1. The prolonged human developmental period1
Human brain development is uniquely slow, and there must be a reason for this pace. Several theorists, including those listed below, have previously discussed this topic at great lengths (e.g., Bogin, 1997; Konner, 2011; Thompson & Nelson, 2011), and I only briefly present some of those established and influential ideas here in order to turn the focus on the importance of early experiences on human brain development. Despite comparable brain size at birth between humans and Neanderthals, our closest extinct relatives, the rate of growth from the neonate toward the adult state was much more rapid in Neanderthal’s than in the human (Ponce de Leon, et al., 2008). That evolutionary pressures have resulted in a different chronological phenotype, that is, a slower rate of development in humans, has profound implications for the adult phenotype. Examinations of the slower brain development in humans have focused on the function of a slower pace and suggested that a slower pace of development conferred greater benefits to the species as an adult (Charvet & Finlay, 2012). Specifically, it has been postulated that the duration of development across species covaries with the ability of the species to adapt in variable social and ecological environments across the life span. In line with this reasoning, humans, who have the most complex and flexible behavioral repertoires, have extremely slow brain development (reviewed in Neubauer, Gunz, Schwarz, Hublin, & Boesch, 2012). Examination of the fossil dental record (molar eruption timing is often used as a marker for developmental stages) shows that the temporal pacing of tooth development was much more rapid in Neanderthals than modern humans (Smith, Toussaint, Reid, Olejniczak, & Hublin, 2007), and comparative studies with modern apes show that a slow and prolonged development in general may be uniquely human (see Figure 1) (Thompson & Nelson, 2011), supporting the notion that a prolonged childhood is adaptive to the species (Konner, 2011)
Figure 1.
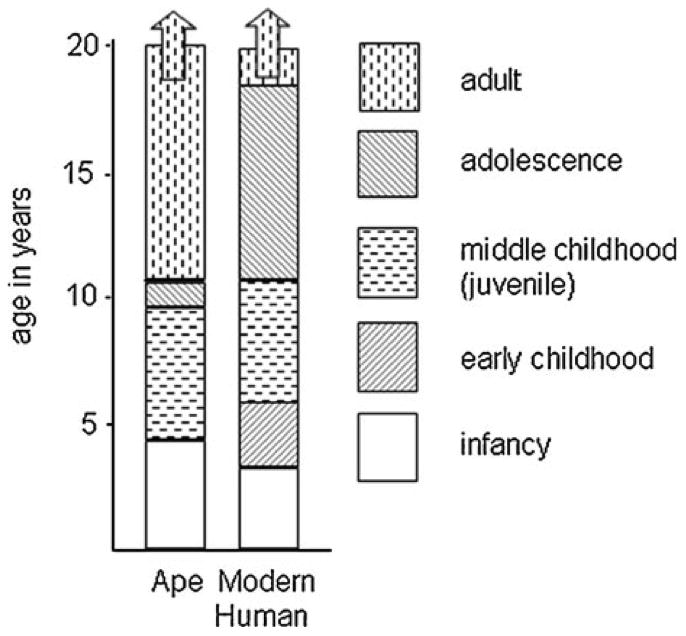
Proportional increases in length of immature states in humans relative to apes. (Copied from Thompson & Nelson, 2011).
Given the survival advantage that the grown adult state would have over an immature state, one might wonder as to what the evolutionary advantage of a prolonged childhood would be. To address this question, it is first worth considering the environmental conditions under which the human species first evolved. Original theories describe environmental conditions under which humans emerged as a harsh one that might have put pressure on the species to evolve intelligent means of surviving the extreme climate. However, recent evidence has shown instead that human evolution coincided with rapid and repeated ecosystem restructuring induced by fluctuating aridity (Magill, Ashley, & Freeman, 2013) - that is, a highly variable climate. From this one might surmise that humans evolved not to be best adapted for extreme environments, but rather for variables ones, perhaps explaining humans’ great potential for adaptation. With this notion in mind, it may make sense that the human species should be capable of adjusting to the environment that is relevant to his/her immediate existence (and not necessarily so to their past relatives). Being optimized to one’s unique environment would require an idiopathic developmental trajectory tuned to the signals present in the immediate environment; that is, it would require learning. Perhaps for this reason the human brain is a highly plastic organ, ready to adapt to environmental demands. Given the complexity of humans’ environments, the longer the learning period, the better attuned the individual should be to his/her environment. It is within this functional context that a long infancy and childhood makes sense in that it allows for greater learning about one’s own environment for optimal performance during adulthood.
This ontogenetic pattern has been demonstrated by empirical work. By longitudinally studying brain development in the same individuals over time from early childhood through adulthood, Shaw and colleagues (2006) have been able to create developmental trajectory “phenotypes” of the thickness of the cerebral cortex of individuals. Whereas cortical thickness itself in adulthood was not associated with intellectual ability, the path that one took to reach adult levels of thickness was. In general, cortical thickness declined as age increased, with a peak in thickness occurring some time during development. Those individuals who had the latest occurring maturation peaks were also the individuals with the highest intellectual ability. In fact, these superior intellectual ability individuals had the most immature (i.e., thinnest) cortices during childhood relative to the other groups. These data support the notion that exhibiting an immature pattern of brain function for a longer period confers adaptive intellectual advantage to the individual.
The experiences that one has during development may serve as strong cues to the system of how his/her world operates, and these cues may exert large influence over the construction of the system. If the environment is enriched and favorable, neurobiology will optimize for growth and advancement. If the environment is harsh, then neurobiology will optimize for thrift and adversity. At the end of this chapter, I will return to this point in discussing an example of early adversity. It is perhaps for this reason that experiences that are incurred early in life exert long-lasting influences over brain development – because they are instructions on how best to respond to the particular environment. Moreover, neural systems tend to be more plastic earlier in development (Lupien, McEwen, Gunnar, & Heim, 2009), with stability and increased resistance to environmental restructuring with increasing age. It has been posited that while a highly plastic brain is beneficial for the developing organism, ongoing plasticity is a disadvantage as described by a plasticity/efficiency trade-off (Lebel, et al., 2012). A highly plastic brain is, by the virtue of its changeability, not very efficient. Once a system settles on its most efficient routes, plasticity diminishes and is replaced by relative stability and efficiency of the system. Therefore, studying early experiences may be particularly useful in understanding the function of the adult brain.
2. Sensitive periods
Neural immaturity during infancy and childhood may be an optimal phenotype under most conditions because neural immaturity confers greater plasticity of the system. Neural plasticity is “an intrinsic property of the human brain” and is the ability of neurobiology to be altered by experiences (Pascual-Leone, Amedi, Fregni, & Merabet, 2005). Since learning occurs throughout the lifetime (Cajal, 1904), neural plasticity is not an “occasional state of the nervous system” (Pascual-Leone, et al., 2005). However, there may be period of time when neural circuits are particularly sensitive to environmental pressures, and these periods are known as sensitive periods.
Sensitive periods are important when considering the influence of the environment because environmental input “that occurs during sensitive periods lays the foundation for future learning” (Knudsen, 2004). The reason why later behavior occurs in the manner it does is because the environment is operating at the level of altering biology. Sensitive periods of any neural system emerge during times of significant organization or reorganization (Lupien, et al., 2009). Because of this fundamental aspect of neural development, sensitive periods are properties of all neural systems, and provide moments of increased plasticity when environmental stimuli can exert large influences. The term “critical period,” which is often used in the literature, refers to a very specific type of sensitive period that results in irreversible change in brain function (Knudsen, 2004). There are many theories of what constitutes a sensitive period. According to Knudsen (2004), there are three prerequisites to a sensitive period. Information from the environment must be sufficiently reliable to exert an effect, the receiving circuit must be adequately connected to process the information, and mechanisms that allow for neuronal change must be in place. Then axon elaboration and synapse formation occur following Hebbian principles, which is followed by synapse and axonal pruning of non-activated routes. The surviving synapses are stabilized via structural modifications.
At the molecular level, sensitive periods have been described as a “sequence of molecular events” (Morishita & Hensch, 2008). Within this framework, a sensitive period begins when there is a large shift in the excitatory and inhibitory balance of a system, which reflects the late ontogenetic development of inhibitory inputs (e.g., GABAergic) relative to excitatory ones (Hensch, et al., 1998). This newly achieved excitatory and inhibitory balance constitutes the sensitive period, a time during which environmental input can exert great change in the system. This heightened period of plasticity ceases (i.e., the sensitive period window closes) with the development of new structural additions (e.g., myelination). It has been shown that genetic knockouts (e.g., Nogo receptor knockout), which result in low myelin integrity, or pharmacologic manipulations (e.g., valproic acid), which can re-introduce a shift in the excitatory/inhibitory balance in adulthood, can both independently operate to re-open a sensitive period of a neural system (Yang, Lin, & Hensch, 2012). Although under natural conditions, mechanisms exist to maintain stability in the system after the sensitive period, experimentally increasing excitatory activation (e.g., electrical) or administration of growth hormones (e.g., brain derived neurotrophic factor) have been shown to shift the excitatory/inhibitory balance and essentially re-open sensitive periods long after they have ended (Huberman & McAllister, 2002; Kilgard & Merzenich, 1998).
3. Early experiences
Although sensitive periods are conceptually a highly useful concept when considering human brain development, they are typically challenging to empirically identify, particularly in the human. In part, the challenge arises from the nature of normative human experiences. In general, it is challenging to empirically control the environments of humans or to even know precisely when events were experienced. Some domains lend themselves more readily to the study of sensitive periods than others. Perceptual and language development in particular have made significant progress in terms of identifying periods in early life when learning systems exhibit the moments of greatest plasticity. Development of vision, in particular the development of binocular depth perception, has been the most well-characterized process (e.g., Hubel & Wiesel, 1970). In humans, as in non-human animals, early-life represents a sensitive period for binocular light experience. Without this regular exposure during childhood, ocular dominance columns fail to establish balanced and organized neural structure and consequently, binocular perception fails to develop (Holmes & Clarke, 2006). Monocular deprivation may naturally occur in some individuals born with conditions like strabismus, a condition where the eyes fail to properly align with one another. Without early intervention, strabismus irreversibly results in amblyopia where the visual cortex ceases to respond to input from the weaker eye, a process resistant to later behavioral intervention. However, with an understanding of this timing, clinical practice has demonstrated that intervention at young ages (depriving the weaker during the sensitive period) can right the input and prevent amblyopia (Holmes & Clarke, 2006; von Noorden & Crawford, 1979). Although this process is typically irreversible in adulthood, animal research has shown that, as predicted by sensitive period models, artificially increasing levels neurotropic factors can re-open the sensitive period and allow for the intervening effects of “patching” in adulthood (reviewed in Bavelier, Levi, Li, Dan, & Hensch; Maya Vetencourt, et al., 2008)
The domain of language development has similarly been successful in identifying sensitive periods for environmental input. As adults, we are proficient in identifying and discriminating sounds (i.e., phonemes) from our native language. However, this behavior is the result of a developmental process involving precisely timed linguistic input during a sensitive period in infancy. During infancy, the auditory system must be exposed to the phonemic sounds that will be used in adulthood if one is to process those sounds with native expertise (Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992; Werker, Gilbert, Humphrey, & Tees, 1981). After this infant period (which can be as brief as the first six months of postnatal life), the auditory systems begin tuning perceptual systems towards the native language and away from other sound systems. It is for this reason that it is very difficult for a native Japanese speaker to perceptually discriminate /la/ from /ra/. At more complex levels of language processing (e.g., learning a second language), research has shown that a second language must be learned within the first decade of life in order for the speaker to be perceived as fluent (Johnson & Newport, 1989). This second example comprises a very wide age range (i.e., 10 years), which is a very liberal sensitive period. It might be that the more complex the input, and therefore the behavioral output, the longer and more forgiving the sensitive period is.
Both of these examples are useful in demonstrating the principle that the brain optimizes development towards the information it receives from early inputs. These are principles evident in classic human developmental theories. Jean Piaget (1954) described cognitive development as a hierarchical process of adaptation and organization as the system learns about and is shaped by the environment of the child. Through this process, the adult should be highly adapted to his/her unique environment. More recently the Predictive-Adaptive-Response model (Gluckman, Hanson, & Spencer, 2005; Nettle, Frankenhuis, & Rickard, 2013) has posited that developmental outcomes are optimized when later environments are concordant with very early environments, even when those environments are unfavorable. This hypothesis has been supported in the domains of maternal mental health (Sandman, Davis, & Glynn, 2012) and prenatal nutrition (Plagemann, 2006). For example, the odds for obesity-related outcomes are greater for an undernourished fetus in the long term because the system had taken cues from the early environment and optimized its biology to develop a “thrifty” phenotype with the expectation of an also undernourished postnatal environment. When the postnatal environment is unexpectedly abundant with nourishment and the system was, in essence, designed by the prenatal environment to store as much of the energy intake as possible, there is an increased risk of obesity-related illness. Presumably under most conditions in human history, environments within one individual’s lifetime would have been highly correlated over time (the prenatal environment was probably a good indicator of the postnatal one), and unlikely to change dramatically. Therefore, the impact of early environments can be large because odds are they are highly predictive of the environment to come.
The more complex a given behavior is, the ability to identify relevant sensitive periods, and for that matter relevant inputs, becomes increasingly more challenging. For example, the identification of human sensitive periods for cognitive, social and affective processes has been less fruitful than in perceptual and language systems. However, animal models have delineated some sensitive periods that may translate to human emotional processes. I will discuss work that has focused on the development of the amygdala-prefrontal cortex (PFC) circuit as an illustrative case. Rat models of early emotional development have demonstrated that the infant state operates in a fundamentally different manner than the adult animal, and as will be discussed, this difference is the result of sensitive periods for amygdala-PFC development. Events that occur during these sensitive periods have long term consequences on adolescent and adult behavior.
4. The Development of Affective Behaviors (Rodent Models)
Amygdala-based learning
The amygdala is a subcortical structure involved in learning about the emotional significance of stimuli (Davis & Whalen, 2001). It is phylogenetically conserved across species, including humans, showing functional responsivity to emotional stimuli across childhood, adolescence, and adulthood (e.g., Baird, Gruber, et al., 1999; Gee et al., 2013; Guyer, Monk, et al., 2008; Hare et al., 2008; Lobaugh, Gibson, et al., 2006; Killgore, Oki, et al., 2001; Monk, McClure, et al., 2003; Pine, 2007; Thomas, Drevets et al., 2001). In adulthood, the amygdala mediates the associative pairing of an initially neutral conditioned stimulus (e.g., tone) with an aversive unconditioned stimulus (e.g., shock) (LeDoux, 2000). In rodent studies, lesioning the amygdala or infusion of a protein synthesis inhibitor (e.g., muscimol) prior to training obliterates this learning (reviewed in Maren, 2001). Once the pairing has been learned, the adult animal shows fear behaviors (e.g., freezing or avoiding) towards the conditioned stimulus. During infancy, the same pairing will result in a seemingly paradoxical preference for the conditioned stimulus (Camp & Rudy, 1988; Moriceau & Sullivan, 2006). The infant will also form a preference for the conditioned stimulus had it been paired with a pleasurable brush stroke (Raineki, Moriceau, & Sullivan, 2010). This ambivalence during the infant period suggests it will form a preference for either pairing. It has been suggested that the preference the infant displays reflects a bias the infant has to approach any learned stimulus. This bias is to the great benefit of any altricial infant, that is an infant who must form an attachment to its caregiver, regardless of the quality of care, to ensure survival. In the case of the rodent learning paradigms, the conditioned stimulus has become, in essence, the maternal stimulus, and the infant has attached to the stimulus. This bias has been observed in other species as well (chick, dog, human), where even in the face of maltreatment, the infant will form a preference towards the attachment figure (Kovach & Hess, 1963; Rajecki, Lamb, & Obmascher, 1978).
The neural basis for this preference lies in the developmental timing of the rodent amygdala. Despite showing early anatomical maturity, the amygdala is functionally dormant under typical conditions and despite retaining the capacity to activate, it typically does not (Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Sullivan, 2001). The amygdala remains functionally dormant because corticosteroids (CORT), which instantiate amygdala firing via dopamine early in life (Barr, et al., 2009), remain at very low levels. During this period of life, which has been called a stress hyporesponsive period, it is very difficult to observe a significant rise in CORT levels in response to a stressor (Sapolsky & Meaney, 1986), which seems to have a functional analogue in the human (Gunnar & Donzella, 2002). As a consequence of low CORT levels, the amygdala remains un-recruited during shock-related learning, leaving other learning systems (e.g., olfactory bulb, piriform cortex) to mediate learning and a preference behavior is expressed rather than an avoidance.
Part of the reason why CORT levels remain low during infancy is because the mother’s presence acts as a social buffer against elevations in CORT (Shionoya et al., 2007). Therefore, there exists a tight neuro-environmental loop, whereby the mother’s presence maintains low CORT levels, which maintains amygdala uninvolvement, which results in preference learning, which promotes attachment to the mother. This period of amygdala uninvolvement spans for the first two weeks of life; after this point, stress-induced elevations in CORT begin and amygdala activations are first observed at which point, adult-like avoidance learning emerges.
This process can be accelerated either by direct administration of CORT (Moriceau, et al., 2004; Roth & Sullivan, 2005) or premature separation from the mother (which elevates CORT) (Moriceau & Sullivan, 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007). In the context of elevated CORT, the amygdala prematurely activates during avoidance learning, and the rat pup exhibits adult-like avoidance learning. Conversely, when the animal is just past the sensitive period (approximately 12–14 postnatal (PN) days old; aka a transitional sensitive period) and normally exhibiting adult-like avoidance learning, placement of the mother back in the nest with the animal will result in infant-like preference learning (Moriceau & Sullivan, 2006; Shionoya, et al., 2007). Therefore, the mother acts as a switch for amygdala-mediated fear learning, and can move around the timing of amygdala-sensitive periods that can include accelerated transitions (see Callaghan & Richardson, 2013) into the mature state. Exposure to the mother in the nest at older ages maintains the immature state of the amygdala, whereas early maternal absence will result in an accelerated development of the amygdala and a resultant premature adult-like phenotype.
Rat pups experiencing chronic maternal separation (3 hours per day from PN2–14) also tend to show enhanced fear learning relative to controls (Callaghan & Richardson, 2011). Typically, young rats will exhibit infantile amnesia, where learned fear memories are prone to poor retention; the learned fear is forgotten within 10 days in PN17 animals. However, following chronic maternal separation, memory is near perfect in these young animals, an effect that seemed to have been mediated by high circulating CORT levels (Callaghan & Richardson, 2012b). These findings in rat pups suggest that early maternal separation has the effect of switching the state of emotional limbic regions to a more adult-like function at earlier ages.
Amygdala-mPFC based learning (Rodent Models)
Early maternal absence will result in ontogenetic changes observed later in life in amygdala-related circuits that connect to the cortex. In adulthood, a robust connection of the amygdala exists bidirectionally with the medial prefrontal cortex (mPFC), which in the rodent includes the infralimbic cortex (IL). One important function of the mPFC is to regulate the activity of the amygdala thereby resulting in a diminished fear response (reviewed in Hartley & Phelps, 2013). For example, during extinction, which is the decrease in fear responding that results when the conditioned stimulus is presented in the absence of the unconditioned stimulus, connections from mPFC to amygdala are necessary. The mPFC likely regulates through input to the basolateral nuclei of the amygdala and the intercalated cells, which inhibit amygdala activity by regulating inputs from the basolateral nuclei to the central nucleus (Akirav, Raizel, & Maroun, 2006; Harris & Westbrook, 1998; Milad & Quirk, 2002) (reviewed in Kim, et al., 2011). Just as the amygdala is sensitive to the effects of early maternal absence, so too are connections with mPFC. Under normal rearing conditions these connections are late to develop (Pattwell, Duhoux et al., 2012), and the mPFC plays no role in extinction behaviors during the juvenile period (Kim, Hamlin, & Richardson, 2009; Kim & Richardson, 2009). However, maternally-separated rat pups recruit mPFC at a much younger age during extinction and therefore function behaviorally as adults do (Callaghan & Richardson, 2011, 2012a). Taken together, these findings suggest that early maternal separation acts to not only lead to accelerations in amygdala function but also in the development of connections between amygdala and mPFC.
Callghan & Richardson (2013) have argued that these neural differences are ontogenetic adaptations that the developing system makes in order to meet the demands of an adverse environment. This begs the question of what the downside to premature development is. One possibility is that there will be a cost later in life, which may be caused by an abbreviated period of plasticity (or immaturity). Support for this hypothesis comes from mouse work that has identified a sensitive period for mPFC-based learning in the “childhood” (i.e., juvenile) phase, that is in the post-weaning/pre-pubertal animal. Exposure to a stimulus (i.e., music) during the juvenile period allowed this stimulus to act as a safety signal in adulthood (that is, presentation of the music in adulthood reduced anxiety) via activation of the mPFC (Yang, et al., 2012). Taken together, these data may indicate that accelerated developmental shifts towards the adult state (because of early life stress) would reduce learning opportunities during development that could serve the individual in adulthood. This framework is consonant with the idea that early adversity may prioritize immediate timeframes over long term ones, as has been suggested in theories that emphasize biological sensitivity to context and life-history (Belsky, Houts, & Fearon, 2010; Boyce & Ellis, 2005; Ellis, Essex, & Boyce, 2005).
5. The Development of Affective Behaviors (Humans)
Although sensitive periods in humans tend to be more challenging to identify in emotional domains, there are data supporting the claim that there are early moments in development when events can exert lasting and potent effects on future behavior. One of the more robust effects has been observed with regard to the influence of the primary caregiver. Humans belong to a class of animals that are altricial, those that are incapable of independent survival at birth. This distinction from precocial animals confers a number of developmental differences including the requirement that the altricial animal form a psychological attachment to the primary caregiver and the caregiver to the infant. Attachment theory holds that the continued presence of a caregiver during the first year of life will result in the formation of an enduring bond between caregiver and offspring (Aisnworth, 1969; Bowlby, 1977). The nature of the care provided (e.g., how sensitive the caregiving is to the child’s needs) can influence the quality of the attachment relationships and thus the emotional health of the individual.
While the quality of care has been posited to affect the quality of the attachment relationship, the quantity of care seems to influence whether an attachment forms or not. The presence or absence of a stable caregiver for a human infant can have devastating consequences for development. Because the effects of caregiver absence can be so large, it might stand to reason that caregiver presence is a “species-expected” stimulus, expected because caregiver presence was almost ensured for every individual during our species’ evolution (Greenough, Black, & Wallace, 1987), and therefore infants would have co-evolved with the expectation of a caregiver. Indeed, caregiver presence (regardless of quality of care) is nearly ubiquitous for human infants. Therefore, for the human infant, a caregiver may be considered a species expected environmental stimulus (discussed in Tottenham, 2012a).
However, there are rare instances of infants who develop in the absence of stable caregiver presence. Infants and children raised in institutional care (e.g., orphanages) have the unusual experience of caregiver deprivation. Institutional care, even the best of circumstances, provides suboptimal caregiving (although it is often a better alternative to other options) because the care is not consistent (multiple staff members, rotating shifts) and is provided by care workers who are responsible for several infants and children at once (Gunnar, Bruce, & Grotevant, 2000; Taneja, et al., 2002; Tirella, et al., 2008; The St. Petersburg-USA Orphanage Research Team, 2008). For children that are adopted out of institutional care by families, there is a wide range of outcomes, where some children struggle more than others and some phenotypes are impacted more than others (discussed in Tottenham, 2012b). Nonetheless, at the group level, previously institutionalized (PI) youth are at elevated risk for mental health difficulties (Bos, et al., 2011; M. R. Gunnar, et al., 2000; Gunnar & van Dulmen, 2007; Nelson, Furtado, Fox, & Zeanah, 2009; Rutter, 1998; Rutter & O’Connor, 2004; Tottenham, 2012b; Zeanah, et al., 2009). Emotion-related difficulties are common following early caregiver deprivation, and research has examined the nature and timing of these difficulties.
Many PI youths are adopted after their first birthday, meaning that they would not have the experience of a stable caregiver’s presence during what the attachment theory literature would identify as a potential sensitive period for attachment learning (Bowlby, 1977). In support of the hypothesis that this early period represents a sensitive period for attachment relationships, many PI child exhibit attachment related difficulties. Following adoption, PI children eventually and typically form attachments to their adoptive parents, but the quality of the attachment is often insecure (Chisholm, 1998; van den Dries, Juffer, van Ijzendoorn, & Bakermans-Kranenburg, 2010).
Although children can form attachments to their adoptive parents, many children may continue to exhibit a behavior that has been called “indiscriminate friendliness,” a fairly enduring behavior that includes reduced reticence and atypical approach behaviors toward all adults, including friendly strangers (Bruce, Tarullo, & Gunnar, 2009; Chisholm, 1998; Gleason, et al.; Hodges & Tizard, 1989). It has been argued that this term is a misnomer as the behavior is not true friendliness, but rather it is “superficial, impersonal, and rarely reciprocal” (Gunnar, et al., 2000). Examination of neurobiological correlates of indiscriminate friendliness has shown that an atypical amygdala response may underlie some of the behaviors associated with indiscriminate friendliness. Youths who grew up with stable caregiving (i.e., their biological parent) showed an amygdala response that significantly discriminated between photos of their mothers versus the photos of another youth’s mother (i.e., a stranger) (Tottenham, Shapiro, Telzer, & Humphreys, 2012). However, PI youths exhibited on average greater indiscriminate friendliness behaviors (despite showing no group differences in attachment to parents), which was more pronounced the later a youth was adopted. They also exhibited indiscriminate amygdala reactivity to photos of both their (adoptive) mothers and to strangers (Olsavsky, et al., 2013). PI youth with lower indiscriminate friendliness scores showed more typical amygdala discriminations between mother and stranger. These behavioral and neural data support the hypothesis that early postnatal life is a sensitive period for learning about the affective specialness of primary caregivers.
Human Amygdala following Early Caregiver Deprivation
The study described above examining amygdala responses highlight the amygdala as a target for early adverse experiences (Teicher, Anderson et al., 2002; Teicher, Anderson et al., 2003), a finding that has been repeatedly observed in non-human animal studies (reviewed in Callaghan & Richardson, 2013; Davidson & McEwen, 2012; Landers & Sullivan, 2013; Pechtel & Pizzagalli, 2011; Tottenham & Sheridan, 2010). Although there are several neural circuits affected by early-life adversity, the amygdala is a suitable choice for discussion because it develops early in postnatal life (Gilmore, et al., 2012; Humphrey, 1968; Ulfig, Setzer, & Bohl, 2003) mediates many of the emotional difficulties exhibited by those who experienced early-life adversity (e.g., hyper-emotionality), and because it is rich with stress hormone receptors particularly in early postnatal life (Avishai-Eliner, Yi, & Baram, 1996; Baram & Hatalski, 1998; Fenoglio, Brunson, Avishai-Eliner, Chen, & Baram, 2004; Moriceau, et al., 2004; Vazquez, et al., 2006). Youths who have experienced early caregiver deprivation tend to be highly anxious (Casey, et al., 2009; Goff, et al., 2012; Tottenham, et al., 2010; Zeanah, et al., 2009), as has been found in other species that have experienced maternal deprivation (Berman, Rasmussen, & Suomi, 1994; Botero, Macdonald, & Miller, 2013; Caldji, et al., 1998; Callaghan & Richardson, 2013; Macri, Laviola et al., 2010; Sabatini, et al., 2007). Youths with a history of early neglect have exhibited enlarged amygdala volumes in samples that have included PI youths (Mehta, et al., 2009; Tottenham, et al., 2010; but see Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012) and youths whose mothers with post-partum depression (and presumably would have provided little direct caregiving)(Lupien, et al., 2011). PI youths have also been shown to exhibit amygdala hyperactivity to highly arousing stimuli (Gee, et al., in press; Tottenham, et al., 2011). These studies finding atypical amygdala responses mirror those found in rodent studies of adverse early caregiving (Raineki, Cortes, Belnoue, & Sullivan, 2012). Non-human primate studies suggest that these changes may be mediated by increases in stress hormones (CRH) in the amygdala following early deprivation (Kanitz, Tuchscherer, Puppe, Tuchscherer, & Stabenow, 2004).
As described earlier, rodent studies have found that maternal absence may accelerate amygdala functional development (Callaghan & Richardson, 2011, 2013; Moriceau, et al., 2004; Moriceau, Roth, & Sullivan, 2010). It is not possible to interpret, based on the data described in humans above, whether humans also experience amygdala accelerations in functional development (discussed in Gee, et al., in press). One reason we cannot make this claim in humans is because the youngest age tested (i.e., 6 years old)(Gee, et al., in press) is probably too late to identify a developmental switch from a quiescent amygdala state to a functional one. The normative switch probably predates the ages tested; to begin to answer this question, preschool age children or younger would need to be scanned to identify the age at which the amygdala becomes functionally active as has been done in rodents (Upton & Sullivan, 2010; Wiedenmayer & Barr, 2001).
Human Amygdala-mPFC Connections
As described earlier, strong structural (Kim & Whalen, 2009) and functional connections (Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Hare, et al., 2008; Kim, et al., 2004; Pezawas, et al., 2005; Roy, et al., 2009) exist between the amygdala and the mPFC in adulthood, which have been interpreted to support regulatory function of the mPFC over the amygdala (reviewed in Hartley & Phelps, 2013; reviewed in Ochsner, Silvers, & Buhle, 2012; Delgado, Olsson, & Phelps, 2006; Phelps, Delgado, Nearing, & LeDoux, 2004). In rodents, it has been shown that these connections are late to develop (showing continued growth through early adulthood)(Cunningham, Bhattacharyya, & Benes, 2002) and functional immaturity through the juvenile period (Kim & Richardson, 2009)). Similar findings have been observed in human development. Childhood and adolescence is a period of large change in fronto-amygdala phenotypes (Gee, et al., 2013; Perlman & Pelphrey, 2011), with amygdala-mPFC connectivity being markedly immature during childhood (Gee, et al., 2013). Specifically, the nature of the connectivity has been shown to exhibit a qualitatively different pattern during childhood (under age 10 years old) than during adolescence and adulthood (Gee, et al., 2013). Unlike adolescents and adults who exhibited inverse connectivity (i.e., negative correlations in functional responses such that when activity in the mPFC increased, activity in the amygdala decreased) in response to emotional stimuli, children exhibited positive connectivity (such that amygdala and mPFC activity activated with the same temporal course). This connectivity difference parallelled an age-related attenuation in amygdala reactivity and mediated normative changes in age-appropriate anxiety (i.e., separation anxiety), suggesting an intimate link between the child-caregiver relationship and the development of the amygdala-mPFC circuit. There seems to be an important developmental shift between childhood and adolescence from amygdala-mPFC immaturity to maturity. Whereas infancy may be an important period for amygdala functional development, childhood may be an important developmental period for connections between the amygdala and mPFC.
Rodent work has shown that early maternal absence has been followed by an acceleration of amygdala-mPFC circuit development (Callaghan & Richardson, 2011; Moriceau & Sullivan, 2006). I speculated earlier that examination of children may preclude the possibility of observing stress-accelerated shifts towards maturity in amygdala functional onset (because this shift most likely occurs prior to childhood). However, childhood measures may allow for the observation of the later occurring shift in amygdala-mPFC connectivity. In contrast to typically raised children who showed connectivity immaturity, children with a history of caregiver deprivation (PI children) have shown evidence of developmental acceleration in connectivity (Gee, et al., in press). Amygdala-mPFC connectivity in PI children was negatively correlated in response to emotional stimuli and resembled the adult-like state (see Figure 2). These group differences between typically raised and PI children were mediated by group differences in cortisol production, consistent with the notion that stress can accelerate amygdala-mPFC circuit development. These phenotypes were observed on average after children had been adopted into families (after termination of institutional care). One interpretation of this timing effect is that the shift in connectivity towards the adult-state is the cascading result of early and/or elevated amygdala activity, which could have instantiated this change in connections with mPFC. Thus, both rodent models and human studies have shown evidence of the same adult-like phenotypes following early maternal absence.
Figure 2.

Mature amygdala-mPFC connectivity following maternal deprivation. Unlike typically-raised (comparison) children who showed immature (positive) amygdala-medial prefrontal cortex (mPFC) connectivity, previously institutionalized (PI) children exhibited the mature pattern of negative amygdala-mPFC coupling, such that PI children resembled adolescents. SEM = standard error of the mean. (Copied from Gee et al. (in press). Proceedings of the National Academy of Sciences).
Ontogenetic Adaptation
At first glance, the adult-like phenotype in PI children may seem counterintuitive. The mature phenotype of negative connectivity has been associated with decreased anxiety (reviewed in Bishop, 2007; Burghy, et al., 2012; Etkin, et al., 2010; Gee, et al., 2013; Hare, et al., 2008), so logic would dictate that PI children, who as a group exhibit mature connectivity should exhibit lower trait anxiety. However, PI children as a group exhibit higher than average trait anxiety (Casey, et al., 2009; Goff, et al., 2012; Juffer & van Ijzendoorn, 2005; Tottenham, et al., 2010; Wiik, et al., 2011; Zeanah, et al., 2009). Examination of the individual differences within groups (typical, PI) helped resolve the seeming discrepancy. Despite higher levels of anxiety at the group level, adult-like amygdala-mPFC phenotypes mitigated anxiety within the PI group (Gee, et al., in press). PI participants with negative (i.e., adult-like) amygdala-PFC connectivity showed lower separation anxiety relative to PI peers with positive connectivity. Therefore, within the PI group, the adult-like connectivity phenotype seemed to confer lower anxiety relative to PI youths with the immature phenotype. As the reader may recall, PI youths also have higher amygdala reactivity overall with which regulatory influences from mPFC would have to contend. For those PI youths for whom an adult-like amygdala-mPFC connectivity was achieved, this state of connectivity may serve as an ontogenetic adaptation to the heightened amygdala reactivity. As pointed out earlier, although this adaptation may serve a very important immediate purpose of helping mitigate strong bottom-up reactivity, there may be long term costs conferred by an abbreviated period of immaturity. Longitudinal studies with these PI youth as adults will be necessary to address this possibility.
6. Summary and Conclusions
The brain retains plasticity throughout life, so experiences at all ages have the potential to shape neural phenotypes. However, experiences that occur during sensitive periods of development have an extraordinary power to shape neural trajectories. Sensitive periods tend to occur early in life, making early experiences particularly important to understand. I highlighted the development of the amygdala and its connections with mPFC as an example of how sensitive periods can be identified and how brain development can be shaped by adverse events that occur during these sensitive moments. Based on the instructions received by the system from early environments, the brain may adapt to optimize for the expectation of a similar environmental condition in the future. Therefore, by examining early environments, we may be able to better understand how the neural architecture of an individual was designed and what it anticipates in the future.
Footnotes
Inspired by Jay Giedd.
References
- Ainsworth MD. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Development. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23(3):758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research. 1996;91(2):159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21(11):471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Houts RM, Fearon RM. Infant attachment security and the timing of puberty: testing an evolutionary hypothesis. Psychol Sci. 2010;21(9):1195–1201. doi: 10.1177/0956797610379867. [DOI] [PubMed] [Google Scholar]
- Berman CM, Rasmussen KL, Suomi SJ. Responses of free-ranging rhesus monkeys to a natural form of social separation. I. Parallels with mother-infant separation in captivity. Child Dev. 1994;65(4):1028–1041. [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bogin B. Evolutionary Hypotheses for Human Childhood. Yearbook of Physical Anthropology. 1997;40:63–89. [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harv Rev Psychiatry. 2011;19(1):15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero M, Macdonald SE, Miller RS. Anxiety-related behavior of orphan chimpanzees (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania. Primates. 2013;54(1):21–26. doi: 10.1007/s10329-012-0327-1. [DOI] [PubMed] [Google Scholar]
- Bowlby J. The making and breaking of affectional bonds. I. Aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. Br J Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Dev Psychopathol. 2009;21(1):157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Textura del Sistema Nervioso del Hombre y de los Vertebrados. Madrid, Spain: Tomo II. Librería de Nicolás Moya; 1904. [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125(1):20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early-life stress affects extinction during critical periods of development: an analysis of the effects of maternal separation on extinction in adolescent rats. Stress. 2012a;15(6):671–679. doi: 10.3109/10253890.2012.667463. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Transl Psychiatry. 2012b;2:e138. doi: 10.1038/tp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord. 2013;3(1):8. doi: 10.1186/2045-5380-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164(1):108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Finlay BL. Embracing covariation in brain evolution: large brains, extended development, and flexible primate social systems. Prog Brain Res. 2012;195:71–87. doi: 10.1016/B978-0-444-53860-4.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Dev. 1998;69(4):1092–1106. [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145(6):2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durman L, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early Developmental Emergence of Human Amygdala-PFC Connectivity after Maternal Deprivation. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1307893110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MM, Fox NA, Drury S, Smyke A, Egger HL, Nelson CA, 3rd, et al. Validity of evidence-derived criteria for reactive attachment disorder: indiscriminately social/disinhibited and emotionally withdrawn/inhibited types. J Am Acad Child Adolesc Psychiatry. 50(3):216–231. e213. doi: 10.1016/j.jaac.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Dev Psychopathol. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, van Dulmen MH. Behavior problems in postinstitutionalized internationally adopted children. Dev Psychopathol. 2007;19(1):129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Benzodiazepine-induced amnesia in rats: reinstatement of conditioned performance by noxious stimulation on test. Behav Neurosci. 1998;112(1):183–192. doi: 10.1037//0735-7044.112.1.183. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps E. Fear Models in Animals and Humans. In: Vasa RA, Roy AK, editors. Pediatric Anxiety Disorders. New York: Springer; 2013. pp. 3–21. [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282(5393):1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. Journal of Child Psychology & Psychiatry. 1989;30(1):77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Clarke MP. Amblyopia. Lancet. 2006;367(9519):1343–1351. doi: 10.1016/S0140-6736(06)68581-4. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, McAllister AK. Neurotrophins and visual cortical plasticity. Prog Brain Res. 2002;138:39–51. doi: 10.1016/S0079-6123(02)38069-5. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. The Journal of Comparative Neurology. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn Psychol. 1989;21(1):60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. Journal of the American Medical Association. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kanitz E, Tuchscherer M, Puppe B, Tuchscherer A, Stabenow B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav Immun. 2004;18(1):35–45. doi: 10.1016/s0889-1591(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1(8):727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental differences in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29(35):10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New Findings on Extinction of Conditioned Fear Early in Development: Theoretical and Clinical Implications. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Konner M. The Evolution of Childhood: Relationships, Emotion, Mind. Cambridge, MA: Belknap Press of Harvard University Press; 2011. [Google Scholar]
- Kovach JK, Hess EH. Imprinting: effects of painful stimulation upon the following response. J Comp Physiol Psychol. 1963;56:461–464. doi: 10.1037/h0047033. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255(5044):606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Dev Neurosci. 2013;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17(2):215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Laviola G, Leussis MP, Anderson SL. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35(3):392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Magill CR, Ashley GM, Freeman KH. Water, plants, and early human habitats in eastern Africa. Proc Natl Acad Sci U S A. 2013;110(4):1175–1180. doi: 10.1073/pnas.1209405109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology & Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Monk CS, Grillon C, Baas JM, McClure EB, Nelson EE, Zarahn E, et al. A neuroimaging method for the study of threat in adolescents. Dev Psychobiol. 2003;43(4):359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18(1):101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Furtado EA, Fox NA, Zeanah CH. The Deprived Human Brain: Developmental deficits among institutionalized Romanian children—and later improvements—strengthen the case for individualized care. American Scientist. 2009;97:222–229. [Google Scholar]
- Nettle D, Frankenhuis WE, Rickard IJ. The evolution of predictive adaptive responses in human life history. Proc Biol Sci. 2013;280(1766):20131343. doi: 10.1098/rspb.2013.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S, Gunz P, Schwarz U, Hublin JJ, Boesch C. Brief communication: Endocranial volumes in an ontogenetic sample of chimpanzees from the Tai Forest National Park, Ivory Coast. Am J Phys Anthropol. 2012;147(2):319–325. doi: 10.1002/ajpa.21641. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, et al. Indiscriminate Amygdala Response to Mothers and Strangers After Early Maternal Deprivation. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York: Basic Books; 1954. [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65(Suppl 3):83–89. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon MS, Golovanova L, Doronichev V, Romanova G, Akazawa T, Kondo O, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci U S A. 2008;105(37):13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki DW, Lamb ME, Obmascher P. Toward a general theory of infantile attachment: a comparative review of aspects of the social bond. Behavioral and Brain Sciences. 1978;1(03):417–436. [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1998;39(4):465–476. [PubMed] [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experienceing maternal separation. Journal of Neuroscience. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychol Sci. 2012;23(1):93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA., 3rd Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A. 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm Behav. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Toussaint M, Reid DJ, Olejniczak AJ, Hublin JJ. Rapid dental development in a Middle Paleolithic Belgian Neanderthal. Proc Natl Acad Sci U S A. 2007;104(51):20220–20225. doi: 10.1073/pnas.0707051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Petersburg-USA Orphanage Research Team. The effects of early social-emotional-relationship experience on the development of young orphanage children. Monographs of the Society for Research in Child Development. 2008;73(3) doi: 10.1111/j.1540-5834.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM. Unique Characteristics of Neonatal Classical Conditioning: The Role of the Amygdala and Locus Coeruleus. Integr Physiol Behav Sci. 2001;36(4):293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja V, Sriram S, Beri RS, Sreenivas V, Aggarwal R, Kaur R. Not by bread alone’: impact of a structured 90-minute play session on development of children in an orphanage. Child Care Health Dev. 2002;28(1):95–100. doi: 10.1046/j.1365-2214.2002.00246.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25(2):397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Nelson AJ. Middle childhood and modern human origins. Hum Nat. 2011;22(3):249–280. doi: 10.1007/s12110-011-9119-3. [DOI] [PubMed] [Google Scholar]
- Tirella L, Chan W, Cermak S, Litvinova A, Salas K, MIller L. Time use in Russian Baby Homes. Child: Care, Health and Development. 2008;34(1):77–86. doi: 10.1111/j.1365-2214.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol. 2012a;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Risk and developmental heterogeneity in previously institutionalized children. J Adolesc Health. 2012b;51(2 Suppl):S29–33. doi: 10.1016/j.jadohealth.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Dev Sci. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan M. A Review of adversity, the amygdala and the hippocampus: A Consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Annals of the New York Academy of Sciences. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries L, Juffer F, van Ijzendoorn MH, Bakermans-Kranenburg MJ. Infants’ physical and cognitive development after international adoption from foster care or institutions in China. J Dev Behav Pediatr. 2010;31(2):144–150. doi: 10.1097/DBP.0b013e3181cdaa3a. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, et al. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Noorden GK, Crawford ML. The sensitive period. Trans Ophthalmol Soc U K. 1979;99(3):442–446. [PubMed] [Google Scholar]
- Werker JF, Gilbert JH, Humphrey K, Tees RC. Developmental aspects of cross-language speech perception. Child Dev. 1981;52(1):349–355. [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behav Brain Res. 2001;126(1–2):147–157. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, et al. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. J Child Psychol Psychiatry. 2011;52(1):56–63. doi: 10.1111/j.1469-7610.2010.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17213–17220. doi: 10.1073/pnas.1200705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]


