Abstract
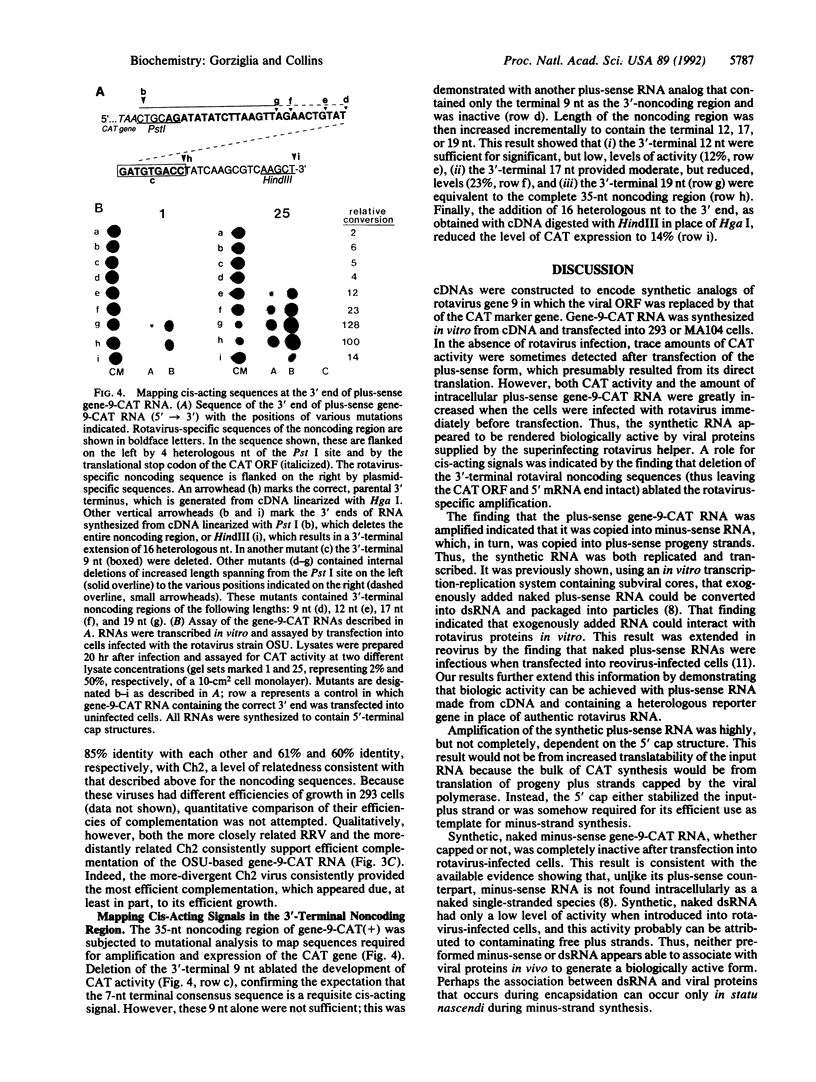
cDNAs were constructed to encode plus- or minus-sense analogs of gene 9 RNA of porcine rotavirus strain OSU in which the bacterial chloramphenicol acetyltransferase (CAT) reporter gene was flanked by the 5'-terminal 44 nucleotides (nt) and 3'-terminal 35 nt of the authentic rotavirus gene. Transfection of plus-sense gene-9-CAT RNA into rotavirus-infected cells resulted in its amplification and in the efficient expression of CAT; this was greatly enhanced by the presence of a 5' cap structure. Amplification was ablated by omitting the rotavirus superinfection or by removing the 3'-terminal 35-nt rotavirus sequence from the RNA. This result indicated that amplification depended both on rotavirus proteins supplied in trans and on cis-acting rotavirus sequences. Minus-sense or double-stranded gene-9-CAT RNA was essentially inactive, indicating that synthetic RNAs can be introduced into the rotavirus replicative cycle in vivo only when provided in the plus sense. However, incorporation of the CAT-bearing RNA into infectious rotavirus was not detected. Two heterologous rotaviruses, the simian RRV and chicken Ch2 strains, efficiently complemented the OSU-based gene-9-CAT RNA, even though the Ch2 strain was only 50%-66% related in the noncoding regions. Mutational analysis of the 35-nt 3'-noncoding region showed that the 3'-terminal 12 or 17 nt were sufficient for reduced (12% or 23%, respectively) levels of amplification, whereas inclusion of the 3'-terminal 19 nt fully restored amplification. Thus, the 3'-terminal cis-acting signals required for amplification include the 7-nt-terminal consensus sequence together with 12 nt of adjoining, less-well-conserved sequence.
Full text
PDF



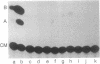
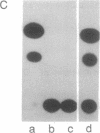
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Charpilienne A., Chilmonczyk S., Estes M. K. Nucleotide sequence of bovine rotavirus gene 1 and expression of the gene product in baculovirus. Virology. 1989 Jul;171(1):131–140. doi: 10.1016/0042-6822(89)90519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos C. O., Patton J. T. Characterization of rotavirus replication intermediates: a model for the assembly of single-shelled particles. Virology. 1989 Oct;172(2):616–627. doi: 10.1016/0042-6822(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Aguirre Y., Hoshino Y., Esparza J., Blumentals I., Askaa J., Thompson M., Glass R. I., Kapikian A. Z., Chanock R. M. VP7 serotype-specific glycoprotein of OSU porcine rotavirus: coding assignment and gene sequence. J Gen Virol. 1986 Nov;67(Pt 11):2445–2454. doi: 10.1099/0022-1317-67-11-2445. [DOI] [PubMed] [Google Scholar]
- Imai M., Akatani K., Ikegami N., Furuichi Y. Capped and conserved terminal structures in human rotavirus genome double-stranded RNA segments. J Virol. 1983 Jul;47(1):125–136. doi: 10.1128/jvi.47.1.125-136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. Molecular biology of rotaviruses. V. Terminal structure of viral RNA species. Virology. 1983 Apr 15;126(1):204–212. doi: 10.1016/0042-6822(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Hoshino Y., Gorziglia M. Sequence of the VP7 gene of chicken rotavirus Ch2 strain of serotype 7 rotavirus. Virology. 1991 Dec;185(2):853–856. doi: 10.1016/0042-6822(91)90558-s. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Hoshino Y., Taniguchi K., Green K. Y., Greenberg H. B., Kapikian A. Z., Chanock R. M., Gorziglia M. Rotavirus VP7 neutralization epitopes of serotype 3 strains. Virology. 1989 Aug;171(2):503–515. doi: 10.1016/0042-6822(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Roner M. R., Sutphin L. A., Joklik W. K. Reovirus RNA is infectious. Virology. 1990 Dec;179(2):845–852. doi: 10.1016/0042-6822(90)90153-i. [DOI] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Schonberg M., Levin D. H., Acs G. The reovirus replicative cycle: conservation of parental RNA and protein. Proc Natl Acad Sci U S A. 1970 Sep;67(1):275–281. doi: 10.1073/pnas.67.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25(10):1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]