Abstract
Protein and peptide drugs administered subcutaneously, such as insulin can be amyloidogenic and result in localized amyloid deposits at the sites of medication injections. These iatrogenic amyloidoses typically present as a localized subcutaneous nodule or skin reaction at the site of administration, and often pose diagnostic challenges. We have analyzed the amyloid proteome in 52 cases of insulin and enfuvirtide associated amyloidosis using laser microdissection/tandem mass spectrometry. We show that the deposits are composed of the drug, as well as other amyloid precursor proteins such as apolipoproteins A-I, A-IV, E and serum amyloid protein. Mass spectrometry-based amyloid sub-typing allows for accurate amyloid diagnosis with resultant therapeutic and prognostic implications. This insight into the amyloid proteome in drug-induced amyloidosis may help further understand pathogenesis of amyloid fibril formation.
Keywords: Drug-induced amyloid, iatrogenic, pharmaceutical amyloidosis
Introduction
The amyloidoses are heterogeneous diseases associated with the deposition of insoluble proteins or peptides with a characteristic beta-diffraction pattern extracellularly. At the current time, over 27 different extracellular fibril proteins are known to cause disease in humans [1]. Iatrogenic amyloidosis is a rare, often not sought diagnosis. Insulin is a well-known agent that has been known to be amyloidogenic [2] and linked to localized amyloidosis (AIns) at sites of recombinant insulin administration [3]. Recently, a peptide anti-retroviral drug, enfuvirtide (Fuzeon®) used in the treatment of HIV infection has also been reported to cause amyloidosis [4]. On occasion, patients with drug-induced amyloidosis can present with other systemic features reminiscent of systemic immunoglobulin-derived (AL) amyloidosis, and may present a diagnostic challenge [5]. Since the treatment of the latter often involves chemotherapy and/or stem cell transplantation, while the former tends to remain localized and therefore needs local therapy, accurate diagnosis is critical. To this end, proteomic analysis of amyloid tissue has proven to be an invaluable tool in amyloid typing [6] and routinely employed at our institution using laser microdissection/tandem mass spectroscopy (LMD-MS/MS). In this paper, we analyze the biochemical composition of amyloid in patients with drug-induced amyloidosis using LMD-MS/MS-based proteomic analysis in order to gain an insight into the amyloid proteome in these cases.
Methods
This study was approved by the Mayo Foundation Institutional Review Board, and conducted in accordance with the state of Minnesota regulations. Between January 2010 and May 2013, we have detected insulin-derived amyloidosis (AIns) in 50 patients, and enfuvirtide-derived amyloidosis in 2 patients. A majority of these patients were seen as consult cases for mass spectroscopy analysis of amyloidosis.
Specimen preparation and LMD/MS-MS proteomic analysis
The methods have previously been published [7]. Briefly, for each case, 10-μm-thick sections of formalin-fixed paraffin-embedded tissues were stained with Congo red. Areas staining positive with Congo red as viewed with a fluorescent light source appeared bright red (Figure 1A). Using LMD, these areas were dissected. Each microdissection contained a volume of at least 60 000 μml3, and three microdissections were analyzed for each case. The microdissected material was collected into 0.5-ml microcentrifuge tube caps containing 35 μl Tris/EDTA/0.002% Zwittergent buffer. Microdissected fragments were subjected to a heat-mediated antigen retrieval method (98 °C for 90 min) before being denatured via sonication and subsequently digested into tryptic peptides overnight using 0.5 ug of trypsin. The resulting digests were then analyzed with nanoflow LC-MS/MS. The MS/MS spectra of each case were matched against a composite protein sequence database using three different search algorithms (Sequest, X!Tandem, and Mascot). The composite database contained the human SwissProt entries but was also augmented with known immunoglobulin variant domains, known amyloidogenic mutations from literature, the enfurvitide amino acid sequence, and common contaminants. Reversed protein sequences were appended to the database for estimating the false discovery rates of the identifications. The peptide identification results were filtered using Scaffold software (Proteome Software, Portland, OR) and then filtered peptides were assembled into protein identifications. Candidate proteins with at least one high-confident (probability of identification >90%) unique peptide identification and at least four MS/MS spectral matches were considered for clinical interpretation.
Figure 1.
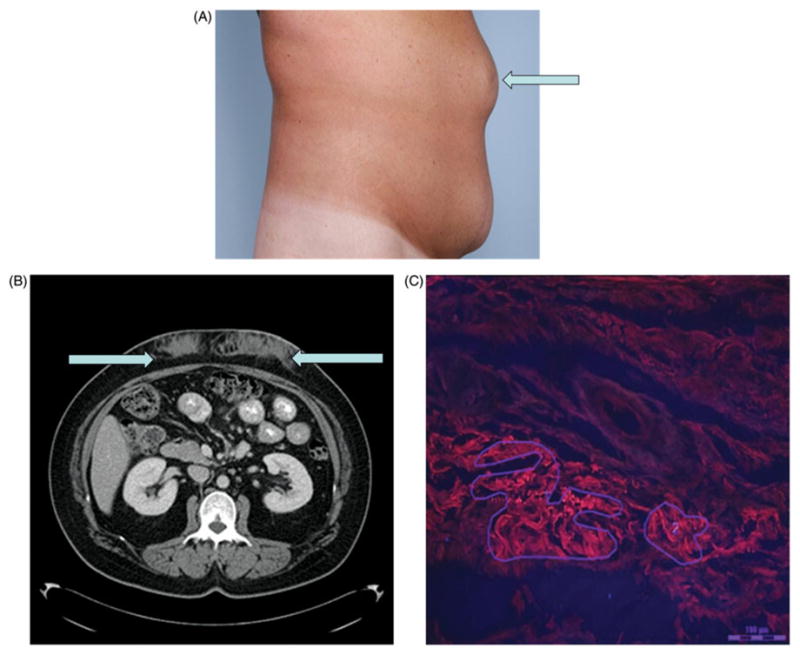
Gross and microscopic appearance of drug-induced amyloidoma. (A) The physical examination findings of insulin amyloidomas. (B) The appearance of insulin amyloidomas in the subcutaneous tissue on transverse cross-section on abdominal computed tomography. (C) Congo red stained biopsy of the abdominal nodule at the site of long-standing insulin administration under fluorescence microscopy. The bright red stained areas represent amyloid deposits and have been outlined for laser microdissection. The scale is set at 100 mm.
For each case, we created a personalized proteomic profile that lists all the confident protein identifications in each of the microdissection along with their respective MS/MS spectral counts. The number of MS/MS spectra matching to a protein is considered as a semi-quantitative measure of its abundance. The most abundant amyloidogenic protein detected across all microdissections and as interpreted in the context of the clinical history is considered to be the amyloid subtype.
Results
Insulin derived amyloid
The following is a clinical vignette of one of the patients with AIns in order to provide an insight in presentation.
A 43-year-old female with type 2 diabetes on recombinant subcutaneous insulin since age 17, noticed nodularities on her abdominal wall at sites of insulin administration. She also noticed increasing requirements of insulin over the course of several months. She had low-grade fevers, nausea and vomiting without identifiable pathology on infectious and gastroenterologic work up. Subsequently, she noticed that after changing the site of insulin administration, her insulin requirement came down by nearly 100 units. Figure 1(B) and (C) show abdominal wall nodularities and the CT scan images of these lesions, respectively. Abdominoplasty was performed to excise these nodules, and histopathologic examination revealed Congo red positive tissue. Laser microdissection and tandem mass spectrometry confirmed the amyloid tissue to have high amounts of insulin.
Table 1 shows patient information, site of biopsy and mass spectrometry results for the 50 cases of amyloidoma at the site of insulin administration. Each of the insulin cases show varying concentrations of insulin within the amyloid deposits. Naturally occurring human pro-insulin is composed of an A-chain and B-chain connected by a C-peptide. Recombinant insulin lacks the C-peptide. In our series, the amyloid deposits showed the complete presence of only the A and B chains without the C-peptide, thus further suggesting that these were a result of recombinant insulin deposition.
Table 1.
Patient details and predominant amyloid constituents of pharmaceutical amyloidosis detected by tandem mass spectrometry.
| Patient | Age/Sex | Site of amyloidoma biopsy | Insulin | Enfuvirtide | Apo AI | Apo AIV | Apo E | SAP |
|---|---|---|---|---|---|---|---|---|
| 1 | 44/F | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 2 | 92/M | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 3 | ?/F | Abdomen | + | NA | +++ | +++ | +++ | − |
| 4 | 36/M | Inguinal lymph node | + | NA | +/− | +++ | +++ | +++ |
| 5 | 68/F | Arm | + | NA | +++ | +++ | +++ | ++ |
| 6 | 42/F | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 7 | 57/M | Abdomen | ++ | NA | ++ | +++ | +++ | + |
| 8 | 49/F | Arm | + | NA | +++ | +++ | +++ | +++ |
| 9 | 55/F | Abdomen | + | NA | − | +++ | +++ | − |
| 10 | 31/M | Arm | + | NA | +++ | +++ | +++ | ++ |
| 11 | 81/M | Thigh | ++ | NA | +++ | +++ | +++ | ++ |
| 12 | 60/M | Abdomen | + | NA | +++ | +++ | +++ | + |
| 13 | 55/F | Abdomen | + | NA | +++ | +++ | +++ | ++ |
| 14 | 66/M | Abdomen | ++ | NA | +++ | +++ | +++ | +++ |
| 15 | 60/M | Thigh | ++ | NA | +++ | +++ | +++ | + |
| 16 | 48/M | Abdomen | + | NA | +++ | +++ | +++ | +/− |
| 17 | 71/M | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 18 | 71/F | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 19 | 64/F | Abdomen | + | NA | +++ | +++ | +++ | ++ |
| 20 | 71/M | Thigh | + | NA | +++ | +++ | +++ | + |
| 21 | 54/M | Abdomen | + | NA | + | +++ | +++ | +++ |
| 22 | 57/M | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 23 | 54/M | Abdomen | + | NA | +++ | +++ | +++ | + |
| 24 | 30/F | Arm | ++ | NA | +++ | +++ | +++ | + |
| 25 | 56/M | Thigh | + | NA | ++ | +++ | +++ | + |
| 26 | 47/F | Abdomen | + | NA | +++ | +++ | +++ | + |
| 27 | 53/M | Abdomen | + | NA | +++ | +++ | +++ | + |
| 28 | 55/M | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 29 | 81/M | Abdomen | +++ | NA | +++ | +++ | +++ | + |
| 30 | 30/M | Thigh | + | NA | +++ | +++ | ++ | ++ |
| 31 | 48/M | Thigh | + | NA | + | +++ | +++ | ++ |
| 32 | 34/M | Thigh | + | NA | +++ | +++ | +++ | + |
| 33 | 76/F | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 34 | 46/M | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 35 | 44/F | Abdomen | + | NA | + | ++ | +++ | + |
| 36 | 62/M | Abdomen | ++ | NA | + | +++ | +++ | + |
| 37 | ?/F | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 38 | 66/F | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 39 | 66/M | Arm | + | NA | +++ | +++ | +++ | +/− |
| 40 | 54/M | Abdomen | + | NA | ++ | + | ++ | +/− |
| 41 | 67/M | Thigh | ++ | NA | ++ | +++ | +++ | + |
| 42 | 62/M | Abdomen | + | NA | +++ | +++ | +++ | + |
| 43 | 73/M | Arm | + | NA | ++ | +++ | +++ | ++ |
| 44 | 80/M | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 45 | 75/F | Thigh | + | NA | +++ | +++ | +++ | ++ |
| 46 | 74/M | Abdomen | ++ | NA | +++ | +++ | +++ | ++ |
| 47 | 82/M | Thigh | + | NA | + | +++ | +++ | ++ |
| 48 | 66/M | Abdomen | ++ | NA | +++ | +++ | +++ | − |
| 49 | 61/F | Abdomen | ++ | NA | ++ | +++ | +++ | + |
| 50 | 46/M | Abdomen | ++ | NA | +++ | +++ | +++ | + |
| 51 | 47/M | Arm | NA | +++ | +++ | +++ | ++ | +++ |
| 52 | 55/F | Abdomen | NA | +++ | +++ | +++ | +++ | +++ |
? – age not available. Columns 4–9 show the amyloid constituents detected on LMD-MS/MS. Number of + signs indicate normalized spectral abundance of protein/peptide in the amyloid. −: not detected, +/−: 1–3, +: 4–10, ++: 11–20, +++: >21, NA – not applicable.
Enfuvirtide derived amyloid
Two patients with skin reactions at the sites of enfuvirtide administration had biopsies that showed Congo red positivity suggestive of amyloidosis. These were seen in consultation in our laboratory for mass spectrometry analysis. Table 1 shows the patient characteristics, site of biopsy and mass spectrometry results in these patients, and as in the insulin amyloid deposits, we show that the amyloid deposits are comprised of the drug itself along with apolipoproteins A-I, A-IV and E and serum amyloid P-component (SAP).
Upon analyzing the representative sequence (peptide) coverage of the protein detected in the microdissected samples, the 50 AIns cases showed insulin sequence coverage at 99–100% probability in 43 cases, and 92–94% probability in 6 cases. Both the enfuvirtide cases showed sequence coverage for Fuzeon® at 100% coverage.
Discussion
Drug-induced amyloidosis, a form of iatrogenic amyloidosis, can occur from subcutaneous injection of protein and peptide drugs. To our knowledge, insulin and enfuvirtide are the only two drugs that have been reported to cause iatrogenic amyloidosis.
Localized AIns was first described in an animal model and a human subject in 1983 [2] where in granulomas were detected at the site of insulin administration showing large clumps of amyloid. Electron microscopy confirmed the fibrillary structure, and immunohistochemical analysis with fluorescein-labeled anti-porcine-insulin antiserum suggested the presence of insulin in these deposits [2]. The exact pathogenesis of AIns is not entirely clear. The insulin protein has been extensively studied in the laboratory as an amyloid protein model for the study of the amyloidogenic process. Amdursky et al. [8] show that insulin can self-assemble to form a crystalline nucleus, which then after a lag time can then assemble into fibrils, central to the amyloidogenic process. These processes are accelerated by an acidic environment and higher concentration of insulin [9]. Further, it is felt that the C-peptide interferes with the insulin fibril formation. Landreh et al have shown that the C-peptide forms a stabilizing complex with the unfolded monomer, and may prevent fibril formation [9].
Additionally, our results show the consistent presence of apolipoproteins A-I, A-IV, E as well as SAP in most if not all the patients in our series. These have been described in different amyloid studies, including AL amyloidosis, senile and hereditary amyloidosis [10–12]. Further, depositions of these apolipoproteins and SAP have also been described in other degenerative processes such as calcific aortic sclerosis [13] and atherosclerosis [14,15]. Bergstrom et al suggest that apolipoproteins A-I, A-IV and E have similar molecular structures, and are intrinsically flexible molecules, and thus may be unusually prone to form amyloid fibrils [10].
Insulin-derived amyloidosis (AIns) has been described in less than 20 cases in medical literature, and reviewed by Yumlu et al. [3]. However, this condition is likely far more prevalent than what is reported in the medical literature given the number of insulin users in the general population. Our series which is the largest series of AIns adds to the existing literature on this condition. AIns tends to be localized to sites of insulin injection, although in one of our patients (patient 4, Table 1), it was found in the inguinal lymph node where insulin injections had been administered into the thigh. This patient went on to have a complete evaluation to look for systemic amyloidosis and was confirmed to not have any other organ involvement. None of the patients in our series had evidence of other organ involvement by amyloid or a specific amyloid syndrome. Patients with AIns may present to the clinician as a direct result of noticing the nodular deposits or symptoms thereof. They may also have associated brittle diabetes as a result of unpredictable release of insulin from these nodules [16,17]. Additionally, inhaled insulin has also been shown to result in amyloid fibril formation in mice lungs with resultant pulmonary toxicity [18]. Treatment for AIns involves surgical excision of amyloidomas, or avoiding the sites of amyloidosis to administer insulin [3]. Further, there has been suggestion that co-administration of C-peptide along with insulin may prevent the formation of amyloid fibrils [9]. Finally, quercetin, a naturally occurring polyphenol has been shown to inhibit the formation of amyloid fibrils as well as destabilization of preformed amyloid fibrils in a bovine insulin model [19].
Enfuvirtide is an HIV fusion inhibiting peptide that is administered subcutaneously. It is known to be associated with a variety of skin reactions [20]. In both of the cases of enfuvirtide-induced amyloidosis which have been individually published as case reports [4,21] we show that the amyloid structure is composed of the enfuvirtide peptide itself. The extensive deposition of the drug proves that it is causative in the amyloidogenesis although the exact pathogenesis of conversion of the enfuvirtide peptide into fibrillar aggregates of amyloid is not clear. Similar to AIns, other amyloid precursor proteins found in the deposits included SAP protein and the apolipoproteins E, A-I and A-IV.
Several patients with pharmaceutical amyloidosis presented with bleeding at the sites of deposits, and this is of importance when interpreting the mass spectrometry results as some proteinaceous components may be remnants of plasma proteins. Because almost all of our cases in this series were seen as consults for amyloid subtyping from other institution, our study is limited by the paucity of clinical data, especially in regards to duration of a site usage for injections, type of insulin formulation used and other user-specific habits that may have given an insight into risk factors associated with the occurrence of drug amyloid.
Conclusions
In conclusion, we highlight two pharmaceutical agents that can result in iatrogenic amyloidosis. These are associated with the direct deposition of the protein or peptide drug, along with other known amyloidogenic or chaperone proteins. The diagnosis of drug-induced amyloidosis must be entertained in patients who develop skin and subcutaneous reactions/masses to drugs, especially those of protein or peptide chemistry. Proteomic analysis of the amyloid deposits can be a very useful adjunct in making an accurate diagnosis, as well as in elucidating the composition of amyloid.
Abbreviations
- AIns
insulin-derived amyloid
- LMD-MS/MS
laser microdissection with tandem mass spectrometry
Footnotes
Declaration of interest
The authors have no conflicts of interests to disclose.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–70. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 2.Storkel S, Schneider HM, Muntefering H, Kashiwagi S. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108–11. [PubMed] [Google Scholar]
- 3.Yumlu S, Barany R, Eriksson M, Rocken C. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655–60. doi: 10.1016/j.humpath.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Morilla ME, Kocher J, Harmaty M. Localized amyloidosis at the site of enfuvirtide injection. Ann Intern Med. 2009;151:515–6. doi: 10.7326/0003-4819-151-7-200910060-00017. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza A, Theis JD, Vrana JA, Buadi F, Dispenzieri A, Dogan A. Localized insulin-derived amyloidosis: a potential pitfall in the diagnosis of systemic amyloidosis by fat aspirate. Am J Hematol. 2012;87:E131–2. doi: 10.1002/ajh.23334. [DOI] [PubMed] [Google Scholar]
- 6.Lavatelli F, Vrana JA. Proteomic typing of amyloid deposits in systemic amyloidoses. Amyloid. 2011;18:177–82. doi: 10.3109/13506129.2011.630762. [DOI] [PubMed] [Google Scholar]
- 7.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 8.Amdursky N, Gazit E, Rosenman G. Formation of low-dimensional crystalline nucleus region during insulin amyloidogenesis process. Biochem Biophys Res Commun. 2012;419:232–7. doi: 10.1016/j.bbrc.2012.01.153. [DOI] [PubMed] [Google Scholar]
- 9.Landreh M, Stukenborg JB, Willander H, Soder O, Johansson J, Jornvall H. Proinsulin C-peptide interferes with insulin fibril formation. Biochem Biophys Res Commun. 2012;418:489–93. doi: 10.1016/j.bbrc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Bergstrom J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, Westermark P. Two different types of amyloid deposits–apolipoprotein A-IV and transthyretin–in a patient with systemic amyloidosis. Lab Invest. 2004;84:981–8. doi: 10.1038/labinvest.3700124. [DOI] [PubMed] [Google Scholar]
- 11.Klein CJ, Vrana JA, Theis JD, Dyck PJ, Dyck PJ, Spinner RJ, Mauermann ML, et al. Mass spectrometric-based proteomic analysis of amyloid neuropathy type in nerve tissue. Arch Neurol. 2011;68:195–9. doi: 10.1001/archneurol.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden AC, Aubry MC, Zhang K, Brady JO, Levin D, Dogan A, Yi ES. Nodular senile pulmonary amyloidosis: a unique case confirmed by immunohistochemistry, mass spectrometry, and genetic study. Hum Pathol. 2010;41:1040–5. doi: 10.1016/j.humpath.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Rojas T, Gil-Dones F, Lopez-Almodovar LF, Padial LR, Vivanco F, Barderas MG. Proteomic profile of human aortic stenosis: insights into the degenerative process. J Proteome Res. 2012;11:1537–50. doi: 10.1021/pr2005692. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CR, Haw A, 3rd, Lopez R, McDonald TO, Callaghan JM, McConville MJ, Moore KJ, et al. Serum amyloid P colocalizes with apolipoproteins in human atheroma: functional implications. J Lipid Res. 2007;48:2162–71. doi: 10.1194/jlr.M700098-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Teoh CL, Griffin MD, Howlett GJ. Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein Cell. 2011;2:116–27. doi: 10.1007/s13238-011-1013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374–6. doi: 10.1016/j.diabres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Shikama Y, Kitazawa J, Yagihashi N, Uehara O, Murata Y, Yajima N, Wada R, et al. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397–401. doi: 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]
- 18.Lasagna-Reeves CA, Clos AL, Midoro-Hiriuti T, Goldblum RM, Jackson GR, Kayed R. Inhaled insulin forms toxic pulmonary amyloid aggregates. Endocrinology. 2010;151:471724. doi: 10.1210/en.2010-0457. [DOI] [PubMed] [Google Scholar]
- 19.Wang JB, Wang YM, Zeng CM. Quercetin inhibits amyloid fibrillation of bovine insulin and destabilizes preformed fibrils. Biochem Biophys Res Commun. 2011;415:675–9. doi: 10.1016/j.bbrc.2011.10.135. [DOI] [PubMed] [Google Scholar]
- 20.Wallace BJ, Tan KB, Pett SL, Cooper DA, Kossard S, Whitfeld MJ. Enfuvirtide injection site reactions: a clinical and histopathological appraisal. Australas J Dermatol. 2011;52:19–26. doi: 10.1111/j.1440-0960.2010.00717.x. [DOI] [PubMed] [Google Scholar]
- 21.Naujokas A, Vidal CI, Mercer SE, Harp J, Kurtin PJ, Fox LP, Thompson MM. A novel form of amyloid deposited at the site of enfuvirtide injection. J Cutan Pathol. 2012;39:220–1. doi: 10.1111/j.1600-0560.2012.01865_2.x. [DOI] [PubMed] [Google Scholar]


