Abstract
Glutamine metabolism is generally regarded as proceeding via glutaminase-catalyzed hydrolysis to glutamate and ammonia, followed by conversion of glutamate to α-ketoglutarate catalyzed by glutamate dehydrogenase or by a glutamate-linked aminotransferase (transaminase). However, another pathway exists for the conversion of glutamine to α-ketoglutarate that is often overlooked, but is widely distributed in nature. This pathway, referred to as the glutaminase II pathway, consists of a glutamine transaminase coupled to ω-amidase. Transamination of glutamine results in formation of the corresponding α-keto acid, namely, α-ketoglutaramate (KGM). KGM is hydrolyzed by ω-amidase to α-ketoglutarate and ammonia. The net glutaminase II reaction is: L-Glutamine + α-keto acid + H2O → α-ketoglutarate + L-amino acid + ammonia. In this mini-review the biochemical importance of the glutaminase II pathway is summarized, with emphasis on the key component KGM. Forty years ago it was noted that the concentration of KGM is increased in the cerebrospinal fluid (CSF) of patients with hepatic encephalopathy (HE) and that the level of KGM in the CSF correlates well with the degree of encephalopathy. In more recent work, we have shown that KGM is markedly elevated in the urine of patients with inborn errors of the urea cycle. It is suggested that KGM may be a useful biomarker for many hyperammonemic diseases including hepatic encephalopathy, inborn errors of the urea cycle, citrin deficiency and lysinuric protein intolerance.
Keywords: ω-Amidase, ammonia, glutaminase II, hepatic encephalopathy, α-ketoglutaramate, urea cycle disorders
Introduction
We are honored to provide a contribution to this special issue dedicated to Professor Roger Butterworth. Not only did Dr. Butterworth run a highly productive and internationally recognized laboratory, but he also found time to mentor numerous young scientists from many parts of the world, including Africa. Dr. Butterworth has played a major role in the ISHEN (International Society for Hepatic Encephalopathy and Nitrogen Metabolism) since its inception in 1971. (Dr. Butterworth was president from 2005 to 2007.) There was always a spirit of warmhearted camaraderie among the senior scientists, junior scientists and students at ISHEN meetings, much of it due to Dr. Butterworth himself. Among his many scientific interests, Dr. Butterworth is a foremost authority on the role of hyperammonemia in acute and chronic liver disease, and on the mechanisms contributing to the neurotoxicity of excess ammonia. His group has contributed considerably to our understanding of the cerebral glutamine-glutamate cycle and the role of neurotransmitters, including monoamines, glutamate and GABA in hepatic encephalopathy (HE). His work has significantly influenced the way that we have come to view nitrogen metabolism in the normal and hyperammonemic brain. Thus, it is great pleasure for us to contribute a mini-review to this special issue that is relevant to the area of hyperammonemic diseases.
Scope of the present review
Here we discuss an aspect of ammonia metabolism that is relatively little studied, yet, we believe, is biomedically very important. First, we discuss the classification of hyperammonemic diseases into primary and secondary hyperammonemias. Secondly, we discuss the glutaminase II pathway. We commence with an historical perspective and then discuss the biological importance of this pathway. We emphasize the metabolic importance of α-ketoglutaramate (KGM) – an overlooked metabolite of glutamine and a crucial component of the glutaminase II pathway – in nitrogen-, sulfur- and 1-carbon metabolism. Thirdly, we discuss the findings that KGM is a biomarker in several hyperammonemic diseases. We suggest that a greater understanding of the biochemistry of KGM will lead to a greater appreciation of nitrogen metabolism under normal and hyperammonemic conditions. We conclude that KGM may serve as a useful clinical biomarker in the diagnosis and treatment of many hyperammonemic diseases.
Classification of hyperammonemic diseases
Hyperammonemia occurs in at least 40 diseases (Cooper and Plum 1987; Brusilow and Horwich 2001; Kuhara et al. 2011a). Brusilow and Horwich (2001) suggested that hyperammonemic diseases may be conveniently classified into primary and secondary hyperammonemias. As discussed by Kuhara et al. (2011a), eight hyperammonemic diseases are classified by Brusilow and Horwich (2001) as primary, whereas other hyperammonemias are classified as secondary. The eight primary hyperammonemias include: 1) carbamoylphosphate synthetase-I deficiency (OMIM 237300), 2) ornithine transcarbamylase deficiency (OMIM 311250), 3) argininosuccinate synthetase deficiency (OMIM 215700; CTLN1), 4) argininosuccinate lyase deficiency (OMIM 207900), 5) arginase deficiency (OMIM 207800), 6) N-acetylglutamate synthase deficiency (OMIM 237310), 7) lysinuric protein intolerance (OMIM 222700), and 8) hyperornithinemia – hyperammonemia – hyperhomocitrullinuria syndrome (OMIM 238970). Thus, six of the primary hyperammonemias are due to defects in urea cycle enzymes (1 to 5) or an enzyme important in the control of the urea cycle activity (6).
In the following section, the glutaminase II pathway will be discussed, as this sets the stage for later discussions in this review on the discovery of KGM as a biomarker in HE (a secondary hyperammonemia) and in urea cycle defects (primary hyperammonemias).
Historical – discovery of the glutaminase II pathway
In the late 1940s Greenstein and co-workers reported that rat tissues contain two glutaminases that they named glutaminase I and glutaminase II. Glutaminase I and II were reported to be activated by phosphate and pyruvate, respectively (e.g. Errera and Greenstein 1949; Greenstein and Price 1949). Glutaminase I is now simply referred to as glutaminase or phosphate-activated glutaminase (PAG) and is present in mammalian tissues in the form of two isozymes encoded by separate genes – namely a liver type glutaminase (LGA; GLS1) and a more widely distributed kidney type (KGA; GLS2). A variant of GLS2 (glutaminase C; GAC) caused by alternative splicing at the C terminal is also present in some tissues and tumors (e.g. Porter et al. 2002; Cassago et al. 2012). The reaction catalyzed by PAG is shown in Eq. 1. The glutamate generated by the PAG reaction may be converted to α-ketoglutarate by the glutamate dehydrogenase reaction (Eq. 2) or by an aminotransferase reaction (Eq. 3). The α-ketoglutarate thus generated may serve as an energy source through its complete oxidation to CO2 through the tricarboxylic acid (TCA) cycle.
| (1) |
| (2) |
| (3) |
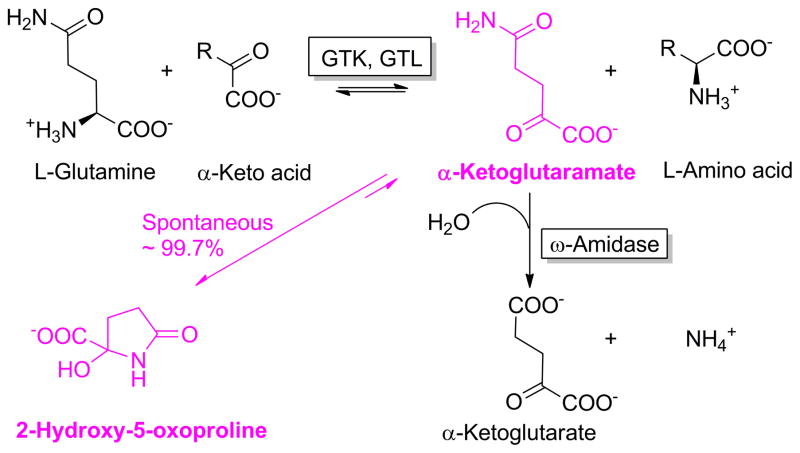
In the early 1950s Meister and colleagues showed that glutaminase II is actually a composite of two enzymes (Meister 1953; Otani and Meister 1957; Meister et al. 1952, 1955). The first enzyme in the glutaminase II pathway is a glutamine transaminase (EC 2.6.1.-)1 that catalyzes the transfer of the amino group of glutamine to a suitable α-keto acid acceptor (Eq. 4). The enzyme partially purified from rat liver was shown to possess broad specificity toward α-keto acids, including pyruvate. The product of glutamine transamination is α-ketoglutaramate (KGM; 2-oxoglutaramate). The second enzyme in the pathway is an amidase (distinct from PAG) that catalyzes the amide hydrolysis of KGM to α-ketoglutarate and ammonia (Eq. 5). This enzyme was named ω-amidase (formal name: ω-amidodicarboxylate amidohydrolase: EC 3.5.1.3). ω-Amidase activity is widespread in nature (Meister 1953). The activity is present in all organs investigated in the rat, with relatively high activity in liver and kidney (Cooper 1988; Cooper and Meister 1981). The overall glutaminase II pathway is shown in Eq. 6. In later work, Cooper and Meister (1972, 1974, 1977, 1981) reported that rat tissues contain at least two glutamine transaminases that they named glutamine transaminase K (GTK, prominent in the kidney) and glutamine transaminase L (GTL, prominent in the liver). Both enzyme activities were found to be present in cytosolic and mitochondrial fractions of rat liver and kidney (Cooper and Meister 1981). The brain contains glutamine transaminase and ω-amidase activities, but the specific activities of these enzymes in the brain are lower than those of kidney and liver (Cooper and Gross 1977: Cooper and Meister 1977: Cooper and Meister 1981)
| (4) |
| (5) |
| (6) |
Meister (1953) noted that KGM reversibly cyclizes to a lactam (2-hydroxy-5-oxoproline). This phenomenon was investigated by Hersh (1971, 1972) who showed that at physiological pH values KGM is 99.7% cyclized to the corresponding lactam with only ~0.3% present in the open-chain conformation. Only the open-chain form of KGM is a substrate of ω-amidase. Presumably, to compensate for the majority of KGM being in an enzymatically unavailable form, the affinity of ω-amidase for the open-chain form is relatively high (Km μM or less) (Hersh 1971; Krasnikov et al. 2009; Jaisson et al. 2009; Chien et al. 2012). Hersh (1971) noted that interconversion between open and cyclized forms is specific base (OH−) catalyzed. At concentrations of ω-amidase typically present in assay mixtures the rate of interconversion between open and closed forms is not rate limiting for enzyme activity at pH values ≥ 8.0 at 30°C. Thus, ω-amidase assays are routinely carried out at pH values of 8 – 8.5 at 30°C or 37°C. The interconversion between open and closed forms of KGM is shown in Fig. 1.
Fig. 1.
The glutamine transaminase – ω-amidase (glutaminase II) pathway for the metabolism of L-glutamine. GTK, glutamine transaminase K; GTL, glutamine transaminase L. Modified from Cooper (2004) to highlight α-ketoglutaramate (KGM) and its cyclic lactam (purple).
The glutamine transaminase reaction (Eq. 4), as is the case for most (but not all) aminotransferase reactions, is freely reversible. However, owing to a) the cyclization of KGM to a lactam, and b) conversion of the open-chain form of KGM to α-ketoglutarate by ω-amidase, the glutamine transaminase reaction is drawn overwhelmingly toward glutamine utilization and amination of α-keto acid substrate.
Relationship between glutamine transaminases and kynurenine aminotransferases (KATs)
Work dating back to the early 1900s identified kynurenine and kynurenate as important metabolites of tryptophan (Heidelberger et al. 1949, and references quoted therein). Two kynurenine aminotransferases, namely kynurenine aminotransferases I and II (KAT I, KAT II), were initially identified as important for the conversion of kynurenine to kynurenate in the brain (e.g. Okuno et al. 1991). [Kynurenate is a neuroprotectant that antagonizes the glutamate receptor and α7 nicotinic acetylcholine receptor (Schwarcz et al. 2012; Albuquerque and Schwarcz 2013).] Subsequently, four mammalian aminotransferases were identified that catalyze transamination between kynurenine and a suitable α-keto acid acceptor. KAT II is identical to α-aminoadipate aminotransferase (Buchli et al. 1995), whereas KAT IV is identical to mitochondrial aspartate aminotransferase (Guidetti et al. 2007). KAT IV has little or no activity with glutamine and will not be discussed further. Sequencing studies revealed that KAT I is identical to GTK (Mosca et al. 1994)2. Interestingly, GTK/KAT I was shown to be present in rat brain in the form of two splice variants – a protein with an intact 32-amino acid mitochondrial leader sequence is targeted to mitochondria, whereas a variant that lacks this sequence is found in the cytosol (Malherbe et al. 1995). We have shown that KAT III is identical to GTL (manuscript in preparation).
Both GTK/KAT I and GTL/KAT III have a remarkably broad amino acid and α-keto acid specificities (Cooper and Meister 1981; Han et al. 2004). Kcat/Km values (min−1.mM−1) reported for recombinant human GTK/KAT I for glutamine, phenylalanine, leucine, kynurenine, tryptophan and methionine (the five “best” amino acid substrates) are 157, 54, 45, 43, 36 and 34, respectively (Han et al. 2004). Note that, of the amino acids tested, glutamine is the most effective amino acid substrate of KATI/GKT. In another study, Han et al. (2009) investigated the amino acid specificity of recombinant mouse GTL/KAT III and found that glutamine is also the most favorable amino acid substrate with a Kcat/Km value of 194 min−1.mM−1. Kcat/Km values for the next six “best” amino acid substrates, namely histidine, methionine, phenylalanine, asparagine, cysteine, and kynurenine were reported to be 171, 162, 147, 126, 114 and 92 min−1.mM−1, respectively. As with GTK/KAT I, of the amino acids tested, glutamine is the most effective amino acid substrate of GTL/KAT III. Finally, Han et al. (2008) investigated the substrate specificity of recombinant human α-aminoadipate aminotransferase/KAT II. As was found for GTK/KAT I and GTL/KAT III, the amino acid and α-keto acid specificity for recombinant human α-aminoadipate aminotransferase/KAT II is broad. Kcat/Km values for aminoadipate, kynurenine, methionine and glutamate were reported to be 196, 126, 124, 119 min−1.mM−1, respectively. The Kcat/Km value for glutamine was lower, but still appreciable (11.8 min−1.mM−1) (Han et al. 2008).
As a general rule, due to the reversibility of the aminotransferase reaction and similarity between α-keto acid and amino acid substrates, if an amino acid is a substrate the corresponding α-keto acid will also be a substrate. Thus, the capacity to metabolize α-keto acids, including the branched chain α-keto acids, phenylpyruvate, and α-keto-γ-methiolbutyrate (KMB) by GTK/KAT I, GTL/KAT III and α-aminoadipate aminotransferase/KAT II is considerable. We shall return to this point later.
In conclusion, rodent and human tissues contain two aminotransferases (i.e. GTK/KAT I and GTL/KAT III) that utilize glutamine as the “best” amino acid substrate of all the amino acids studied. In addition, other aminotransferases, such as α-aminoadipate aminotransferase/KAT II, can catalyze transamination reactions with glutamine to a limited extent. Evidently, mammalian tissues have a high capacity to transaminate glutamine to KGM. Glutamine concentrations in mammalian tissues are typically in the 1.5 – 5 mM range (Bergmeyer 1974). Reported values for the concentration of kynurenine in brain range from <0.05 μM in adult rat brain (calculated from the data in Beal et al. 1992) to about 2 μM in the cerebral cortex of the adult rat (Zheng et al. 2012). In the study of Beal et al. (1992) the reported concentration of kynurenine in the whole rat brain is about 11 μM at E8 declining to ~1 μM at P0 and declining even further by P1. Beal et al. (1992) also reported that the concentration of kynurenate in whole rat brain is ~25 μM at E15, declining with gestation, but spiking at ~25 μM just before birth, and declining further to < 3 μM by P7. The concentration of glutamine and kynurenine in the extracellular fluid of adult rat brain have been reported to be ~ 116 μM (Mena et al. 2005) and ~90 nM (Notarengelo et al. 2012), respectively. In other studies, the concentration of kynurenine in rat plasma has been reported to be 1.6 μM (Pawlak et al. 2003). In the study of Pawlak et al. (2003) levels of kynurenine in a variety of rat tissues ranged from ~3 pmol/g (kidney) to ~6.3 pmol/g (muscle). Thus, while there is no doubt that transamination of kynurenine is important physiologically in the catabolism of tryptophan and the production of the neuroprotectant kynurenate, transamination of glutamine in mammalian tissues is likely to be far more important quantitatively than transamination of kynurenine. The remainder of the review will be restricted to a discussion of GTK/KAT I and GTL/KAT III as glutamine transaminases.
N flux through the glutaminase II pathway in vitro and in vivo
Bourke et al. (1971a,b) presented evidence that the glutaminase II pathway [i.e. glutamine transaminase plus ω-amidase (Eq. 6)] is a significant source of ammonia in slices of healthy portions of renal cortex obtained from patients undergoing nephrectomy. In other studies, these authors used γ-glutamylmethylamide (GGM) to investigate the glutaminase II pathway in dog kidney in situ (Bourke et al. 1971c). It was reasoned that, inasmuch as GGM is not a substrate for PAG but is a substrate for the glutaminase II pathway, GGM is a useful proxy for studying the glutaminase II pathway in vivo (the expected product of the glutaminase II pathway using GGM in place of glutamine is methylamine rather than ammonia). It was found that during infusion of GGM into the renal artery of anesthetized dogs, only small amounts of methylamine were generated during alkalosis. However, during acidosis substantial quantities of methylamine were generated (Bourke et al. 1971c).
In later work, Nissim et al. (1991) incubated isolated human kidney cells with L[amine-15N]glutamine and confirmed the presence of the glutaminase II pathway in human kidney cells. Mass spectral analysis revealed the production of KGM and 15N-labeled alanine and ammonia. The labeled alanine presumably arose through transamination of glutamine or glutamate (generated from glutamine by the PAG reaction) with pyruvate, whereas the labeled ammonia presumably arose through linked transamination reactions coupled to the glutamate dehydrogenase reaction [Flow of N: Gln (α-amino group) → [Ala ⇆ Glu] → ammonia]. However, despite these positive findings, the authors concluded that while the glutaminase II pathway is present in isolated human kidney cells, the pathway is quantitatively minor compared to the glutamate dehydrogenase reaction for the production of ammonia in these cells (Nissim et al. 1991). In other work from the same laboratory, LLC-PK1 kidney cells were incubated with either L-[amide-15N]glutamine or L-[amine-15N]glutamine. It was concluded that at physiological pH value (i.e. 7.4) the mitochondrial PAG reaction is the major source of ammonia, whereas during acute acidosis the major source of ammonia is the glutamate dehydrogenase reaction (Sahai et al. 1991). Interestingly, in these experiments KGM was detected as a metabolite of glutamine and the concentration of KGM increased during acute acidosis (Sahai et al. 1991). This finding is in accord with the relatively slow rate of ring opening of KGM lactam at low pH values.
In other work, Häussinger et al. (1985) perfused isolated rat liver with a mixture of KMB and phenylpyruvate and showed that these α-keto acids are rapidly transaminated with glutamine. Moreover, the amide nitrogen of glutamine was fully recovered in ammonia, glutamate, alanine and urea. This result is explained by ω-amidase acting on the transamination product of glutamine (i.e. KGM) to generate ammonia, which is then incorporated into glutamate (via the glutamate dehydrogenase reaction), alanine (via transamination of glutamate with pyruvate) and urea (via transamination of glutamate with oxaloacetate and incorporation of nitrogen from the resulting aspartate into urea). Inhibitor studies with α-cyanocinnamate (a mitochondrial inhibitor of the monocarboxylate transporter) suggested that about 2/3 of glutamine transamination occurred in the cytosol and about 1/3 in the mitochondria. Interestingly, in the experiments of Häussinger et al. (1985) flux through the glutaminase II pathway exceeded that of the PAG pathway, possibly as a result of competition between the two pathways for glutamine (Häussinger et al. 1985). Admittedly, in the experiments of Häussinger et al. (1985) the concentration of α-keto acid substrates for the glutamine transaminase reaction was non-physiological, but the experimental findings shows that the capacity of the glutaminase II pathway in liver is considerable.
Additional data in the literature suggest that the mammalian glutaminase II pathway is of high capacity. Thus, the α-keto acid analogues of valine, leucine, isoleucine, methionine or phenylalanine were shown to replace the corresponding essential amino acid to varying extents in sustaining the growth of rats (Chow and Walser 1974). It is likely that the glutamine transaminase(s) contributed to the transamination of the branched-chain α-keto acids in this study, and played a major role in converting phenylpyruvate and KMB to phenylalanine and methionine, respectively. In other studies, Walser et al. (1973) noted that perfused isolated liver and perfused isolated hindquarters (muscle) from 48-h fasted rats have the capacity to rapidly remove the α-keto acid analogues (at mM concentrations) of valine, leucine, isoleucine, methionine, or phenylalanine. Removal of the α-keto acids was accompanied by increased efflux of the corresponding amino acids. Interestingly, in liver that was freeze-clamped after the perfusion there was a marked increase in α-ketoglutarate and a significant decrease in glutamine, especially at high α-keto acid concentrations. Rat tissues contain mitochondrial and cytosolic isozymes of the branched-chain amino acid aminotransferases (BCATm and BCATc, respectively) (Sweatt et al. 2004). Inasmuch as muscle contains appreciable BCATm activity (Sweatt et al. 2004) it is likely that a major portion of the branched-chain amino acids efflux from muscle after perfusion with the corresponding α-keto acids was due to the action of BCATm. However, it is also likely that the glutamine transaminase(s) were responsible for the transamination of the α-keto analogues of methionine and phenylalanine (and possibly for transamination of some of the branched-chain α-keto acids) in the muscle. Immunohistochemical studies showed little or no BCATm or BCATc is present in rat liver (Sweatt et al. 2004). Therefore, the increased α-ketoglutarate and decreased glutamine in the perfused rat liver noted for all five α-keto acids by Walser et al. 1973) was almost certainly due in large part to the action of glutaminase II.
Thus, there is ample evidence that the glutaminase II pathway operates in a) isolated human kidney tissue, b) various types of isolated kidney cells, c) isolated rat liver and skeletal muscle perfused with α-keto acids, and d) the dog kidney perfused in situ with γ-glutamylmethylamide. But how relevant are these findings to the in vivo situation at the level of the whole body in humans? A crucial, but overlooked, publication by Darmaun et al. (1986) answers this question. These authors administered L[15N]glutamate, L-[amine-15N]glutamine and L-[amide-15N]glutamine to human volunteers and measured the fate of the label in the circulation. The authors showed that turnover of the amine moiety of glutamine is greater than that of the amide moiety of glutamine. Thus, the data appear to show that the transamination pathway (Eq. 4) is quantitatively more important than the PAG pathway (Eq. 1) for the metabolism of glutamine in humans. On the other hand, the authors raised the caveat that because aminotransferase-catalyzed reactions are generally reversible their findings do not prove that nitrogen fluxes from the amine of glutamine are necessarily greater than those from the amide nitrogen of glutamine or even result in a net amine turnover. However, as noted above, unlike most aminotransferase reactions, those involving glutamine are largely irreversible. Thus, the work of Darmaun et al. (1986) strongly suggests that in humans the glutaminase II pathway (Eq. 6) may be quantitatively as important as the PAG pathway and possibly even more so at the level of whole body nitrogen metabolism in the adult human.
In conclusion, several lines of evidence suggest that mammalian organs, including human liver and kidney, have the capacity to generate ammonia at a high rate from glutamine via the glutaminase II pathway (i.e. glutamine transaminase plus ω-amidase), which under some circumstances may even exceed that of the PAG pathway.
Clinical studies of α-keto acids as nitrogen-sparing compounds – the role of transamination
Given the ready ability of α-keto acids to undergo transamination in vivo, it is perhaps not surprising that these compounds have been used as nitrogen-sparing compounds in animal models of liver disease and kidney disease, and in some clinical settings (Walser and Williamson 1981). The clinical use of α-keto acid analogues of several essential amino acids (often administered as their sodium or calcium salts) was pioneered by Walser and colleagues. For example, Walser et al. (1973) showed that the α-keto acid analogues of valine, leucine, isoleucine, methionine and phenylalanine (and in limited studies, the α-keto acid analogues of histidine and tryptophan) could be used as nitrogen-sparing compounds in patients with severe chronic uremia. Oral administration was shown to be safe and to have the ability to diminish the rate at which urea appears in urine and other body fluids in these patients. In other studies, Walser and colleagues infused a mixture of the α-keto acid analogues of valine, leucine, isoleucine, methionine and phenylalanine (plus the remaining essential amino acids) into obese patients who were undergoing prolonged starvation (Sapir et al. 1974). Rapid amination of the infused α-keto acids occurred, as indicated by significant increases in plasma concentrations of valine, leucine, isoleucine, alloisoleucine, phenylalanine, and methionine. The concentrations of glutamine, glycine, serine, glutamate, and taurine fell significantly (Sapir et al. 1974). Loss of glutamine and glutamate presumably arose through increased transamination of these amino acids with the infused α-keto acids.
In other studies, Walser and colleagues investigated the effects of α-keto acids in patients with liver disease (Maddrey et al. 1976) and concluded that “keto analogues of essential amino acids are converted to the corresponding amino acids in patients with portal-systemic encephalopathy, and that such therapy may be of benefit”. In later studies by the Walser group it was shown that the ornithine salts of the branched-chain α-keto acids markedly improved electroencephalographic abnormalities and clinical grade of encephalopathy in patients with portal-systemic encephalopathy (Herlong et al. 1980) (but see Walker et al. 1982).
Since the 1980s the use of α-keto acids of essential amino acids as nitrogen-sparing compounds in liver and kidney diseases has declined, possibly as a result of 1) the inordinate expense of preparing these compounds in quantities large enough for clinical studies, and 2) possible instability problems. Nevertheless, the clinical studies from the 1970s and 1980s emphasize the scope of transamination reactions involving the α-keto acid analogues of essential amino acids in humans and the central importance of glutamate and glutamine in this process.
Physiological role of glutamine transaminases as repair enzymes
Many enzymes occasionally make mistakes and utilize the “wrong” substrate or convert the “natural” substrate to an inappropriate product. In some cases the mistake can lead to a drain of an important metabolite or to the production of a toxic substance. It is becoming increasingly apparent that several enzymes have evolved to repair or pre-empt these enzymatic “mistakes”. [For recent reviews see Van Schaftingen et al. 2013; Linster et al. 2013.] However, the concept of repair (salvage) enzymes is not new. We previously suggested that glutamine transaminases act as salvage enzymes to correct for non-specific transamination reactions (Cooper 1988; 2004; Cooper and Meister 1977 Cooper and Meister 1981). For example, the cytosolic and mitochondrial isozymes of aspartate aminotransferase can catalyze transamination to some extent of amino acids other than aspartate and glutamate. Such “inappropriate” amino acid substrates of mitochondrial aspartate aminotransferase include, for example, cysteine, methionine, isoleucine and the aromatic amino acids (Miller and Litwack 1971). Cytosolic aspartate aminotransferase can catalyze transamination of phenylalanine and tyrosine to some extent (Shrawder and Martinez-Carrion 1972). We have pointed out that the glutamine transaminases can correct these mistakes, thereby preventing the build up of potentially neurotoxic α-keto acids and a possible drain on the essential carbon of indispensable amino acids (Cooper 1988, 2004; Cooper and Meister 1977, 1981).
Because most aminotransferase reactions are freely reversible, the broad specificity toward α-keto acids exhibited by GTK and GTL is consistent with the broad specificity toward L-amino acids. At first sight this lack of specificity might preclude GTK and GTL as efficient α-keto acid scavengers. However, glutamine is far more abundant in mammalian tissues than other “good” in vitro amino acid substrates of GTK and GTL, such as the aromatic amino acids, leucine, methionine and cysteine (and as noted above, kynurenine). Moreover, importantly, as noted above, the aminotransferase reaction with glutamine as amine donor is drawn in the direction of glutamine utilization and α-keto acid amination by cyclization of KGM and deamidation of KGM by ω-amidase. Thus, the broad α-keto acid specificity of the glutamine transaminases makes biological sense. This property provides a means of converting many α-keto acids originating as “mistakes” back to the parent amino acid in an essentially irreversible manner at the expanse of easily obtainable glutamine.
As noted above, good amino acid substrates of the glutamine transaminases include glutamine, methionine, phenylalanine, tyrosine, histidine, tryptophan, leucine, α-aminobutyrate and cysteine. The corresponding α-keto acids are also good substrates. In addition, kynurenine is a good amino acid substrate and glyoxylate and α-ketosuccinamate are good α-keto acid substrates. Amino acids (e.g. glutamate, aspartate) and α-keto acids (e.g. α-ketoglutarate, oxaloacetate) with charged side groups are generally poor substrates. For discussions on the specificity of GTK/KAT I and GTL/KAT III see Cooper and Meister 1972, 1974, 1977, 1981; Han et al. 2004, 2009.
Role of the glutaminase II pathway in closure of the methionine salvage pathway
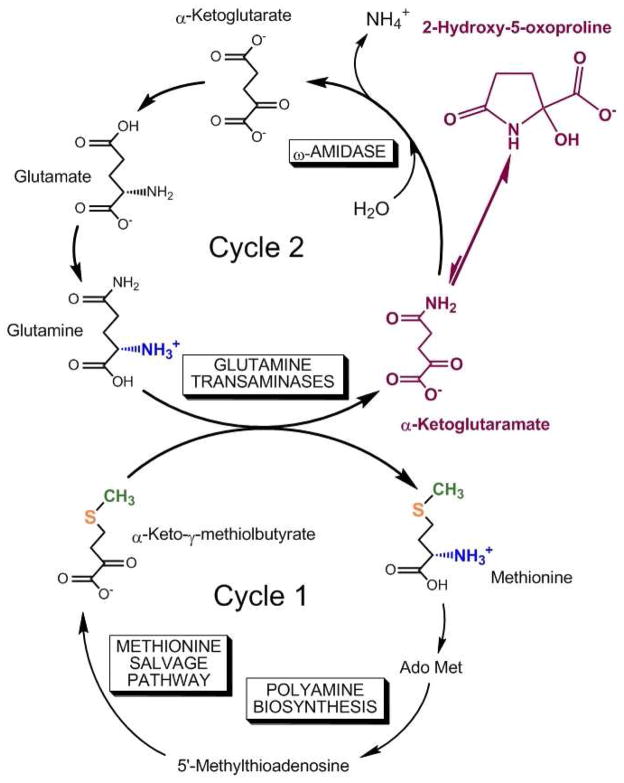
During polyamine biosynthesis 1) the C1 of methionine is lost as CO2, 2) carbons C2–C4 and the α amine are incorporated into polyamines, and 3) the methyl and sulfur are incorporated into 5′-methylthioadenosine (MTA). Nature has gone to remarkable lengths to salvage the methionine used for polyamine biosynthesis. The methionine salvage pathway is of ancient lineage and has been conserved with minor variations in archaea, bacteria, fungi, plants and vertebrates. This salvage pathway, brilliantly elucidated by Abeles and colleagues at Brandeis University in the 1990s, converts MTA to KMB through a remarkable series of reactions, some of which had no precedent in nature (Wray and Abeles 1995; Dai et al. 1999, 2001). The methionine salvage pathway is closed by transamination of KMB to methionine. In mammals and many other organisms the preferred amine donor is glutamine, which then requires removal of the generated KGM by the action of ω-amidase. In the salvaged methionine, the original methyl group and the sulfur are retained, the amino group is obtained from the non-essential amino acid glutamine and carbons 1–4 are formed anew from the ribose moiety of MTA. We have pointed out that the methionine salvage pathway (cycle 1) can be linked to a second cycle (cycle 2) through a process that we have termed the glutamine-methionine bi-cycle (Fig. 2) (Krasnikov et al. 2009). In cycle 2, KGM is converted to α-ketoglutarate by ω-amidase. The α-ketoglutarate is then converted to glutamate by the glutamate dehydrogenase reaction or via transamination. Finally glutamate is converted to glutamine, which in turn is converted to KGM by transamination, completing cycle 2. The central role of KGM and its lactam in the glutamine-methionine bi-cycle is highlighted in Fig. 2.
Fig. 2.
The glutamine-methionine bi-cycle. Cycle 1: In the methionine salvage pathway, the original sulfur (orange) and methyl group (green) of methionine are retained. The amine group of methionine is formed via a transamination reaction with glutamine and the C1–C4 of methionine is obtained anew from the ribose moiety of 5′-methylthioadenosine (MTA). Transamination of glutamine results in formation of α-ketoglutaramate (KGM), which exists overwhelmingly in a cyclic lactam form (2-hydroxy-5-oxoproline). Cycle 2: The α-ketoglutarate generated from KGM by the action of ω-amidase is converted to glutamate by the action of glutamate dehydrogenase/α-ketoglutarate-utilizing aminotransferases and thence to glutamine by glutamine synthetase. As in Fig. 1 KGM and its cyclic lactam are highlighted (magenta). Modified from Krasnikov at al. (2009).
Metabolism of circulating α-keto acids – presence of -amidase and GTK in epithelial tissue
A considerable amount of work has been carried out on interorgan trafficking and uptake of amino acids (e.g. Wipf et al. 2002). In mammals approximately 45 amino acid transporters have been described, many of which when defective are involved in disease processes (Bröer and Palacín 2011 and references cited therein). In contrast, relatively few studies have focused on the interorgan trafficking and transport of the corresponding α-keto acids. Three studies will be mentioned here. The monocarboxylate transporter was shown to be responsible for the uptake of branched-chain α-keto acids into rat liver cells (Patel et al. 1980), and for the uptake of pyruvate, α-ketoisocaproate, α-ketobutyrate and KMB across the blood-brain barrier (Steele 1986). A mitochondrial transporter for the branched-chain α-keto acids in rat brain has also been described (Hutson and Rannels 1985). This paucity of information on α-keto acid transporters is surprising given that a) excessive accumulation of certain α-keto acids (e.g. phenylpyruvate, branched-chain α-keto acids) is deleterious to brain development, and b) the concentrations of α-keto acids in blood/plasma are often >10 μM. For example, the concentrations of the keto acid analogues of the branched-chain amino acids in human plasma are in the range of 12 – 22 μM (Hoffer et al. 1993; Pailla et al. 2000). Similar values have been reported for rat plasma (Hutson and Harper 1981; Olson et al. 2013). By comparison the concentrations of the branched-chain amino acids in human plasma are ~80 – 350 μM (e.g. Stegink et al. 1991).
Several studies have shown that in the kidney, GTK is especially enriched in the proximal tubules (e.g. Jones et al. 1988; Cooper et al. 1993; Kim et al. 1997). In addition, the specific activity of both GTK and ω-amidase is much higher in the isolated rat choroid plexus than in a homogenate of the whole rat brain (Cooper et al.. 1993) Thus, it is possible that the two enzymes of the glutaminase II pathway [i.e. GTK (and possibly GTL) and ω-amidase] are metabolically linked in most (if not all) tissues. In support of this possibility, in a recent study we showed that both GTK and ω-amidase are enriched in the epithelial cells of human prostate, pancreas and bladder cells as well as in cancerous tissues derived from these tissues (Cooper et al. 2013).
In summary, the glutaminase II pathway is strategically positioned in epithelial cells in several tissues to convert a variety of α-keto acids transported from body fluids to the corresponding amino acids (Cooper et al. 2013).
Identification of the putative tumor suppressor Nit2 as ω-amidase
In 2007 Lin et al. reported that a protein named Nit 2 (Nitrilase-like protein 2) is a putative tumor suppressor. Northern blot analysis showed that the gene is transcribed in all sixteen human tissues and cell types investigated with highest message levels in liver and kidney (Lin et al. 2007). Overexpression of Nit2 in HeLa cells was found to inhibit cell growth through G2 arrest. This finding is in contrast to that reported for the more thoroughly studied tumor suppressor (and phylogenetically related) protein Nit1, which is pro-apoptotic (Huebner et al. 2011). At the time of the study by Lin et al. (2007) the biological function of Nit2 was unknown although the protein was listed in mammalian genome databanks as a likely amidase.
Nit2 and Nit1 are the sole representatives of branch 10 of the nitrilase superfamily (Pace and Brenner 2001). Despite a detailed investigation with reactive peptides designed to covalently bind to the cysteine residue of the canonical Glu-Cys-Lys triad of the putative active site, the in vivo substrate(s) of Nit1 and Nit2 remained elusive (Barglow et al. 2008). However, in 2009 our group (Krasnikov et al. 2009) and Van Schaftingen’s group (Jaisson et al. 2009) published back-to-back articles in Biochimie showing by independent methods that Nit2 is identical to ω-amidase. [The enzyme specificity of Nit1 remains elusive.] The reported widespread occurrence of Nit2 mRNA in human tissues, with highest levels in liver and kidney (Lin et al.. 2007), is in accord with the previous findings of Cooper (1988) who showed that the specific activity of ω-amidase (KGM as substrate) is present in all ten rat tissues investigated, with highest specific activity in liver and kidney.
The finding that ω-amidase is a putative tumor suppressor raises an interesting problem. It is well known that many rapidly dividing cells rely on glutamine as a source of 1) energy and 2) nitrogen for the synthesis of DNA and polyamines (Roediger 1982; Zielke et al. 1978; Mallet et al. 1986). Many rapidly dividing cancer cells also have a strong requirement for glutamine (e.g. Abou-Khalil et al. 1983; Polet and Feron 2013; Erickson et al. 2010; Koppenol et al. 2011; Ward et al. 2012; Durán et al. 2012). Glutamine is readily converted to α-ketoglutarate, which is then oxidized through the tricarboxylic acid cycle making available considerable energy to the cell. As noted above, conversion of glutamine to α-ketoglutarate may occur through hydrolysis to glutamate catalyzed by PAG (Eq. 1), followed by conversion of glutamate to α-ketoglutarate catalyzed by glutamate dehydrogenase (Eq. 2) or various aminotransferases (Eq. 3). These reactions are generally considered to constitute the main pathway by which metabolism of glutamine is initiated. However, glutamine may be converted to α-ketoglutarate via the glutaminase II pathway (Eq. 6), and we have shown that glutamine transaminase and ω-amidase activities are present in a variety of human tumors (unpublished data). The advantage of the glutaminase II pathway is that glutamine can be converted of α-ketoglutarate through a net reaction that can occur under hypoxic conditions. Evidently, however, ω-amidase/Nit2 may be a “double-edged” sword. On the one hand, ω-amidase may be a tumor suppressor, perhaps via a domain distinct from that of the amidase active site. Possibly, KGM may be an oncometabolite that is removed by high levels of ω-amidase. On the other hand, ω-amidase may be beneficial to the survival of the tumor cell by providing α-ketoglutarate. A resolution of this apparent contradiction requires further study.
KGM levels in vivo
Given that a) glutamine transamination in mammals is extensive, and b) the action of ω-amidase may be limited at physiological pH values owing to relatively slow opening of the lactam to the open-chain form, KGM is predicted to be a natural metabolite and to be present in tissues and body fluids at readily detectable levels. Indeed, the concentration of KGM in normal human cerebrospinal fluid (CSF) and rat tissues is in the μM range (Duffy et al. 1974a; Cooper et al. 1980). KGM levels are also detectable in normal human urine at a concentration of ~2 μmol/mmol creatinine (Kuhara et al. 2011b).
KGM levels in the CSF of liver disease patients correlate with degree of HE
Excess ammonia has long been recognized as neurotoxic in patients with HE and that astrocyte morphology is compromised (reviewed by Cooper and Plum 1987). Extrahepatic tissues, including the brain, do not contain a complete urea cycle and rely on the glutamine synthetase reaction (Eq. 7) to detoxify endogenous and exogenous ammonia. In the brain, glutamine synthetase is found exclusively within astrocytes (Martinez-Hernandez et al. 1977; Norenberg and Martinez-Hernandez 1979). The concentration of ammonia in whole brain is ~180 μM (Cooper and Plum 1987) and likely even lower in astrocytes. The reported Km for brain glutamine synthetase is 180 μM (Pamiljans et al. 1962). Thus, cerebral glutamine synthetase is not saturated with respect to ammonia under normoammonemic conditions. [It is possible that transport of glutamine from the astrocytes is also compromised in HE (Desjardins et al. 2012).] Accordingly, cerebral glutamine concentrations are elevated during hyperammonemia (Cudalbu et al. 2012).
It is now increasingly recognized that excess glutamine in astrocytes contributes to hyperammonemia-induced encephalopathy in liver disease patients. Brusilow and colleagues have presented considerable evidence that an osmotic response to increased glutamine synthesis contributes to the swelling of astrocytes in liver disease (reviewed in Brusilow et al. 2010). Although additional mechanisms are likely to contribute to the neuropathology associated with liver disease (e.g. Butterworth 2013; Holecek 2013; Heins and Zwingmann 2010), nuclear magnetic resonance studies show that not only is cerebral glutamine concentration increased in HE patients (e.g. Chavarria et al. 2013) but cerebral osmotic swelling can also be detected, especially in acute liver failure patients (e.g. Mardini et al. 2011; Keiding and Pavese 2011)
| [7] |
Despite the well established role of hyperammonemia in HE, levels of ammonia in blood and CSF do not always correlate well with the degree of encephalopathy (Plum 1971; Brusilow and Cooper 2011). Brennan and Plum (1971) reported that CSF obtained from HE patients contained dialyzable material (i.e. compound(s) of relatively low molecular weight) that when infused into the CSF of free-ranging rats induced a behavioral depression. Based on the above-mentioned findings of Plum (1971) and Brennan and Plum (1971), Duffy and colleagues considered that the glutaminase II pathway may be involved in the pathogenesis of HE (Vergara et al. 1974; Duffy et al. 1974b). As noted above, the glutaminase II pathway is present in mammalian, including human brain (see Siguira 1957; Yoshida 1967; Van Leuven 1975, 1976; Lockwood and Duffy 1977; Cooper and Gross 1977).
Duffy and colleagues hypothesized that increased ammonia levels in patients with liver disease will lead to increased levels of glutamine in brain, which in turn will lead to increased transamination of glutamine to KGM (Vergara et al. 1974; Duffy et al. 1974b). Vergara et al. (1974) showed that the normal level of KGM in human CSF is about 3 – 4 μM (Table 1). This value is little changed in patients with liver disease who exhibit no overt neurological symptoms. However, in hyperammonemic and comatose liver disease patients a large increase in KGM in the CSF was noted. There was also a marked increase in CSF ammonia and glutamine. Interestingly, the relative increase in CSF glutamine in the comatose patients compared to control CSF (~4–5-fold) was less than that observed for KGM (>10 fold) (Table 1). In another publication from the same group a larger number of liver disease patients was studied (Duffy et al. 1974). The authors found that the concentration of KGM in the CSF of patients with liver disease correlated well with the degree of encephalopathy (Table 2). There was also a good correlation between the concentration of KGM and the concentration of glutamine in the CSF (r = 0.696; n = 21; p <0.001) and between the concentration of KGM and ammonia in the CSF (r = 0.660; n = 19; p <0.001) (Duffy et al. 1974b). Based on a) the findings of Brennan and Plum (1972), b) the findings of increased KGM in the CSF of patients with HE, and c) the good correlation between degree of HE and concentration of KGM in HE, Duffy and colleagues considered the possibility that KGM may be a small molecular-weight toxic metabolite of ammonia/glutamine contributing to the encephalopathy associated with liver disease. To obtain evidence for this hypothesis the authors infused solutions of KGM into the lateral cerebral ventricles of free-ranging rats for 12 hours (5 pm to 5 am) at a rate of 3 μl/min (the estimated rate of CSF formation in the rat). Infusion of 100 mM KGM in mock CSF caused immediate myoclonus and circling. Infusion of mock CSF caused a significant decline (35%) in the animals’ locomotor activity. Infusion of 10 mM KGM in mock CSF resulted in a further decline in locomotor activity (p <0.01) (Duffy et al. 1974). The authors noted that the 10 mM KGM infused is 100 times that of the maximum amount found in the CSF of HE patients (~1 mM) and more than 3 orders of magnitude greater than the concentration in normal CSF. However, Duffy et al. (1974) pointed out that the depression of locomotor activity is not simply due to an osmotic effect. Infusion of equimolar sodium chloride had no effect. It should, however, be noted that the only currently available method for synthesizing KGM is via oxidation of L-glutamine with L-amino acid oxidase and separation of the resultant KGM from unreacted glutamine by passage through a Dowex 50 (H+) column (Meister 1953). Under these conditions glutamine has a tendency to undergo cyclization to 5-oxoproline plus ammonia. In the experience of one of the present authors (AJLC), even the best preparations of KGM prepared by the enzymatic oxidation of glutamine contain a few percent of 5-oxoproline – a potential precursor for glutamate and GABA in the brain.
Table 1.
Nitrogen-containing Metabolites in Human Cerebrospinal Fluid (Mean ± S.D.)a
| Condition (n) | Concentration (μM) | |||
|---|---|---|---|---|
| Ammonia | Glutamate | Glutamine | α-Ketoglutaramate (KGM) | |
| Non hepatic controls (9) | 67.7 ± 45.6 | 3.6 ± 2.9 | 422 ± 47 | 3.6 ± 3.2 |
| Liver disease without coma (5) | 37.9 ± 21.1 | 2.4 ± 1.4 | 576 ± 226 | 2.6 ± 2.3 |
| Hepatic coma (8) | 1551 ± 62 | 7.6 ± 4.2 | 2056 ± 679 | 49 ± 22 |
Data from Vergara et al. 1977
Table 2.
Concentration of CSF α-ketoglutaramate (KGM) in controls versus patients with hepatic encephalopathy (HE) (Mean ± SEM)a
| Condition (n) | Concentration (μM) |
|---|---|
| Non-HE controls (17) | 6.1 ± 1.8 |
| HE Grade 0–1+ (7) | 7.1 ± 2.5 |
| HE Grade 2–3+ (15) | 33.7 ± 3.4 |
| HE Grade 4+ (6) | 76.7 ± 11.5 |
Data from Duffy et al. (1974). The classification of HE used by the authors is as follows: Grade 0, no neurological symptoms; Grade 1, mild mental impairment, hypocapnia; Grade 2, asterixis, lethargy, confusion; Grade 3, stupor, doll’s eyes, gegenhalten; Grade 4, coma. Control CSF was obtained from patients with neurological diseases other than HE.
The mechanism responsible for this increased KGM in the CSF of patients with HE is unknown, but may be related to the increased glutamine in the CSF (Table 1). The increased glutamine in the CSF is presumably a reflection of increased glutamine in the brains of HE patients (e.g. Record et al. 1976; Lavoie et al. 1987; Mardini et al. 2011). But does this increased brain glutamine lead to increased glutamine transamination? Because aminotransferases catalyze a ping-pong type of reaction the Km value for an amino acid will depend on both the nature and the concentration of the α-keto acid co-substrate. The Km value for glutamine will increase as the α-keto acid level is increased reaching the Ks (true Michaelis constant) value at infinite concentration of α-keto acid. But α-keto acid substrate inhibition is often observed. Thus, it is difficult to assess the affinity of the glutamine transaminases for glutamine in vivo. Nevertheless, some reasonable estimate may be made. Han et al. (2004) reported a Km value for glutamine for recombinant human GTK/KATI (the most prominent glutamine transaminase in human brain) of 2.8 mM in the presence of 16 mM α-ketobutyrate. Assuming that this Km value for glutamine is close to the in vivo value then it is likely that GTK is not saturated with glutamine (the concentration of glutamine in normal whole brain is about 5 mM). It should also be noted that other amino acids (e.g. methionine and phenylalanine) also compete with glutamine. Although the levels of methionine and phenylalanine are increased in autopsied brain from patients dying of fulminant hepatic failure (Record et al. 1976) the increase in glutamine is far greater. Thus, the increase in glutamine in the brains of HE patients will favor increased transamination of glutamine assuming a constant supply of α-keto acid. However, the status of α-keto acid substrates in HE brain is not known. Nevertheless, given the increased concentration of methionine and phenylalanine in human HE brain (Record et al. 1976) and unchanged or increased cerebral α-ketoglutarate levels in animal models of hyperammonemia (reviewed in Cooper and Plum 1987) it is likely that increased transamination of methionine and phenylalanine with α-ketoglutarate will generate increased levels of KMB and phenylpyruvate, which in turn will contribute to increased glutamine transamination. Increased transamination of glutamine will result in increased KGM levels, but since the rate of ring opening of the lactam form of KGM is somewhat slow at physiological pH (pH 7.2 – 7.4) a new steady state favoring increased levels of KGM will be attained in HE brain. Finally, Hersh (1971) reported a 72% inhibition of ω-amidase-catalyzed hydrolysis of KGM in the presence of 50 mM ammonia. We reported a 50% inhibition in the presence of 10 mM ammonia (Cooper et al. 1985). Brain ammonia levels are unlikely to much exceed 1 mM in liver disease (Cooper 2013). Nevertheless, it is possible that some product inhibition of the ω-amidase-catalyzed hydrolysis of cerebral KGM may occur under extreme hyperammonemic conditions.
In conclusion, excess ammonia in the brain leads to increased glutamine and to compromised astrocytes. Although this excess glutamine leads to an osmotic stress, other mechanisms contributing to astrocyte dysfunction may occur. One possibility is that a metabolite of glutamine such as KGM may contribute. However, the evidence that KGM is a neurotoxin that contributes to hyperammonemia-induced HE is not compelling. It is of interest, however, that KGM in the CSF is a very good biomarker for HE. An understanding of the mechanisms that give rise to increased KGM in the CSF of hyperammonemic patients should provide clues as to how cerebral nitrogen metabolism is altered in HE and perhaps lead to more effective treatments.
Urinary KGM levels are increased in patients with a defect in the urea cycle
The work of Duffy and colleagues has largely been ignored, possibly because KGM is not available commercially and/or because of the invasive procedure required to obtain CSF samples. We decided to “take up the story” once again, but this time to use non-invasively obtained urine samples. [Methods for the detection of KGM in blood or plasma are currently under development (Halámková et al. 2012).] A considerable number of urine samples from Japanese newborns, infants and adults are analyzed by a gas chromatography-mass spectrometry (GC/MS) technique in the laboratory of one of the present authors (TK) at Kanazawa Medical University in Japan to screen for many inborn errors of metabolism (Kuhara 2007). A collaboration was begun between the two present authors to investigate urinary levels of KGM in patients with a defect of the urea cycle. GC/MS procedures require a suitable internal standard (IS). We used KGM labeled in the γ position with a methyl group as the IS (Kuhara et al. 2011b). γ-Methyl KGM (M-KGM), as is the case with KGM, exists overwhelmingly as a lactam. As observed previously (Cooper et al. 1980), when KGM standards were trimethylsilylated a tris-trimethylsilyl (TMS) derivative of KGM lactam was formed (Kuhara et al. 2011b). Urine samples were spiked with M-KGM, treated with urease, deproteinized with ethanol, dried, trimethylsilylated, and subjected to GC-MS analysis (Tables 3 – 5). [In these tables, mean ± SD are provided; p values were established using the t test or Mann-Whitney U test.]
Table 3.
Urinary KGM and Blood Ammonia Levels in Children with Ornithine Transcarbamylase (OTC) Deficiencya
| Patient ID | Age | KGM (mmol/mol creatinine) | Blood NH3 (μM) |
|---|---|---|---|
| OTC1 | 7 days | 47.6 | 530 |
| OTC2 | 1.2 years | 42.8 | 302 |
| OTC3 | 1.5years | 13.0 | 155 |
| OTC3 | 1.5years | 4.6 | 65 |
| OTC4 | 2 years | 11.5 | 120–180 |
| OTC5 | 3.15 years | 35.4 | 100 |
| OTC5 | 3.2 years | 13.0 | 41 |
| Controls | 4 days – 3 years 11months | 2.54 ± 1.18 b(range: 0.71 – 5.63; n = 35) | <40 |
Different from the OTC deficiency values; p = 1.5 × 10−6
Table 5.
Urinary KGM and Blood Ammonia Levels in Patients with Other Urea Cycle Disordersa
| Patient number | Age | KGM (mmol/mol creatinine) | Blood NH3 (μM) |
|---|---|---|---|
| Argininosuccinate synthase (ASS) deficiency | |||
| ASSD1 | 9 days | 6.96 | Not determined |
| ASSD2 | 1 year | 34.3 | 304 |
| Argininosuccinate lyase (ASL) deficiency | |||
| ASL1 | 0.8year | 12.2 | 126 |
| Arginase deficiency | |||
| ARG1 | 0.13 year | 42.7 | 1160 |
From Kuhara et al. (2011a). Reference values for KGM in normal urine are shown in Tables 3 and 4.
Table 3 shows urinary KGM levels in 5 young hyperammonemic patients with ornithine transcarbamylase (OTC) deficiency. A highly significant increase in urinary KGM levels compared to controls was noted. In each case of OTC deficiency the urinary KGM level was considerably higher than the highest control value. High values of urinary KGM were also noted for young patients with carbamyl phosphate synthetase I (CPS I) deficiency (Table 4). In three of the four patients the urinary KGM was higher than all values in the normal range. In patient 2, treatment resulted in urinary KGM within the normal range. In patient 3, urinary KGM was high normal but the urine was obtained just prior to death. Finally, urinary KGM levels were markedly elevated in two patients with argininosuccinate synthase (ASS) deficiency, in one patient with argininosuccinate lyase (ASL) deficiency, and in one patient with arginase (ARG) deficiency (Table 5). In conclusion, of the 13 young patients with a defect in the urea cycle, 12 had markedly elevated KMG levels (p < 0.000001 relative to control values). As noted, the one exception was agonal at the time of urine sampling.
Table 4.
Urinary KGM and Blood Ammonia Levels in Patients with Carbamyl Phosphate Synthetase I (CPS-I) Deficiencya
| Patient ID | Age | KGM (mmol/mol creatinine) | Blood NH3(μM) |
|---|---|---|---|
| CPS1 | 2 days | 9.9 | 568 |
| CPS2 | 4 days | 11.2 | 588 |
| CPS2 b | 5 days | 2.1 | Not determined |
| CPS3 | 6 days | 3.7 | Very high, died day 6 |
| CPS4 | 5 days | 13.0 | 410 (maximum) |
| Controls | 4 days – 3 years 11months | 2.54 ± 1.18 b(range: 0.71 – 5.63; n = 35) | <40 |
From Kuhara et al. (2011b).
Treatment was begun at day 4.
Different from the CPS-I deficiency values; p = 0.00022.
As noted above, lysinuric protein intolerance (LPI) is a primary hyperammonemic disease. LPI is caused by a mutation in the cationic anion transporter SLC7A7 of the basolateral membrane of the intestine and kidney tubule cells (Ogier de Baulny et al. 2012). LPI results in a defect in the transport of not only lysine, but also of cationic components of the urea cycle such as ornithine and arginine. Thus, the urea cycle is severely compromised in this disease. KGM levels were found to be markedly elevated in urine samples from two hyperammonemic patients with LPI (Table 6).
Table 6.
Urinary KGM and Blood Ammonia Levels in Two Children with Lysinuric Protein Intolerancea
| Patient ID | Age | KGM (mmol/mol creatinine) | Blood NH3 (μM) |
|---|---|---|---|
| LP11 | 2 years | 8.12 | > 40 |
| LP12 | 4.1 years | 22.8 | 89 |
| LP12 | 4.13 years | 6.52 | ND |
Data from Kuhara et al. (2011b). ND, not determined. Reference values for KGM in normal urine are shown in Tables 3 and 4.
Urinary KGM levels in other hyperammonemic diseases
Citrin deficiency is associated with secondary hyperammonemia. Deficiency of citrin (which is the hepatic isoform of the mitochondrial aspartate-glutamate carrier and which is known to participate in gluconeogenesis and the synthesis of urea, nucleotides, and protein) is a relatively recently established disease entity (Saheki and Kobayashi 2002; Kuhara et al. 2011a). Mutation in the gene coding for citrin (SLC25A13) often causes hyperammonemia and leads to two clinical presentations previously thought to be distinct: adult-onset type II citrullinemia (CTLN2, OMIM 603471) and neonatal intrahepatic cholestasis (NICCD, OMIM 605814). In general, clinical features in citrin deficiency include jaundice, hypoglycemia, a fatty liver, growth retardation, and transient hyperammonemia (Saheki and Kobayashi 2002). Some patients with neonatal NICCD suffer from severe type II citrullinemia one or several decades later in life. This later onset type II citrullinemia is associated with hyperammonemia, citrullinemia, and neuropsychiatric symptoms, such as disorientation, aberrant behavior, coma, and in the most severe cases, death (Saheki and Kobayashi 2002).
The KGM/creatinine ratio was determined in 7 urine samples obtained from 6 hyperammonemic infants with citrin deficiency (aged 2 months to 5 months 15 days). The range of urinary KGM/creatinine values in these patients (4.06 – 15) was significantly higher than the range of values in age-matched controls (1.17 – 4.20). The KGM/creatinine ratio was also determined in 5 urine samples obtained from 4 hyperammonemic older patients aged 11 years to 36 years with citrin deficiency. The range of urinary KGM/creatinine values in these patients (1.58 – 8.75) was significantly higher than the range of values in age-matched controls (0.53 – 2.25). In summary, among the 12 urine samples from 10 citrin-deficient patients, in only two determinations was the KGM/creatinine ratio within normal limits. Thus, urinary KGM is a good biomarker for hyperammonemia associated with citrin deficiency (Kuhara et al. 2011a).
Secondary hyperammonemia occurs in propionic acidemia and methylmalonic acidemia (Cathelineau et al. 1981; Filipowicz et al. 2006; Scholl-Bürgi et al. 2012). The urinary KGM/creatinine ratio (mmol/mol) was determined in two patients with propionic acidemia and in two patients with methylmalonic acidemia (Kuhara et al. 2011b). In one patient with severe propionic acidemia and severe hyperammonemia the KGM/creatinine ratio was normal at age 3 days (2.99) and high normal at age 42 days (4.00). In another patient with a milder form of propionic acidemia (aged 6 d) the blood ammonia and the urinary KGM/creatinine ratio (1.48) were normal. In one moderately hyperammonemic patient with methylmalonic acidemia the ratio of KGM/creatinine was high normal at age 1 year 4 months (4.19) and normal (2.75) at 1 year 5 months. In another patient with methylmalonic acidemia both the ammonia and KGM/creatinine ratio (2.59) were normal. Although the number of cases of patients with acidemias was limited in the study of Kuhara et al. (2011b), urinary KGM may not be a good biomarker for secondary hyperammonemia resulting from excess accumulation of propionyl-CoA or methylmalonyl-CoA. The relatively low urinary KGM in propionic acidemia may be due to a block in the tricarboxylic acid cycle resulting in low amounts of 5-carbon units and normal to low glutamine levels (Filipowicz et al. 2006; Scholl-Bürgi et al. 2012).
Conclusion
KGM in the CSF is a biomarker for the degree of encephalopathy in patients with liver disease. The concentration of KGM in the CSF correlates with ammonia and glutamine levels. Although the exact mechanism is unknown, the increased CSF KGM in encephalopathic patients with liver disease is probably due in part to a) increased brain glutamine and b) increased α-keto acid substrate for the glutamine transaminases. Although urinary KGM has not been evaluated in patients with liver disease it is a biomarker for other hyperammonemic diseases such as urea cycle disorders, lysinuric protein intolerance and citrin deficiency.
In the studies of urinary KGM described in this review only urine samples from patients with hyperammonemic diseases were evaluated (Kuhara et al. 2011b). No urinary specimens were evaluated from patients with diseases other than hyperammonemic diseases. Thus, as noted by Kuhara et al. (2011b) it is not yet possible to judge the specificity and discriminatory power of urinary KGM as a biomarker solely of hyperammonemic diseases. Nevertheless, it is hoped that the article by Kuhara et al. (2011b) and the present review will alert the clinical and biomedical community to the fact that urinary KGM is a biomarker for hyperammonemic diseases provided that the tricarboxylic acid cycle is not severely compromised. An increased understanding of the factors leading to increased urinary KGM levels in hyperammonemic diseases may lead to an increased understanding of deranged nitrogen metabolism in these diseases and possibly to improved therapies.
Acknowledgments
Some of the author’s work mentioned in this review was supported by NIH grant DK 16739.
Abbreviations used
- ARG
arginase
- ASS
argininosuccinate synthase
- BCAT
branched-chain amino acid aminotransferase
- CSF
cerebrospinal fluid
- CPS
carbamyl phosphate synthetase I
- GC/MS
gas chromatography-mass spectrometry
- GGM
γ-glutamylmethylamide
- GTK
glutamine transaminase K
- GTL
glutamine transaminase L
- HE
hepatic encephalopathy
- KAT
kynurenine aminotransferase
- KGA
kidney type glutaminase
- KGM
α-ketoglutaramate
- KMB
α-keto-γ-methiolbutyrate
- LGA
liver type glutaminase
- LPI
lysinuric protein intolerance
- MTA
5′-methylthioadenosine
- NICCD
neonatal intrahepatic cholestasis
- Nit 1
nitrilase-like protein 1
- Nit 2
nitrilase-like protein 2
- PAG
phosphate-activated glutaminase
- OTC
ornithine transcarbamylase
Footnotes
Enzymes that catalyze the transfer of an amino group from an amino acid to an α-keto acid were originally named transaminases. The word transaminase has been largely replaced by the word aminotransferase, especially for those enzymes that utilize as substrates L-glutamate and α-ketoglutarate. However, the word transaminase continues to be used for those enzymes that catalyze the transfer of the amino group of glutamine to a suitable α-keto acid acceptor.
Perry et al. (1993) sequenced rat kidney GTK (a pyridoxal 5′-phosphate-containing enzyme) and later the human orthologue (Perry et al. 1995). Because GTK has considerable cysteine S-conjugate β-lyase activity the authors referred to GTK as cysteine S-conjugate β-lyase. As a result, the gene for GTK is listed in human and rodent genome databanks as CCBL1 (Cysteine S-Conjugate Beta-Lyase isozyme 1) and antibody products are listed under this name. GTK and KAT I are listed as synonyms. The CCBL1 nomenclature is unfortunate because the cysteine S-conjugate β-lyase activity is presumably not the physiological role of this enzyme. Moreover, many additional pyridoxal 5′-phosphate-containing enzymes can also catalyze cysteine S-conjugate β-lyase reactions (Cooper et al. 2011). A second gene listed in mammalian genomes is referred to as CCBL2, a gene closely related to CCBL1. CCBL2 encodes GTL/KAT III.
Conflict of interest: No potential conflict of interest relevant to this article is presented.
References
- Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol. 2013;85:1027–1032. doi: 10.1016/j.bcp.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Khalil WH, Yunis AA, Abou-Khalil S. Prominent glutamine oxidation activity in mitochondria of hematopoietic tumors. Cancer Res. 1983;43:1990–1993. [PubMed] [Google Scholar]
- Barglow KT, Saikatendu KS, Bracey MH, Huey R, Morris GM, Olson AJ, Stevens RC, Cravatt BF. Functional proteomic and structural insights into molecular recognition in the nitrilase family enzymes. Biochemistry. 2008;47:13514–13523. doi: 10.1021/bi801786y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Swartz KJ, Isacson O. Developmental changes in brain kynurenic acid concentrations. Brain Res Dev Brain Res. 1992;68:136–139. doi: 10.1016/0165-3806(92)90256-v. [DOI] [PubMed] [Google Scholar]
- Bergmeyer U, editor. Methods of Enzymatic analysis. Verlag ChemieWeiheim, Academic Press; New York: 1974. p. 2285. Second English Edition. [Google Scholar]
- Bourke E, Fine A, Scott J. Mechanism of ammoniagenesis in human kidney. Biochem J. 1971a;125(4):94P. doi: 10.1042/bj1250094pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke E, Fine A, Scott JM. Glutaminase II pathway in human kidney. Nat New Biol. 1971b;233:249–250. doi: 10.1038/newbio233249a0. [DOI] [PubMed] [Google Scholar]
- Bourke E, Frindt G, Rubio-Paez D, Schreiner GE. Effect of chronic alkalosis and acidosis on glutaminase II path in the dog kidney in vivo. Am J Physiol. 1971c;220:1033–1036. doi: 10.1152/ajplegacy.1971.220.4.1033. [DOI] [PubMed] [Google Scholar]
- Brennan RW, Plum F. A cerebrospinal fluid transfer model for hepatic and uremic encephalopathy. Trans Am Neurol Assoc. 1971;96:210–11. [PubMed] [Google Scholar]
- Bröer S, Palacín M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 2011;436:193–211. doi: 10.1042/BJ20101912. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Horwich AL. Urea cycle enzymes Chapter 85. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. McGraw-Hill; New York: 2001. [Google Scholar]
- Brusilow SW, Cooper AJL. Encephalopathy in acute liver failure resulting from acetaminophen intoxication: new observations with potential therapy. Crit Care Med. 2011;39:2550–2553. doi: 10.1097/CCM.0b013e31822572fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow SW, Koehler RC, Traystman RJ, Cooper AJL. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchli R, Alberati-Giani D, Malherbe P, Köhler C, Broger C, Cesura AM. Cloning and functional expression of a soluble form of kynurenine/α-aminoadipate aminotransferase from rat kidney. J Biol Chem. 1995;270:29330–29335. doi: 10.1074/jbc.270.49.29330. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10:522–528. doi: 10.1038/nrgastro.2013.99. [DOI] [PubMed] [Google Scholar]
- Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, Pereira HM, Garratt RC, Dias SM, Ambrosio AL. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA. 2012;109:1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathelineau L, Briand P, Ogier H, Charpentier C, Coude FX, Saudubray JM. Occurrence of hyperammonemia in the course of 17 cases of methylmalonic acidemia. J Pediatr. 1981;99:279–280. doi: 10.1016/s0022-3476(81)80478-7. [DOI] [PubMed] [Google Scholar]
- Chavarria L, Alonso J, García-Martínez R, Simón-Talero M, Ventura-Cots M, Ramírez C, Torrens M, Vargas V, Rovira A, Córdoba J. Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J Cereb Blood Flow Metab. 2013;33:272–277. doi: 10.1038/jcbfm.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CH, Gao QZ, Cooper AJL, Lyu JH, Sheu SY. Structural insights into the catalytic active site and activity of human Nit2/ω-amidase: kinetic assay and molecular dynamics simulation. J Biol Chem. 2012;287:25715–25726. doi: 10.1074/jbc.M111.259119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KW, Walser M. Substitution of five essential amino acids by their alpha-keto analogues in the diet of rats. J Nutr. 1974;104:1208–1214. doi: 10.1093/jn/104.9.1208. [DOI] [PubMed] [Google Scholar]
- Cooper AJL. Glutamine aminotransferases and ω-amidases. In: Kvamme E, editor. Glutamine and Glutamate in Mammals. Vol. 1. CRC Press, Inc; Boca Raton, Florida: 1988. pp. 33–52. [Google Scholar]
- Cooper AJL. The role of glutamine transaminase K (GTK) in sulfur and α-keto acid metabolism in the brain, and possible bioactivation of neurotoxicants. Neurochem Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Cooper AJL. Possible treatment of end-stage hyperammonemic encephalopathy by inhibition of glutamine synthetase. Metab Brain Dis. 2013;28:119–125. doi: 10.1007/s11011-012-9338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Gross M. The glutamine transaminase-ω-amidase system in rat and human brain. J Neurochem. 1977;28:771–778. doi: 10.1111/j.1471-4159.1977.tb10626.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Meister A. Isolation and properties of highly purified glutamine transaminase. Biochemistry. 1972;11:661–671. doi: 10.1021/bi00755a001. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Meister A. Isolation and properties of a new glutamine transaminase from rat kidney. J Biol Chem. 1974;249:2554–2561. [PubMed] [Google Scholar]
- Cooper AJL, Meister A. The glutamine transaminase - ω-amidase pathway. CRC Critical Reviews in Biochemistry. 1977;4:281–303. doi: 10.3109/10409237709102560. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Meister A. Comparative studies of glutamine transaminases from rat tissues. Comp Biochem Physiol. 1981;69B:137–145. [Google Scholar]
- Cooper AJL, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Dhar AK, Kutt H, Duffy TE. Determination of 2-pyrrolidone-5-carboxylic and α-ketoglutaramic acids in human cerebrospinal fluid by gas chromatography. Anal Biochem. 1980;103:118–126. doi: 10.1016/0003-2697(80)90245-6. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Duffy TE, Meister A. α-Keto acid ω-amidase from rat liver. Methods Enzymol. 1985;113:350–358. doi: 10.1016/s0076-6879(85)13048-x. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Abraham DG, Gelbard AS, Lai JC, Petito CK. High activities of glutamine transaminase K (dichlorovinylcysteine β-lyase) and ω-amidase in the choroid plexus of rat brain. J Neurochem. 1993;61:1731–1741. doi: 10.1111/j.1471-4159.1993.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Krasnikov BF, Niatsetskaya ZV, Pinto JT, Callery PS, Villar MT, Artigues A, Bruschi SA. Cysteine S-conjugate β-lyases: important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids. 2011;41:7–27. doi: 10.1007/s00726-010-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Dorai T, Dorai B, Krasnikov BF, Li J, Hallen A, Pinto JT. Role of Glutamine transaminases in nitrogen, sulfur, selenium and 1-carbon metabolism. In: Rajendram R, Preedy VR, Patel VB, editors. Glutamine in health and disease. Springer; New York: 2013. in press. [Google Scholar]
- Cudalbu C, Lanz B, Duarte JM, Morgenthaler FD, Pilloud Y, Mlynárik V, Gruetter R. Cerebral glutamine metabolism under hyperammonemia determined in vivo by localized 1H and 15N NMR spectroscopy. J Cereb Blood Flow Metab. 2012;32:696–708. doi: 10.1038/jcbfm.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wensink PC, Abeles RH. One protein, two enzymes. J Biol Chem. 1999;274:1193–1195. doi: 10.1074/jbc.274.3.1193. [DOI] [PubMed] [Google Scholar]
- Dai Y, Pochapsky TC, Abeles RH. Mechanistic studies of two dioxygenases in the methionine salvage pathway of Klebsiella pneumoniae. Biochemistry. 2001;40:6379–6387. doi: 10.1021/bi010110y. [DOI] [PubMed] [Google Scholar]
- Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol. 1986;251:E117–E126. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Du T, Jiang W, Peng L, Butterworth RF. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012;60:690–696. doi: 10.1016/j.neuint.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Duffy TE, Cooper AJL, Meister A. Identification of α-ketoglutaramate in rat liver, kidney, and brain. Relationship to glutamine transaminase and ω-amidase activities. J Biol Chem. 1974a;249:7603–7606. [PubMed] [Google Scholar]
- Duffy TE, Vergara F, Plum F. α-Ketoglutaramate in hepatic encephalopathy. Res Publ Assoc Res Nerv Ment Dis. 1974b;53:39–52. [PubMed] [Google Scholar]
- Durán RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA. Glutaminase: A hot spot for regulation of cancer cell metabolism? Oncotarget. 2010;1:734–740. doi: 10.18632/oncotarget.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errera M, Greenstein JP. Phosphate-activated glutaminase in kidney and other tissues. J Biol Chem. 1949;178:495–502. [PubMed] [Google Scholar]
- Filipowicz HR, Ernst SL, Ashurst CL, Pasquali M, Longo N. Metabolic changes associated with hyperammonemia in patients with propionic acidemia. Mol Genet Metab. 2006;88:123–130. doi: 10.1016/j.ymgme.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Greenstein JP, Price VE. α-Keto acid-activated glutaminase and asparaginase. J Biol Chem. 1949;178:695–705. [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Halámková L, Mailloux S, Halámek J, Cooper AJ, Katz E. Enzymatic analysis of α-ketoglutaramate - a biomarker for hyperammonemia. Talanta. 2012;100:7–11. doi: 10.1016/j.talanta.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Li J, Li J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur J Biochem. 2004;71:4804–4814. doi: 10.1111/j.1432-1033.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- Han Q, Cai T, Tagle DA, Robinson H, Li J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Biosci Rep. 2008;28:205–215. doi: 10.1042/BSR20080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol. 2009;29:784–793. doi: 10.1128/MCB.01272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D, Stehle T, Gerok W. Glutamine metabolism in isolated perfused rat liver. The transamination pathway. Biol Chem Hoppe Seyler. 1985;366:527–536. doi: 10.1515/bchm3.1985.366.1.527. [DOI] [PubMed] [Google Scholar]
- Heidelberger C, Gullberg ME, Morgan AF, Lepkovsky S. Tryptophan metabolism; concerning the mechanism of the mammalian conversion of tryptophan into kynurenine, kynurenic acid, and nicotinic acid. J Biol Chem. 1949;179:143–150. [PubMed] [Google Scholar]
- Heins J, Zwingmann C. Organic osmolytes in hyponatremia and ammonia toxicity. Metab Brain Dis. 2010;25:81–89. doi: 10.1007/s11011-010-9170-5. [DOI] [PubMed] [Google Scholar]
- Herlong HF, Maddrey WC, Walser M. The use of ornithine salts of branched-chain ketoacids in portal-systemic encephalopathy. Ann Intern Med. 1980;93:545–550. doi: 10.7326/0003-4819-93-4-545. [DOI] [PubMed] [Google Scholar]
- Hersh LB. Rat liver ω-amidase: Purification and properties. Biochemistry. 1971;10:2884–2891. doi: 10.1021/bi00791a014. [DOI] [PubMed] [Google Scholar]
- Hersh LB. Rat liver ω-amidase. Kinetic evidence for an acyl-enzyme intermediate. Biochemistry. 1972;11:2251–2256. doi: 10.1021/bi00762a007. [DOI] [PubMed] [Google Scholar]
- Hoffer LJ, Taveroff A, Robitaille L, Mamer OA, Reimer ML. α-Keto and α-hydroxy branched-chain acid interrelationships in normal humans. J Nutr. 1993;123:1513–1521. doi: 10.1093/jn/123.9.1513. [DOI] [PubMed] [Google Scholar]
- Holecek M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis. 2013 Aug 31 ; doi: 10.1007/s11011-013-9428-9. 2013 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner K, Saldivar JC, Sun J, Shibata H, Druck T. Hits, Fhits and Nits: beyond enzymatic function. Adv Enzyme Regul. 2011;51:208–217. doi: 10.1016/j.advenzreg.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson SM, Harper AE. Blood and tissue branched-chain amino and α-keto acid concentrations: effect of diet, starvation, and disease. 1981;34:173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Rannels SL. Characterization of a mitochondrial transport system for branched chain α-keto acids. J Biol Chem. 1985;260:14189–14193. [PubMed] [Google Scholar]
- Jaisson S, Veiga-da-Cunha M, Van Schaftingen E. Molecular identification of ω-amidase, the enzyme that is functionally coupled with glutamine transaminases, as the putative tumor suppressor Nit2. Biochimie. 2009;91:1066–1071. doi: 10.1016/j.biochi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Jones TW, Qin C, Schaeffer VH, Stevens JL. Immunohistochemical localization of glutamine transaminase K, a rat kidney cysteine conjugate β-lyase, and the relationship to the segment specificity of cysteine conjugate nephrotoxicity. Mol Pharmacol. 1988;34:621–627. [PubMed] [Google Scholar]
- Keiding S, Pavese N. Brain metabolism in patients with hepatic encephalopathy studied by PET and MR. Arch Biochem Biophys. 2103;536:131–142. doi: 10.1016/j.abb.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cha SH, Abraham DG, Cooper AJL, Endou H. Intranephron distribution of cysteine S-conjugate beta-lyase activity and its implication for hexachloro-1,3-butadiene-induced nephrotoxicity in rats. Arch Toxicol. 1997;71:131–41. doi: 10.1007/s002040050367. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Krasnikov BF, Chien C-H, Nostramo R, Pinto JT, Nieves E, Callaway M, Sun J, Huebner K, Cooper AJL. Identification of the putative tumor suppressor Nit2 as ω-amidase, an enzyme metabolically linked to glutamine and asparagine transamination. Biochimie. 2009;91:1072–1080. doi: 10.1016/j.biochi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara T. Noninvasive human metabolome analysis for differential diagnosis of inborn errors of metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:42–50. doi: 10.1016/j.jchromb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Kuhara T, Ohse M, Inoue Y, Cooper AJL. A GC/MS-based metabolomic approach for diagnosing citrin deficiency. Anal Bioanal Chem. 2011a;400:1881–1894. doi: 10.1007/s00216-011-4766-0. [DOI] [PubMed] [Google Scholar]
- Kuhara T, Inoue Y, Ohse M, Krasnikov BF, Cooper AJL. Urinary 2-hydroxy-5-oxoproline, the lactam form of α-ketoglutaramate, is markedly increased in urea cycle disorders. Anal Bioanal Chem. 2011b;400:1843–1851. doi: 10.1007/s00216-011-4688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J, Giguère JF, Layrargues GP, Butterworth RF. Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem. 1987;49:692–697. doi: 10.1111/j.1471-4159.1987.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chung MY, Chen WB, Chien CH. Growth inhibitory effect of the human NIT2 gene and its allelic imbalance in cancers. FEBS J. 2007;274:2946–2956. doi: 10.1111/j.1742-4658.2007.05828.x. [DOI] [PubMed] [Google Scholar]
- Linster CL, Van Schaftingen E, Hanson AD. Metabolite damage and its repair or pre-emption. Nat Chem Biol. 2013;9:72–80. doi: 10.1038/nchembio.1141. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Duffy TE. Glutamine transaminase and ω-amidase: species variations in brain activity and effect of portacaval shunting. J Neurochem. 1977;28:673–675. doi: 10.1111/j.1471-4159.1977.tb10443.x. [DOI] [PubMed] [Google Scholar]
- Maddrey WC, Weber FL, Jr, Coulter AW, Chura CM, Chapanis NP, Walser M. Effects of keto analogues of essential amino acids in portal-systemic encephalopathy. Gastroenterology. 1976;71:190–195. [PubMed] [Google Scholar]
- Malherbe P, Alberati-Giani D, Köhler C, Cesura AM. Identification of a mitochondrial form of kynurenine aminotransferase/glutamine transaminase K from rat brain. FEBS Lett. 1995;367:141–144. doi: 10.1016/0014-5793(95)00546-l. [DOI] [PubMed] [Google Scholar]
- Mallet RT, Kelleher JK, Jackson MJ. Substrate metabolism of isolated jejunal epithelium: conservation of three-carbon units. Am J Physiol. 1986;250:C191–C198. doi: 10.1152/ajpcell.1986.250.2.C191. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Mardini H, Smith FE, Record CO, Blamire AM. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol. 2011;54:1154–1160. doi: 10.1016/j.jhep.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Meister A. Preparation and enzymatic reactions of the keto analogues of glutamine and asparagine. J Biol Chem. 1953;200:571–589. [PubMed] [Google Scholar]
- Meister A, Sober HA, Tice SV, Fraser PE. Transamination and associated deamidation of asparagine and glutamine. J Biol Chem. 1952;197:319–330. [PubMed] [Google Scholar]
- Meister A, Levintow L, Greenfield RE, Abendschein PA. Hydrolysis and transfer reactions catalyzed by ω-amidase. J Biol Chem. 1955;215:441–460. [PubMed] [Google Scholar]
- Mena FV, Baab PJ, Zielke CL, Huang Y, Zielke HR. Formation of extracellular glutamate from glutamine: exclusion of pyroglutamate as an intermediate. Brain Res. 2005;1052:88–96. doi: 10.1016/j.brainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Miller JE, Litwack G. Purification, properties, and identity of liver mitochondrial tyrosine aminotransferase. J Biol Chem. 1971;246:3234–3240. [PubMed] [Google Scholar]
- Mosca M, Cozzi L, Breton J, Speciale C, Okuno E, Schwarcz R, Benatti L. Molecular cloning of rat kynurenine aminotransferase: identity with glutamine transaminase K. FEBS Lett. 1994;353:21–24. doi: 10.1016/0014-5793(94)01003-x. [DOI] [PubMed] [Google Scholar]
- Nissim I, Wehrli S, States B, Nissim I, Yudkoff M. Analysis and physiological implications of renal 2-oxoglutaramate metabolism. Biochem J. 1991;277:33–38. doi: 10.1042/bj2770033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Notarangelo FM, Wu HQ, Macherone A, Graham DR, Schwarcz R. Gas chromatography/tandem mass spectrometry detection of extracellular kynurenine and related metabolites in normal and lesioned rat brain. Anal Biochem. 2012;421:573–581. doi: 10.1016/j.ab.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier de Baulny H, Schiff M, Dionisi-Vici C. Lysinuric protein intolerance (LPI): a multi organ disease by far more complex than a classic urea cycle disorder. Mol Genet Metab. 2012;106:12–17. doi: 10.1016/j.ymgme.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Okuno E, Nakamura M, Schwarcz R. Two kynurenine aminotransferases in human brain. Brain Res. 1991;542:307–312. doi: 10.1016/0006-8993(91)91583-m. [DOI] [PubMed] [Google Scholar]
- Olson KC, Chen G, Lynch CJ. Quantification of branched-chain keto acids in tissue by ultra fast liquid chromatography-mass spectrometry. Anal Biochem. 2103;439:116–122. doi: 10.1016/j.ab.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani TT, Meister A. ω-Amide and ω-amino acid derivatives of α-ketoglutaric and oxalacetic acids. J Biol Chem. 1957;224:137–148. [PubMed] [Google Scholar]
- Pace HC, Brenner C. The nitrilase superfamily: classification, structure and function. Genome Biol. 2001;2(1) doi: 10.1186/gb-2001-2-1-reviews0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailla K, Blonde-Cynober F, Aussel C, De Bandt JP, Cynober L. Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem. 2000;46:848–853. [PubMed] [Google Scholar]
- Pamiljans V, Krishnaswamy PR, Dumville G, Meister A. Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry. 1962;1:153–158. doi: 10.1021/bi00907a023. [DOI] [PubMed] [Google Scholar]
- Patel TB, Waymack PP, Olson MS. The effect of the monocarboxylate translocator inhibitor, α-cyanocinnamate, on the oxidation of branched chain α-keto acids in rat liver. Arch Biochem Biophys. 1980;201:629–365. doi: 10.1016/0003-9861(80)90552-4. [DOI] [PubMed] [Google Scholar]
- Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175–189. [PubMed] [Google Scholar]
- Perry SJ, Schofield MA, MacFarlane M, Lock EA, King LJ, Gibson GG, Goldfarb PS. Isolation and expression of a cDNA coding for rat kidney cytosolic cysteine conjugate β-lyase. Mol Pharmacol. 1993;43:660–665. [PubMed] [Google Scholar]
- Perry S, Harries H, Scholfield C, Lock T, King L, Gibson G, Goldfarb P. Molecular cloning and expression of a cDNA for human kidney cysteine conjugate β-lyase. FEBS Lett. 1995;360:277–280. doi: 10.1016/0014-5793(95)00123-q. [DOI] [PubMed] [Google Scholar]
- Plum F. The CSF in hepatic encephalopathy. Exp Biol Med. 1971;4:34–41. [PubMed] [Google Scholar]
- Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–165. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- Porter LD, Ibrahim H, Taylor L, Curthoys NP. Complexity and species variation of the kidney-type glutaminase gene. Physiol Genomics. 2002;9:157–166. doi: 10.1152/physiolgenomics.00017.2002. [DOI] [PubMed] [Google Scholar]
- Record CO, Buxton B, Chase RA, Curzon G, Murray-Lyon IM, Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- Sahai A, Nissim I, Tannen RL. Pathways of acute pH regulation of ammoniagenesis in LLC-PK1 cells: study with [15N]glutamine. Am J Physiol. 1991;261:F481–487. doi: 10.1152/ajprenal.1991.261.3.F481. [DOI] [PubMed] [Google Scholar]
- Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD) J Hum Genet. 2002;47:333–341. doi: 10.1007/s100380200046. [DOI] [PubMed] [Google Scholar]
- Sapir DG, Owen OE, Pozefsky T, Walser M. Nitrogen sparing induced by a mixture of essential amino acids given chiefly as their keto-analogues during prolonged starvation in obese subjects. J Clin Invest. 1974;54:974–980. doi: 10.1172/JCI107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl-Bürgi S, Sass JO, Zschocke J, Karall D. Amino acid metabolism in patients with propionic acidaemia. J Inherit Metab Dis. 2012;35:65–70. doi: 10.1007/s10545-010-9245-9. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawder E, Martinez-Carrion M. Evidence of phenylalanine transaminase activity in the isoenzymes of aspartate transaminase. J Biol Chem. 1972;247:2486–2492. [PubMed] [Google Scholar]
- Siguira M. A pharmacological aspect on glutamic acid metabolism in brain: transamination and associated deamidation of glutamine to α-keto acid in brain. Japan J Pharmacol. 1957;7:1–5. doi: 10.1254/jjp.7.1. [DOI] [PubMed] [Google Scholar]
- Stegink LD, Filer LJ, Jr, Brummel MC, Baker GL, Krause WL, Bell EF, Ziegler EE. Plasma amino acid concentrations and amino acid ratios in normal adults and adults heterozygous for phenylketonuria ingesting a hamburger and milk shake meal. Am J Clin Nutr. 1991;53:670–675. doi: 10.1093/ajcn/53.3.670. [DOI] [PubMed] [Google Scholar]
- Steele RD. Blood-brain barrier transport of the α-keto acid analogs of amino acids. Fed Proc. 1986;45:2060–2064. [PubMed] [Google Scholar]
- Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–E76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- Van Leuven F. Highly purified glutamine transaminase from rat brain. Physical and kinetic properties. Eur J Biochem. 1975;58:153–158. doi: 10.1111/j.1432-1033.1975.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Van Leuven F. Glutamine transaminase from brain tissue. Further studies on kinetic properties and specificity of the enzyme. Eur J Biochem. 1976;65:271–274. doi: 10.1111/j.1432-1033.1976.tb10414.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E, Rzem R, Marbaix A, Collard F, Veiga-da-Cunha M, Linster CL. Metabolite proofreading, a neglected aspect of intermediary metabolism. J Inherit Metab Dis. 2013;36:427–434. doi: 10.1007/s10545-012-9571-1. [DOI] [PubMed] [Google Scholar]
- Vergara F, Plum F, Duffy TE. α-Ketoglutaramate: increased concentrations in the cerebrospinal fluid of patients in hepatic coma. Science. 1974;183:81–83. doi: 10.1126/science.183.4120.81. [DOI] [PubMed] [Google Scholar]
- Walser M, Williamson JR, editors. Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids. Elsevier/North Holland; New York: 1981. [Google Scholar]
- Walser M, Lund P, Ruderman NB, Coulter AW. Synthesis of essential amino acids from their α-keto analogues by perfused rat liver and muscle. J Clin Invest. 1973;52:2865–2877. doi: 10.1172/JCI107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S, Götz R, Czygan P, Stiehl A, Lanzinger G, Sieg A, Raedsch R, Kommerell B. Oral keto analogs of branched-chain amino acids in hyperammonemia in patients with cirrhosis of the liver. A double-blind crossover study. Digestion. 1982;24:105–111. doi: 10.1159/000198784. [DOI] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf D, Ludewig U, Tegeder M, Rentsch D, Koch W, Frommer WB. Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem Sci. 2002;27:139–147. doi: 10.1016/s0968-0004(01)02054-0. [DOI] [PubMed] [Google Scholar]
- Wray JW, Abeles RH. The methionine salvage pathway in Klebsiella pneumoniae and rat liver. Identification and characterization of two novel dioxygenases. J Biol Chem. 1995;270:3147–3153. doi: 10.1074/jbc.270.7.3147. [DOI] [PubMed] [Google Scholar]
- Yoshida T. Purification and some properties of soluble and mitochondrial glutamine-ketoacids transaminase isozymes. Vitamins. 1967;35:227–234. (Japanese) [Google Scholar]
- Zheng X, Kang A, Dai C, Liang Y, Xie T, Xie L, Peng Y, Wang G, Hao H. Quantitative analysis of neurochemical panel in rat brain and plasma by liquid chromatography-tandem mass spectrometry. Anal Chem. 2012;84:10044–10051. doi: 10.1021/ac3025202. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Ozand PT, Tildon JT, Sevdalian DA, Cornblath M. Reciprocal regulation of glucose and glutamine utilization by cultured human diploid fibroblasts. J Cell Physiol. 1978;95:41–48. doi: 10.1002/jcp.1040950106. [DOI] [PubMed] [Google Scholar]