models [83, 86, 87]. A clinical trial may be beneficial to determine if apigenin is a potential chemopreventive or chemotherapeutic agent for prostate cancer. Additionally, since serum IGF-1 levels were consistently elevated in clinical subjects diagnosed with prostate cancer [58–62], IGF-1 may be found to be a valuable serum biomarker in the detection of prostate cancer.
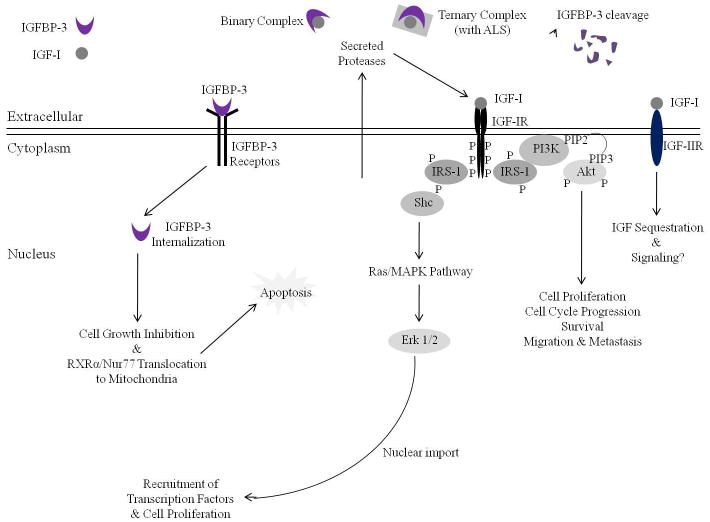
Figure 1. Apigenin targets insulin-like growth factor (IGF) axis and intracellular signaling to induce growth inhibition and apoptosis in prostate cancer cells.
Most IGF-1 within the extracellular intravascular compartment is sequestered in a binary complex with IGFBP-3 or in a ternary complex with IGFBP-3 and an ALS. Proteases secreted by the prostate cancer cell cleave IGFBP-3, causing the release of IGF-1. Free IGF-1 can then bind IGF-1R, causing its autophosphorylation and activation. Mediated through the phosphorylation and activation of IRS-1 scaffold protein, two primary intracellular signaling pathways are then activated, the MAPK/ERK pathway and the PI3K/Akt pathway, leading to cell cycle progression, cell proliferation, and cell survival. Apigenin can modulate this process by increasing both circulating and intracellular levels of IGFBP-3; decreasing circulating levels of IGF-1; inhibiting the secretion of proteases, such as PSA; inhibiting IGF-1R activation and downstream signaling; and inducing cell growth inhibition and apoptosis in prostate cancer cells.
Acknowledgments
We sincerely apologize to those investigators whose work could not be cited due to space constraints. Original apigenin work was supported by the United States Public Health Service Grants R01 CA108512 and R01 AT002709 and funds from the Cancer Research and Prevention Foundation to SG. MAB is supported by the Metabolism Training Program Ruth L. Kirschstein National Research Service Award for Pre-Doctoral Fellows (5T32DK007319).
Footnotes
Disclosure
This manuscript is an extended/updated version of a previous manuscript published in Biomedical Research 23: SI55-68, 2012.
References
- 1.American Cancer Society. [accessed July 23, 2012];Prostate Cancer. Available at: http://www.cancer.org/Cancer/ProstateCancer/
- 2.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 3.Meinbach DS, Lokeshwar BL. Insulin-like growth factors and their binding proteins in prostate cancer: cause or consequence? Urol Oncol. 2006;24:294–306. doi: 10.1016/j.urolonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig SA, Atreya HS. Defining the pathway to insulin-like growth factor system targeting in cancer. Biochem Pharmacol. 2010;80:1115–1124. doi: 10.1016/j.bcp.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran VG, Court F, Duputié A, et al. H19 antisense RNA can up-regulate Igf2 transcription by activation of a novel promoter in mouse myoblasts. PLoS One. 2012;7:e37923. doi: 10.1371/journal.pone.0037923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada PM, Lee K-W. Perspectives in mammalian IGFBP-3 biology: local vs systemic action. Am J Physiol Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- 9.Life JS, Kipgen WW. Guidelines for the Diagnosis and Treatment of Adult Growth Hormone Deficiency. Cenegenics Medical Institute; [accessed July 23, 2012]. Available at: http://www.cenegenicsfoundation.org/pdf_files/Final_Version-Guidelines_hGH_Rx.pdf. [Google Scholar]
- 10.O’Dell SD, Day IN. Insulin-like growth factor II (IGF-2) Int J Biochem Cell Biol. 1998;30:767–771. doi: 10.1016/s1357-2725(98)00048-x. [DOI] [PubMed] [Google Scholar]
- 11.Juul A, Bang P, Hertel NT, et al. Serum insulin-like growth factor I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocr Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 12.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the IGF-1 receptor. Endocr Rev. 1995;16:143–153. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 13.Tartare-Deckert S, Sawka-Verhelle D, Murdaca J, Van Obberghen E. Evidence for a differential interaction of SHC and the insulin receptor substrate-1 (IRS-1) with the insulin-like growth factor-I (IGF-1) receptor in the yeast two-hybrid system. J Biol Chem. 1995;270:23456–23460. doi: 10.1074/jbc.270.40.23456. [DOI] [PubMed] [Google Scholar]
- 14.Baserga R. The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res. 2009;315:727–732. doi: 10.1016/j.yexcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 16.O’Gorman DB, Weiss J, Hettiaratchi A, Firth SM, Scott CD. Insulin-like growth factor-II/mannose-6-phosphate receptor overexpression reduces growth of choriocarcinoma cells in vitro and in vivo. Endocrinology. 2002;143:4287–4294. doi: 10.1210/en.2002-220548. [DOI] [PubMed] [Google Scholar]
- 17.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 18.Rosenzweig SA. What’s new in the IGF-binding proteins? Growth Horm IGF Res. 2004;14:329–336. doi: 10.1016/j.ghir.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clemmons DR. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr Rev. 2001;22:800–817. doi: 10.1210/edrv.22.6.0449. [DOI] [PubMed] [Google Scholar]
- 20.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170:63–70. doi: 10.1677/joe.0.1700063. [DOI] [PubMed] [Google Scholar]
- 21.Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol. 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- 22.Galanis M, Firth SM, Bond J, et al. Ligand-binding characteristics of recombinant amino-and carboxyl-terminal fragments of human insulin-like growth factor-binding protein-3. J Endocrinol. 2001;169:123–133. doi: 10.1677/joe.0.1690123. [DOI] [PubMed] [Google Scholar]
- 23.Ahlsén M, Carlsson-Skwirut C, Jonsson AP, Cederlund E, Bergman T, Bang P. A 30-kDa fragment of insulin-like growth factor (IGF) binding protein-3 in human pregnancy serum with strongly reduced IGF-1 binding. Cell Mol Life Sci. 2007;64:1870–1880. doi: 10.1007/s00018-007-7201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schedlich LJ, Graham LD, O’Han MK, et al. Molecular basis of the interaction between IGFBP-3 and retinoid X receptor: role in modulation of RAR-signaling. Arch Biochem Biophys. 2007;465:359–369. doi: 10.1016/j.abb.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Fowlkes JL, Serra DM. Characterization of glycosaminoglycan-binding domains present in insulin-like growth factor-binding protein-3. J Biol Chem. 1996;271:14676–14679. doi: 10.1074/jbc.271.25.14676. [DOI] [PubMed] [Google Scholar]
- 26.Coverley JA, Martin JL, Baxter RC. The effect of phosphorylation by casein kinase 2 on the activity of insulin-like growth factor-binding protein-3. Endocrinology. 2000;141:564–570. doi: 10.1210/endo.141.2.7306. [DOI] [PubMed] [Google Scholar]
- 27.Firth SM, Baxter RC. Characterization of recombinant glycosylation variants of insulin-like growth factor binding protein-3. J Endocrinol. 1999;160:379–387. doi: 10.1677/joe.0.1600379. [DOI] [PubMed] [Google Scholar]
- 28.Wood WI, Cachianes G, Henzel WJ, et al. Cloning and expression of the growth hormone-dependent insulin-like growth factor-binding protein. Mol Endocrinol. 1988;2:1176–1185. doi: 10.1210/mend-2-12-1176. [DOI] [PubMed] [Google Scholar]
- 29.Firth SM, Baxter RC. The role of glycosylation in the action of IGFBP-3. Prog Growth Factor Res. 1995;6:223–229. doi: 10.1016/0955-2235(95)00009-7. [DOI] [PubMed] [Google Scholar]
- 30.Banudevi S, Senthilkumar K, Sharmila G, Arunkumar R, Vijayababu MR, Arunakaran J. Effect of zinc on regulation of insulin-like growth factor signaling in human androgen-independent prostate cancer cells. Clin Chim Acta. 2010;411:172–178. doi: 10.1016/j.cca.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Leal SM, Liu Q, Huang SS, Huang JS. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J Biol Chem. 1997;272:20572–20576. doi: 10.1074/jbc.272.33.20572. [DOI] [PubMed] [Google Scholar]
- 32.Ricort JM, Lombet A, Lassarre C, Binoux M. Insulin-like growth factor binding protein-3 increases intracellular calcium concentrations in MCF-7 breast carcinoma cells. FEBS Lett. 2002;527:293–297. doi: 10.1016/s0014-5793(02)03250-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, Liu B, Ma L, et al. Cellular internalization of insulin-like growth factor binding protein-3: distinct endocytic pathways facilitate re-uptake and nuclear localization. J Biol Chem. 2004;279:469–476. doi: 10.1074/jbc.M307316200. [DOI] [PubMed] [Google Scholar]
- 34.Singh B, Charkowicz D, Mascarenhas D. Insulin-like growth factor-independent effects mediated by a C-terminal metal-binding domain of insulin-like growth factor binding protein-3. J Biol Chem. 2004;279:477–487. doi: 10.1074/jbc.M307322200. [DOI] [PubMed] [Google Scholar]
- 35.Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit. J Biol Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- 36.Schedlich LJ, Nilsen T, John AP, Jans DA, Baxter RC. Phosphorylation of insulin-like growth factor binding protein-3 by deoxyribonucleic acid-dependent protein kinase reduces ligand binding and enhances nuclear accumulation. Endocrinology. 2003;144:1984–1993. doi: 10.1210/en.2002-220798. [DOI] [PubMed] [Google Scholar]
- 37.Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 38.Cobb LJ, Liu B, Lee KW, Cohen P. Phosphorylation by DNA-dependent protein kinase is critical for apoptosis induction by insulin-like growth factor binding protein-3. Cancer Res. 2006;66:10878–10884. doi: 10.1158/0008-5472.CAN-06-0585. [DOI] [PubMed] [Google Scholar]
- 39.Oufattole M, Lin SW, Liu B, Mascarenhas D, Cohen P, Rodgers BD. Ribonucleic acid polymerase II binding subunit 3 (Rpb3), a potential nuclear target of insulin-like growth factor binding protein-3. Endocrinology. 2006;147:2138–2146. doi: 10.1210/en.2005-1269. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Lee HY, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 41.Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem. 2005;280:16942–16948. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- 42.Lee KW, Cobb LJ, Paharkova-Vatchkova V, Liu B, Milbrandt J, Cohen P. Contribution of the orphan nuclear receptor Nur77 to the apoptotic action of IGFBP-3. Carcinogenesis. 2007;28:1653–1658. doi: 10.1093/carcin/bgm088. [DOI] [PubMed] [Google Scholar]
- 43.Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology. 1999;140:1984–1989. doi: 10.1210/endo.140.5.6721. [DOI] [PubMed] [Google Scholar]
- 44.Lopaczynski W, Hruszkewycz AM, Lieberman R. Preprostatectomy: a clinical model to study stromal-epithelial interactions. Urology. 2001;57:194–199. doi: 10.1016/s0090-4295(00)00973-0. [DOI] [PubMed] [Google Scholar]
- 45.Ryan CJ, Haqq CM, Simko J, et al. Expression of insulin-like growth factor-1 receptor in local and metastatic prostate cancer. Urol Oncol. 2007;25:134–140. doi: 10.1016/j.urolonc.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Grimberg A. P53 and IGFBP-3: apoptosis and cancer protection. Mol Genet Metab. 2000;70:85–98. doi: 10.1006/mgme.2000.3008. [DOI] [PubMed] [Google Scholar]
- 47.Kojima S, Mulholland DJ, Ettinger S, Fazli L, Nelson CC, Gleave ME. Differential regulation of IGFBP-3 by the androgen receptor in the lineage-related androgen-dependent LNCaP and androgen-independent C4-2 prostate cancer models. Prostate. 2006;66:971–986. doi: 10.1002/pros.20420. [DOI] [PubMed] [Google Scholar]
- 48.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75:1046–1053. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 49.Iwamura M, Sluss PM, Casamento JB, Cockett AT. Insulin-like growth factor I: action and receptor characterization in human prostate cancer cell lines. Prostate. 1993;22:243–252. doi: 10.1002/pros.2990220307. [DOI] [PubMed] [Google Scholar]
- 50.Kaur P, Shukla S, Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29:2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schayek H, Seti H, Greenberg NM, Sun S, Werner H, Plymate SR. Differential regulation of insulin-like growth factor-I receptor gene expression by wild type and mutant androgen receptor in prostate cancer cells. Mol Cell Endocrinol. 2010;323:239–245. doi: 10.1016/j.mce.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krueckl SL, Sikes RA, Edlund NM, et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 53.Furukawa J, Wraight CJ, Freier SM, et al. Antisense oligonucleotide targeting of insulin-like growth factor-I receptor (IGF-IR) in prostate cancer. Prostate. 2010;70:206–218. doi: 10.1002/pros.21054. [DOI] [PubMed] [Google Scholar]
- 54.Marelli MM, Moretti RM, Procacci P, Motta M, Limonta P. Insulin-like growth factor-I promotes migration in human androgen-independent prostate cancer cells via the αvβ3 integrin and PI3K/Akt signaling. Int J Oncol. 2006;28:723–730. [PubMed] [Google Scholar]
- 55.DiGiovanni J, Kiguchi K, Frijhoff A, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majeed N, Blouin MJ, Kaplan-Lefko PJ, et al. A germ line mutation that delays prostate cancer progression and prolongs survival in a murine prostate cancer model. Oncogene. 2005;24:4736–4740. doi: 10.1038/sj.onc.1208572. [DOI] [PubMed] [Google Scholar]
- 57.Takahara K, Tearle H, Ghaffari M, Gleave ME, Pollak M, Cox ME. Human prostate cancer xenografts in lit/lit mice exhibit reduced growth and androgen-independent progression. Prostate. 2011;71:525–537. doi: 10.1002/pros.21268. [DOI] [PubMed] [Google Scholar]
- 58.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 59.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 60.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB Baltimore Longitudinal Study on Aging. Serum levels of insulin-like growth factor I (IGF-1), IGF-2, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab. 2000;85:4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 61.Shi R, Berkel HJ, Yu H. Insulin-like growth factor-I and prostate cancer: a meta-analysis. Br J Cancer. 2001;85:991–996. doi: 10.1054/bjoc.2001.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodson K, Tangrea JA, Pollak M, et al. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Res. 2003;63:3991–3994. [PubMed] [Google Scholar]
- 63.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103:76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 64.Chokkalingam AP, Gao YT, Deng J, et al. Insulin-like growth factors and risk of benign prostatic hyperplasia. Prostate. 2002;52:98–105. doi: 10.1002/pros.10096. [DOI] [PubMed] [Google Scholar]
- 65.Nam RK, Trachtenberg J, Jewett MA, et al. Serum insulin-like growth factor-I levels and prostatic intraepithelial neoplasia: a clue to the relationship between IGF-1 physiology and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1270–1273. doi: 10.1158/1055-9965.EPI-04-0430. [DOI] [PubMed] [Google Scholar]
- 66.Aksoy Y, Aksoy H, Bakan E, Atmaca AF, Akçay F. Serum insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in localized, metastasized prostate cancer and benign prostatic hyperplasia. Urol Int. 2004;72:62–65. doi: 10.1159/000075275. [DOI] [PubMed] [Google Scholar]
- 67.Roddam AW, Allen NE, Appleby P, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–471. W83–W88. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124:2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer. 2006;95:112–117. doi: 10.1038/sj.bjc.6603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nimptsch K, Platz EA, Pollak MN, et al. Plasma insulin-like growth factor 1 is positively associated with low-grade prostate cancer in the Health Professionals Follow-up Study 1993–2004. Int J Cancer. 2011;128:660–667. doi: 10.1002/ijc.25381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control. 2005;16:255–262. doi: 10.1007/s10552-004-3484-8. [DOI] [PubMed] [Google Scholar]
- 72.Severi G, Morris HA, MacInnis RJ, et al. Circulating insulin-like growth factor-I and binding protein-3 and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1137–1141. doi: 10.1158/1055-9965.EPI-05-0823. [DOI] [PubMed] [Google Scholar]
- 73.Allen NE, Key TJ, Appleby PN, et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16:1121–1127. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 74.Weiss JM, Huang WY, Rinaldi S, et al. IGF-1 and IGFBP-3: risk of prostate cancer among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2007;121:2267–2273. doi: 10.1002/ijc.22921. [DOI] [PubMed] [Google Scholar]
- 75.Borugian MJ, Spinelli JJ, Sun Z, et al. Prostate cancer risk in relation to insulin-like growth factor (IGF)-I and IGF-binding protein-3: a prospective multiethnic study. Cancer Epidemiol Biomarkers Prev. 2008;17:252–254. doi: 10.1158/1055-9965.EPI-07-2694. [DOI] [PubMed] [Google Scholar]
- 76.Mikami K, Ozasa K, Nakao M, et al. Prostate cancer risk in relation to insulin-like growth factor (IGF)-I and IGF-binding protein-3: a nested case-control study in large scale cohort study in Japan. Asian Pac J Cancer Prev. 2009;10 (Suppl):57–61. [PubMed] [Google Scholar]
- 77.Pollak M. Targeting insulin and insulin-like growth factor signaling in oncology. Curr Opin Pharmacol. 2008;8:384–392. doi: 10.1016/j.coph.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Nagata Y, Sonoda T, Mori M, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr. 2007;137:1974–1979. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 79.Svehliková V, Bennett RN, Mellon FA, et al. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L. ] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52:982–986. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- 81.Schlupper D, Giesa S, Gebhardt R. Influence of biotransformation of luteolin, luteolin 7-O-glucoside, 3′,4′-dihydroxyflavone and apigenin by cultured rat hepatocytes on antioxidative capacity and inhibition of EGF receptor tyrosine kinase activity. Planta Med. 2006;72:596–603. doi: 10.1055/s-2006-931555. [DOI] [PubMed] [Google Scholar]
- 82.Franzen CA, Amargo E, Todorović V, et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res. 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 83.Shukla S, Gupta S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: an in vitro and in vivo study. Mol Carcinog. 2009;48:243–252. doi: 10.1002/mc.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19:2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- 85.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 86.Shukla S, MacLennan GT, Fu P, Gupta S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharm Res. 2012;29:1506–1517. doi: 10.1007/s11095-011-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]