Abstract
Background
During laparoscopic cholecystectomy, common bile duct (CBD) injury is a rare but severe complication. To reduce the risk of injury, near-infrared (NIR) fluorescent cholangiography using indocyanine green (ICG) has recently been introduced as a novel method to visualize the biliary system during surgery. To date, several studies have shown feasibility of this technique. However, liver background fluorescence remains a major problem during fluorescent cholangiography. The aim of the current study was to optimize ICG dose and timing for NIR cholangiography using a quantitative intraoperative camera system during open hepatopancreatobiliary (HPB) surgery. Subsequently, these results were validated during laparoscopic cholecystectomy using a laparoscopic fluorescence imaging system.
Methods
27 patients who underwent NIR imaging using the Mini-FLARE image-guided surgery system during open HPB surgery were analyzed to assess optimal dosage and timing of ICG administration. ICG was intravenously injected preoperatively at doses of 5, 10, and 20 mg, and imaged at either 30 min (early) or 24 h (delayed) post-injection. Next, the optimal doses found for early and delayed imaging were applied to 2 groups of 7 patients (n=14) undergoing laparoscopic NIR fluorescent cholangiography during laparoscopic cholecystectomy.
Results
Median liver-to-background contrast was 23.5 (range: 22.1–35.0), 16.8 (range: 11.3–25.1), 1.3 (range: 0.7–7.8), and 2.5 (range: 1.3–3.6) for the 5 mg/30 min, 10 mg/30 min, 10 mg/24 h and 20 mg/24 h respectively. Fluorescence intensity of the liver was significantly lower in the 10 mg delayed imaging dose group compared to the early imaging 5 mg and 10 mg dose groups (P = 0.001), which resulted in a significant increase in CBD-to-liver contrast ratio compared to the early administration groups (p < 0.002). These findings were qualitatively confirmed during laparoscopic cholecystectomy.
Conclusion
This study shows that a prolonged interval between ICG administration and surgery permits optimal NIR cholangiography with minimal liver background fluorescence.
Keywords: Near-Infrared Fluorescence Imaging, Image-Guided Surgery, Intraoperative cholangiography, Indocyanine Green, Laparoscopic cholecystectomy
INTRODUCTION
Laparoscopic cholecystectomy is the most frequently performed abdominal surgical procedure, and one of the most common operations in Europe and the United States (1,2). Surgical morbidity has been reported as 2–4%, however the incidence of major complications that require acute re-intervention is much lower (3). A rare but serious complication of laparoscopic cholecystectomy is bile duct injury (BDI), with a reported incidence of 0.3–1.5% (4,5). BDI is a feared surgical complication since it is associated with severe morbidity, impaired survival, and poor long-term quality of life (6).
To reduce the risk of BDI, large retrospective and prospective studies have looked at the benefit of routine intraoperative radiographic cholangiography (IOC) for detection of CBD stones and to identify or prevent bile duct injury. Whether this procedure should be performed routinely is still an active subject of debate, as systematic reviews are inconclusive (7). However, several of the larger retrospective studies observe a decrease in frequency and severity of BDI when IOC is performed (8). Limiting factors for performing radiographic laparoscopic cholangiography include: 1) it requires specific expertise in the technique and its interpretation, 2) it involves the use of ionizing radiation, 3) it is time-consuming, and 4) it creates a risk for bile leakage and duct injury itself, since puncturing and cannulation of the cystic duct is required. These limitations justify the quest for alternative, less complicated techniques to visualize biliary anatomy during cholecystectomy.
Recently, near-infrared (NIR) fluorescent cholangiography using indocyanine green (ICG) has been presented as a novel, less invasive, and non-ionizing method to visualize the biliary system during surgery (9,10,11). Circulating ICG is cleared by the liver and almost exclusively excreted into the bile (12). Following intravenous injection of ICG, the extrahepatic bile ducts can be clearly visualized using a laparoscopic fluorescence imaging system. The NIR fluorescence window (700–900 nm) has several advantages for image-guided surgery, such as minimal autofluorescence of the surgical field and several millimeters penetration of the fluorescent light through overlying tissue (13,14). In addition, as NIR light is invisible to the human eye, illumination does not alter the surgical field. In the largest series of patients published so far on the use of this technique during laparoscopic cholecystectomy, NIR fluorescent cholangiography delineated biliary anatomy in the majority of patients before dissection of Calot’s triangle (9).
Although hepatic excretion pharmacokinetics enable the use of ICG for fluorescence imaging of the extrahepatic bile ducts, at the same time, retention of ICG in the liver can cause significant background fluorescence signal that impairs discrimination of bile ducts from liver tissue. To date, studies using ICG for NIR fluorescence cholangiography have used variable doses that were injected only at time-points directly prior to surgery (15). We hypothesize that the excretion of ICG from the liver, and thereby the interval between intravenous ICG injection and surgery, would be one of the key determinants for optimal differentiation of bile duct signal from its surrounding tissue during fluorescence cholangiography. The aim of the current study was to compare early and delayed imaging protocols using different doses of ICG to optimize near-infrared cholangiography using a quantitative intraoperative camera system during open hepatopancreatobiliary (HPB) surgery. These results were subsequently validated during laparoscopic cholecystectomy using a laparoscopic fluorescence imaging system.
MATERIALS AND METHODS
Intraoperative Near-Infrared Imaging Systems
Intraoperative imaging during open surgery was performed using the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARE) image-guided surgery system, as described previously (16). Briefly, the system consists of 2 wavelength isolated light sources: a “white” light source, generating 26,600 lx of 400 to 650 nm light, and a “near-infrared” light source, generating 7.7 mW/cm2 of 760 nm light. The Mini-FLARE imaging system was positioned 30 centimeters above the surgical field. Color video and NIR fluorescence images are simultaneously acquired and displayed in real time using custom optics and software that separate the color video and NIR fluorescence images.
Laparoscopic imaging was performed using two D-light fluorescence laparoscopes (Tricam SLII and Image 1 High Definition, Karl Storz Endoscopes, Germany) through a standard 12-mm subumbilical trocar port. In this imaging system, 760-nm light, emitted by an internal light source, is guided through a special fluid light cable to a 30 degrees infrared-optimized rigid laparoscope containing an optical filter system. The system was used for intraoperative conventional imaging (white light mode) and real-time fluorescence imaging (760-nm light, ICG mode) and allowed easy switching between white light mode and ICG mode by using a foot pedal. No overlay of conventional and fluorescent images was possible, but anatomical orientation could be maintained due to the easy switching between light modes. Images are recorded using a charge-coupled device camera.
Preparation and administration of Indocyanine Green
ICG (25 mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) and resuspended in 10 cc of sterile water for injection to yield a 2.5-mg/ml (3.2 mM) stock solution. Of this stock solution 2, 4, or 8 mL, corresponding to doses of 5, 10, or 20 mg, was administered intravenously as a bolus.
Patients
To assess optimal dosage and timing of ICG administration for NIR fluorescent cholangiography, patients from two HPB imaging trials for the identification of pancreatic and liver tumors (NTR2213 and NTR2214 (Netherlands Trial Registrar, Dutch Cochrane Center, Amsterdam)) were analyzed separately. All patients in which the biliary anatomy was exposed were included. This resulted in a cohort of 27 patients (Table 1). Patients received an intravenous bolus of 5 or 10 mg of ICG in the operating room 30 min before incision (early imaging) or 10 or 20 mg at 24 h prior to surgery (delayed imaging). NIR fluorescence signal of the bile ducts and liver was quantified using the Mini-FLARE imaging system.
Table 1.
Study subject characteristics (N = 41).
| Characteristic | Median [Range] or (%) |
|---|---|
| Age | 61 [26 – 76] |
| BMI | 25 [19 – 40] |
| Sex | Male: 14 (34%) Female: 27 (66%) |
| Open surgery (N = 27) | |
| Procedure: | |
| - Resection of colorectal liver metastases | 20 (74%) |
| - Resection of pancreatic carcinoma | 7 (26%) |
| ICG dose/administration-imaging interval: | |
| - 5 mg / 30 min | 3 (11%) |
| - 10 mg / 30 min | 4 (15%) |
| - 10 mg / 24 h | 17 (63%) |
| - 20 mg / 24 h | 3 (11%) |
| Laparoscopic surgery (N=14) | |
| Procedure: | |
| - Laparoscopic cholecystectomy | 14 (100%) |
| ICG dose/administration-imaging interval: | |
| - 5 mg / 30 min | 7 (50%) |
| - 10 mg / 24 h | 7 (50%) |
Abbreviations: BMI: body mass index; ICG: indocyanine green
Subsequently, to validate the findings from open surgery, a total of 14 patients planning to undergo laparoscopic cholecystectomy were included to receive the optimal doses of ICG for either early (n=7) or delayed (n=7) imaging. Laparoscopic images were acquired using the Tricam SLII fluorescence laparoscope or Image 1 High Definition fluorescence laparoscope (Karl Storz Endoscopes, Germany). This trial has been registered as NTR3686 in the Netherlands Trial Registrar, Dutch Cochrane Center, Amsterdam. All patients provided informed consent. Exclusion criteria were pregnancy, lactation, or an allergy to iodine, shellfish, or indocyanine green. The studies were approved by the Local Medical Ethics Committee of the Leiden University Medical Center and were performed in accordance with the ethical standards of the Helsinki Declaration of 1975.
Statistical Analysis
For statistical analysis, SPSS statistical software package (Version 17.0, Chicago, IL) was used. Graphs were generated using GraphPad Prism Software (Version 5.01, La Jolla, CA). Signal-to-background ratios (SBR) were calculated by dividing the fluorescent signal of the CBD by fluorescent signal of surrounding adipose tissue or the liver. Signal-to-background (SBG), ratios were reported as median with range. To test differences between groups, the Kruskal-Wallis one-way analysis of variance test and the Dunn’s Multiple Comparison Test (only computed if overall P < 0.05) were used. P < 0.05 was considered significant.
RESULTS
Study Subjects
Patient characteristics are detailed in Table 1. Twenty-seven patients underwent open HPB surgery, of which 20 were planned for liver resection for colorectal metastases and 7 were planned for surgery with suspected ampullary or pancreatic head carcinoma. Subsequently, 14 patients that were planned for elective laparoscopic cholecystectomy for cholecystolithiasis were included to undergo laparoscopic NIR fluorescence cholangiography.
Optimization of ICG dose and administration timing
Intraoperative NIR fluorescence imaging was performed using the quantitative mini-FLARE imaging system to evaluate the effect of ICG dosage and post-injection imaging time for open surgery (Figure 1). The effect of the injected ICG dosage and timing on fluorescence brightness was determined by comparing median signal-to-background ratios (SBR) between different groups (Figures 2 and 3).
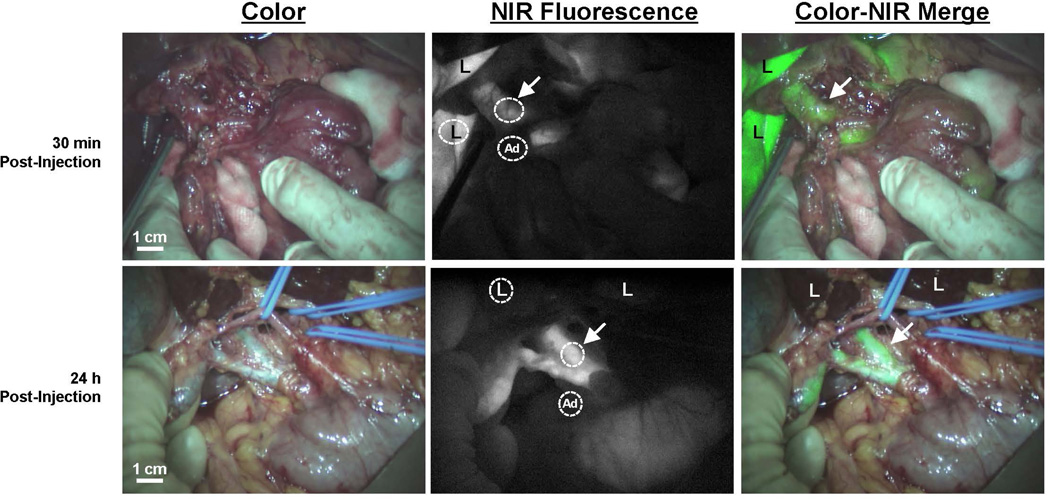
Figure 1. NIR fluorescence imaging of the common bile ducts.
Color video (left panel), NIR fluorescence (middle panel), and a color-NIR overlay (right panel) of intraoperative bile duct imaging. Upper row shows a patient undergoing pancreatoduodenectomy, 30 min after administration of 10 mg ICG. Bottom row shows a patient who underwent liver resection for colorectal metastases, 24 h after administration of 10 mg ICG. Arrows indicate the position of the common bile duct; “L” indicates the position of the liver and “Ad” indicates the localization of adipose tissue surrounding the biliary three. Circles are shown to give an example of the region-of-interests of liver, CBD and surrounding adipose tissue.
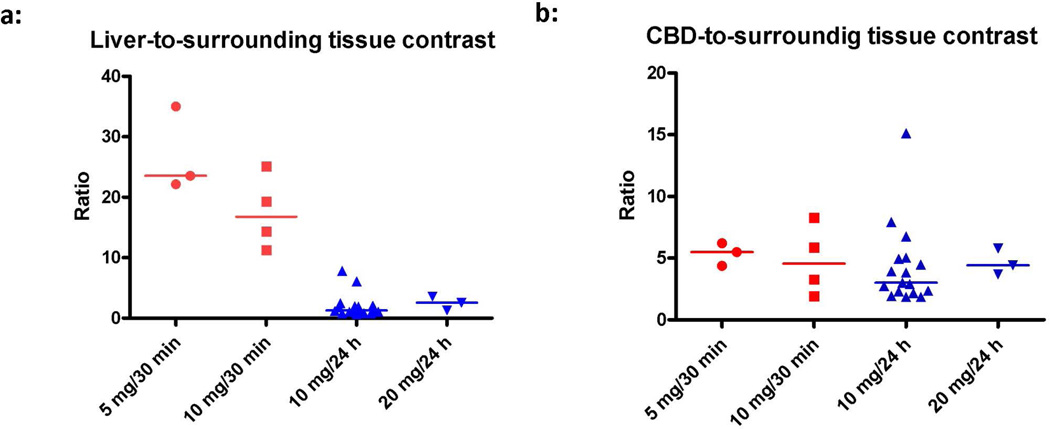
Figure 2. Evaluation of the effect of ICG dose and post-injection imaging time.
Effect of dose and timing was determined by comparing contrast ratios between concentration groups. Signal-to-background ratios (SBR) for the liver and CBD were calculated. A background region-of-interest was drawn on surrounding (fatty) tissue. Figure A shows the effect on liver contrast ratio per dose group. Fluorescence intensity of the liver was significantly lower in the 10 mg delayed imaging dose group compared to the early imaging 5 mg and 10 mg dose groups (P = 0.001), while CBD signal remains the same (Figure B). Points represent individual patient values and the line indicates the median.
Figure 3. Contrast ratio of CBD versus liver background.
Signal-to-background ratios (SBR) for the CBD versus the liver were calculated. A significant increase in contrast ratio was found in the delayed imaging 10 mg dose group compared to the early imaging 5 and 10 mg dose groups (P < 0.002). Points represent individual patient values and the line indicates the median.
Median liver-to-background contrast was 23.5 (range: 22.1–35.0), 16.8 (range: 11.3–25.1), 1.3 (range: 0.7–7.8), and 2.5 (range: 1.3–3.6) for the 5 mg/30 min, 10 mg/30 min, 10 mg/24 h, and 20 mg/24 h respectively (Figure 2a). Liver background contrast was significantly lower in the 10 mg delayed imaging dose group compared to the early imaging 5 mg and 10 mg dose groups (P < 0.001).
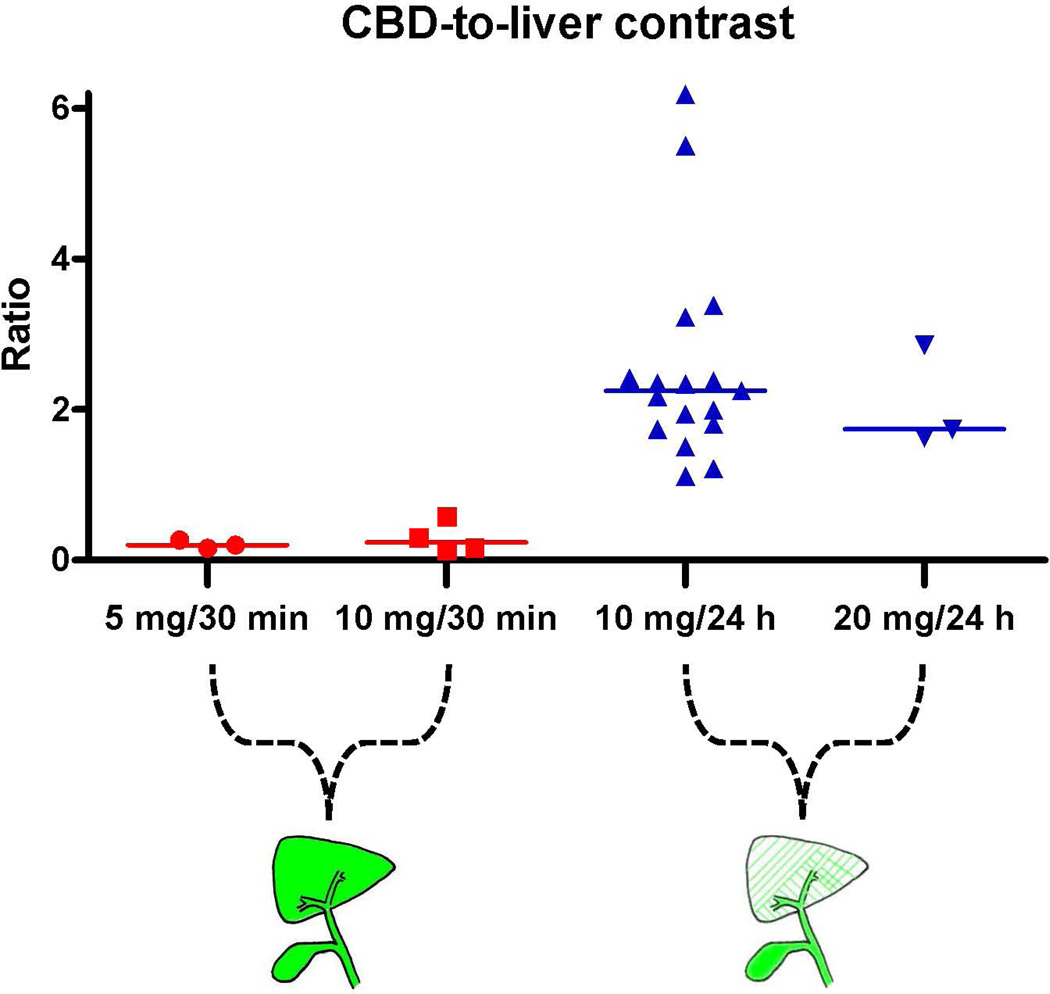
To assess the influence of the liver background signal, the ratio between CBD and liver signal was determined. Median SBR, expressed as CBD-to-liver ratio, were 0.2 (range: 0.2–0.3), 0.2 (range: 0.1–0.6), 2.3 (range: 1.1–6.2), and 1.7 (range: 1.6–2.9) for the 5 mg/30 min, 10 mg/30 min, 10 mg/24 h, and 20 mg/24 h, respectively. Again, a significant difference was found between the early imaging 5 and 10 mg dose groups and the delayed imaging 10 mg dose group (P < 0.002) (figure 3). An example of this difference in contrast ratio is illustrated in Figures 1 and 4.
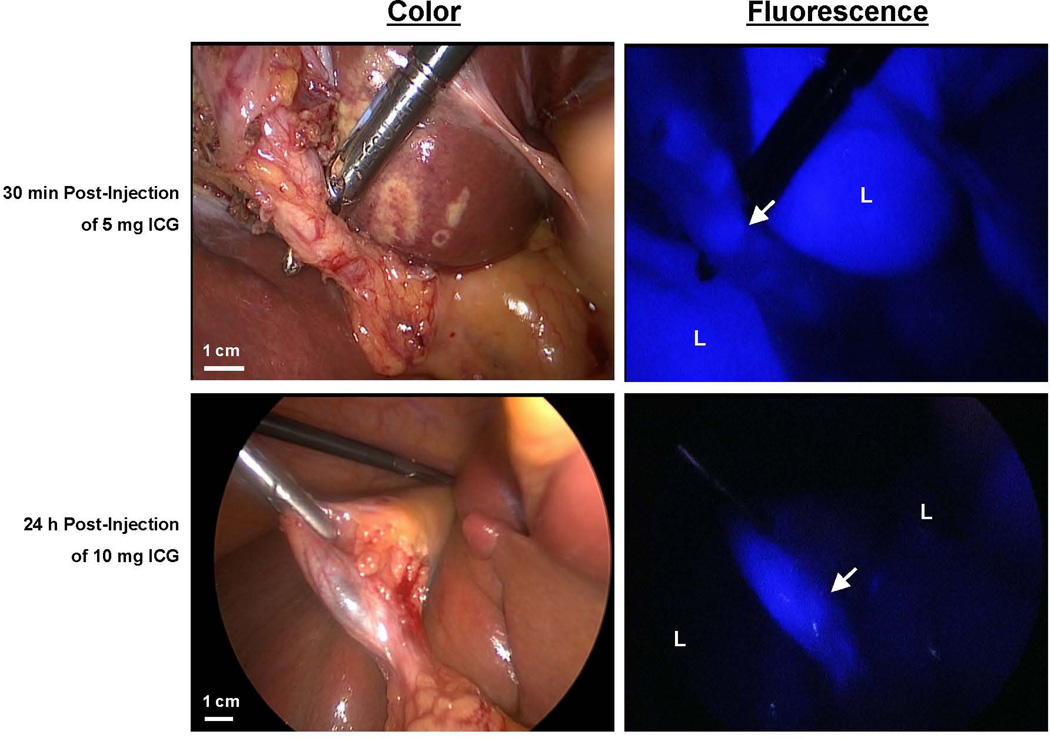
Figure 4. Laparoscopic fluorescence imaging of the biliary anatomy.
Color video (left panel) and NIR fluorescence (right panel; pseudocolored in blue) of laparoscopic bile duct imaging in two patients undergoing laparoscopic cholecystectomy. 5 or 10 mg ICG was injected respectively 30 min or 24 h before surgery. The arrow indicates localization of the cystic duct; “L” indicates the position of the liver. There is clearly less liver signal observed in the 10 mg/24 h group.
Most importantly, when considering the ratio between the CBD and surrounding adipose tissue, no differences were found between dose groups (P = 0.47) (Figure 2B). This indicates that the fluorescence intensity of the CBD itself did not differ between dose groups.
Validation during laparoscopic cholecystectomy
Subsequently 14 patients undergoing laparoscopic cholecystectomy were included and given the optimal doses found for early (5 mg/30 min, n = 7) and delayed (10 mg/24 h, n = 7) imaging. The first 8 patients were imaged using the Tricam SLII laparoscopic system, which has recently been reported (11). However, brightness of the fluorescence images in both dose groups was considerably lower compared to the images obtained with the Mini-FLARE system. To decrease this shortcoming a newly developed, next-generation Image 1 “High Definition” laparoscopic system was used in the latter 6 patients. The improved sensitivity of the CCD chip not only increased fluorescence brightness but also allowed better anatomical orientation in fluorescence mode. Intraoperative NIR fluorescence cholangiography permitted detection of the cystic duct in all patients. Although the laparoscopic NIR fluorescence imaging system was unable to quantify the fluorescence signal intensity, qualitative data analyses revealed a much higher liver signal in the 5 mg/30 min group compared to the 10 mg/24 h group (Figure 4). These results were in concordance with our quantitative findings during open surgery using the Mini-FLARE system as can be seen in Figures 1, 2, and 3.
DISCUSSION
The current study demonstrates feasibility and optimization of NIR fluorescence cholangiography during both open and laparoscopic HPB surgery. To our knowledge this is the first clinical study comparing the effect on dosage and timing of ICG administration for bile duct imaging using intraoperative NIR fluorescence.
However, further optimization of the technology is still necessary. ICG is cleared from blood by the liver within seconds and appears in bile within minutes (17). The ratio of CBD to liver NIR fluorescence peaks or plateaus (depending on species) within 2–6 h. We purposely chose to test the extremes of clearance time (30 min vs. 24 h) in this clinical study. However, future studies must explore intermediate time points in the hope that more clinically convenient timing, e.g., in the pre-op area for same day surgery, can be found.
NIR fluorescent light has several advantages for intraoperative imaging. For example, NIR light is invisible to the human eye, and therefore does not alter the look of the surgical field. Further advantages of NIR light include high tissue penetration (up to several millimeters) and low autofluorescence. Moreover, NIR fluorescence imaging is an inherently safe technique, as there is no ionizing radiation and no direct tissue contact, in contrast to intraoperative cholangiography or ultrasonography.
Visualization of the biliary anatomy can be challenging during laparoscopic cholecystectomy. Previous performed studies have shown feasibility of NIR fluorescence imaging for safe exploration of biliary anatomy (9,10,18–25). Recently, feasibility of fluorescent cholangiography has even been evaluated during robotic single-site cholecystectomy (26). However optimal dosage and timing were, thus far, not investigated. After systemic injection, ICG is completely absorbed by the liver within several minutes. Subsequently, ICG is excreted in the bile, which allows NIR fluorescence visualization of the biliary anatomy. Inherent to this, liver background remains a major problem during NIR fluorescent cholangiography. This study shows that a dose of 10 mg ICG injected 24 h prior to surgery provides a significantly lower liver background signal while the CBD signal remains stable (Figures 2 and 3). Therefore, the contrast ratio between bile duct versus liver background is considerably higher, which allows optimal visualization of the extrahepatic bile ducts (Figures 1 and 4). A limitation of this method is the time window of approximately 24 h that is needed between intravenous injection of the contrast agent and start of surgery. This could be a disadvantage during day-case admissions of patients for an elective laparoscopic cholecystectomy. Similarly, this time window cannot be used for patients suffering from acute cholecystitis in which early surgery is preferred. Still, in these cases we argue that administration of ICG should be performed as early as possible following admission to allow the majority of the contrast agent to be cleared from the liver before NIR fluorescent cholangiography is performed.
Currently, ICG and methylene blue are the only two NIR contrast agents that are approved for other clinical applications, however methylene blue is not suitable for bile duct imaging due to predominantly renal clearance in humans (14). Novel NIR contrast agents have been developed, but presently lack regulatory approval. Our group has previously compared the use of different contrast agents for NIR fluorescent cholangiography in both a small and a large preclinical animal model (17). This study showed that IRDye 800CW provided prolonged visualization of the biliary anatomy with reduced liver background fluorescence when compared to both ICG and IR-786. A significant advantage of NIR imaging is that it can provide real-time guidance. However, to enable the surgeon to work under direct image guidance, navigation in relation to the surgical anatomy is obligatory. Further technical developments of (laparoscopic) NIR imaging systems are in progress aiming to improve the real-time intraoperative display of NIR fluorophores (27,28). A recently published case report of laparoscopic NIR fluorescence imaging uses a camera system that is able to overlay the fluorescence images of the biliary tract with conventional color images in real time (29). Moreover, this case report also clearly shows the issue of liver background fluorescence when using ICG injected shortly before surgery.
In conclusion, the current study demonstrates feasibility of NIR fluorescent cholangiography for both open and laparoscopic surgery. Until novel NIR contrast agents become available for clinical use, only ICG can be applied for this purpose. A dosage of 10 mg administered 24 h before surgery appears to produce optimal results for NIR fluorescent cholangiography during both open and laparoscopic surgery. When permitted by logistics, we would recommend using a prolonged interval between ICG administration and intraoperative bile duct imaging.
ACKNOWLEDGEMENTS
We thank David Burrington Jr. for editing.
Dr. J.V. Frangioni: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation.
Funding: This work was supported in part by the Dutch Cancer Society grant UL2010-4732. Research reported in this publication was also supported by National Institutes of Health grant R01-CA-115296. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This study is accepted for oral presentation during the 21st International Congress of the EAES in Vienna, 19–22 June 2013.
DISCLOSURES
F.P.R. Verbeek, B.E. Schaafsma, Q.R.J.G. Tummers, J.R. van der Vorst, W.J. van der Made, C.I. Baeten, B.A. Bonsing, C.J.H. van de Velde, A.L. Vahrmeijer and R.J. Swijnenburg have no conflicts of interest or financial ties to disclose.
REFERENCES
- 1.NIH Consensus conference. Gallstones and laparoscopic cholecystectomy. JAMA. 1993;269:1018–1024. [PubMed] [Google Scholar]
- 2.Perissat J. Laparoscopic cholecystectomy: the European experience. Am J Surg. 1993;165:444–449. doi: 10.1016/s0002-9610(05)80938-9. [DOI] [PubMed] [Google Scholar]
- 3.Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9–14. doi: 10.1016/s0002-9610(05)80397-6. [DOI] [PubMed] [Google Scholar]
- 4.Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 2001;136:1287–1292. doi: 10.1001/archsurg.136.11.1287. [DOI] [PubMed] [Google Scholar]
- 5.Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98:391–396. doi: 10.1002/bjs.7335. [DOI] [PubMed] [Google Scholar]
- 6.Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639–1644. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 7.Ford JA, Soop M, Du J, Loveday BP, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99:160–167. doi: 10.1002/bjs.7809. [DOI] [PubMed] [Google Scholar]
- 8.Ausania F, Holmes LR, Ausania F, Iype S, Ricci P, White SA. Intraoperative cholangiography in the laparoscopic cholecystectomy era: why are we still debating? Surg Endosc. 2012;26:1193–1200. doi: 10.1007/s00464-012-2241-4. [DOI] [PubMed] [Google Scholar]
- 9.Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–1377. doi: 10.1002/bjs.7125. [DOI] [PubMed] [Google Scholar]
- 10.Aoki T, Murakami M, Yasuda D, Shimizu Y, Kusano T, Matsuda K, Niiya T, Kato H, Murai N, Otsuka K, Kusano M, Kato T. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Surg. 2009;17:590–594. doi: 10.1007/s00534-009-0197-0. [DOI] [PubMed] [Google Scholar]
- 11.Schols RM, Bouvy ND, Masclee AA, van Dam RM, Dejong CH, Stassen LP. Fluorescence cholangiography during laparoscopic cholecystectomy: a feasibility study on early biliary tract delineation. Surg Endosc. 2013;27:1530–1536. doi: 10.1007/s00464-012-2635-3. [DOI] [PubMed] [Google Scholar]
- 12.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schols RM, Bouvy ND, van Dam RM, Stassen LP. Advanced intraoperative imaging methods for laparoscopic anatomy navigation: an overview. Surg Endosc. 2013;27:1851–1859. doi: 10.1007/s00464-012-2701-x. [DOI] [PubMed] [Google Scholar]
- 14.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, Frangioni JV, van de Velde CJ, Swijnenburg RJ, Vahrmeijer AL. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci. 2012;19:626–637. doi: 10.1007/s00534-012-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mieog JS, Troyan SL, Hutteman M, Donohue KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Löwik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Towards Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery. 2008;144:39–48. doi: 10.1016/j.surg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutteman M, van der Vorst JR, Mieog JS, Bonsing BA, Hartgrink HH, Kuppen PJ, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Near-Infrared Fluorescence Imaging in Patients Undergoing Pancreaticoduodenectomy. Eur Surg Res. 2011;47:90–97. doi: 10.1159/000329411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuhashi N, Kimura F, Shimizu H, Imamaki M, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Takeuchi D, Takayashiki T, Suda K, Igarashi T, Miyazaki M. Usefulness of intraoperative fluorescence imaging to evaluate local anatomy in hepatobiliary surgery. J Hepatobiliary Pancreat Surg. 2008;15:508–514. doi: 10.1007/s00534-007-1307-5. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno S, Isaji S. Indocyanine green (ICG) fluorescence imaging-guided cholangiography for donor hepatectomy in living donor liver transplantation. Am J Transplant. 2010;10:2725–2726. doi: 10.1111/j.1600-6143.2010.03288.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishizawa T, Tamura S, Masuda K, Aoki T, Hasegawa K, Imamura H, Beck Y, Kokudo N. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:e1–e4. doi: 10.1016/j.jamcollsurg.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg. 2009;144:381–382. doi: 10.1001/archsurg.2009.9. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi Y, Ishizawa T, Masuda K, Sato S, Kaneko J, Aoki T, Beck Y, Sugawara Y, Hasegawa K, Kokudo N. Hepatobiliary surgery guided by a novel fluorescent imaging technique for visualizing hepatic arteries, bile ducts, and liver cancers on color images. J Am Coll Surg. 2011;212:e33–e39. doi: 10.1016/j.jamcollsurg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Tagaya N, Shimoda M, Kato M, Nakagawa A, Abe A, Iwasaki Y, Oishi H, Shirotani N, Kubota K. Intraoperative exploration of biliary anatomy using fluorescence imaging of indocyanine green in experimental and clinical cholecystectomies. J Hepatobiliary Pancreat Surg. 2009;17:595–600. doi: 10.1007/s00534-009-0195-2. [DOI] [PubMed] [Google Scholar]
- 25.Ishizawa T, Kaneko J, Inoue Y, Takemura N, Seyama Y, Aoki T, Beck Y, Sugawara Y, Hasegawa K, Harada N, Ijichi M, Kusaka K, Shibasaki M, Bandai Y, Kokudo N. Application of fluorescent cholangiography to single-incision laparoscopic cholecystectomy. Surg Endosc. 2011;25:2631–2636. doi: 10.1007/s00464-011-1616-2. [DOI] [PubMed] [Google Scholar]
- 26.Buchs NC, Pugin F, Azagury DE, Jung M, Volonte F, Hagen ME, Morel P. Real-time near-infrared fluorescent cholangiography could shorten operative time during robotic single-site cholecystectomy. Surg Endosc. 2013 doi: 10.1007/s00464-013-3005-5. [DOI] [PubMed] [Google Scholar]
- 27.Ashitate Y, Stockdale A, Choi HS, Laurence RG, Frangioni JV. Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy. J Surg Res. 2011 Jul;176(1):7–13. doi: 10.1016/j.jss.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, Laurence RG, Frangioni JV. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery. 2010;148:87–95. doi: 10.1016/j.surg.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherwinter DA. Identification of anomolous biliary anatomy using near-infrared cholangiography. J Gastrointest Surg. 2012;16:1814–1815. doi: 10.1007/s11605-012-1945-z. [DOI] [PubMed] [Google Scholar]