Abstract
Background and Purpose
Non-invasive imaging identifying a predictive biomarker of the bleeding risk of unruptured intracranial aneurysms (UIA) is needed. We investigated a potential biomarker of UIA instability, myeloperoxidase (MPO), in human aneurysm tissue.
Methods
Human brain aneurysms were harvested following clipping, and histologically and biochemically evaluated for the presence of MPO. Of the tissue collected, 3 were from ruptured aneurysms and 20 were from UIAs. For each UIA, its 5-year aneurysm rupture risk (ARR) was determined using the PHASES model.
Results
All ruptured aneurysms were MPO-positive. Of the UIAs, half were MPO-positive. The median 5-year ARR was higher for MPO-positive UIA (2.28%) than MPO-negative UIA (0.69%) and the distributions were statistically different (p<0.005, Wilcoxon-Mann-Whitney test). The likelihood for MPO-positive UIA was significantly associated (p=0.031) with ARR (Odds Ratio: 4.79; 95% confidence limits: 1.15–19.96).
Conclusion
MPO is associated with PHASES estimated risk of aneurysm rupture and may potentially be used as an imaging biomarker of aneurysm instability.
Keywords: myeloperoxidase, intracranial aneurysm, inflammation
Introduction
Inflammation of the cerebral aneurysm wall has been identified as an important characteristic predictive of aneurysm progression to rupture (reviewed in 1). Numerous UIA pathology studies have shown the presence of neutrophil-derived enzymes (e.g. elastase, NGAL-matrix metalloproteinases-9 complexes) in aneurysms. These enzymes promote medial matrix degradation and are associated with vascular instability.2,3
Myeloperoxidase (MPO) is a secretable oxidoreductase of azurophilic granules of polymorphonuclear cells (primarily neutrophils). In the presence of H2O2, which is generated by neutrophilic respiratory burst, secreted MPO produces chlorinating bactericidal species such as hypochlorous acid. In addition to a well-known role in host defense system against microorganisms, MPO has been recently implicated in the initiation and destabilization of atherosclerotic plaques (reviewed in 4).
Previously, we demonstrated that the enzymatic activity of MPO can be used as a highly selective and sensitive target for inflammation imaging using clinical modalities (MRI, SPECT).5–7 Rabbit model studies validated the use of a gadolinium(III)-chelate MPO activity-sensing MR imaging agent as a probe for the detection and localization of inflammation in a saccular aneurysm model.8 The local accumulation of the imaging agent correlated with the presence of MPO activity at the site of aneurysm inflammation. The resulting MR signal intensity changes were quantifiable at clinically-relevant MR field-strength and gadolinium(III) dosages.8
Although these results are promising, there is no direct evidence of the presence of MPO in human aneurysm tissues. The present study was designed to investigate the association between MPO in human aneurysms with known risk factors for rupture.
Materials and Methods
MPO Levels in Human Aneurysm Tissue and Histology
Human aneurysm tissue was resected during microsurgical clipping of brain aneurysms under an Institutional Review Board-approved protocol. Following secure clip placement, the aneurysm dome was resected, collected in sterile saline, embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham NC) and snap frozen in liquid nitrogen. The specimens were stored at −80°C until histological processing (see protocols in on-line Data Supplement). When the tissue specimen was sufficiently large, approximately 50% of the tissue was used for MPO activity measurement. Two experienced neuroradiologists blinded to the pathology results reviewed angiographic data to categorize the aneurysm morphology and measure the diameter of the aneurysm dome.
Statistical Analysis
The presence or absence of MPO in each aneurysm based on histology score was binary coded. For each UIA, its 5-year aneurysm rupture risk (ARR) was determined using the PHASES model.9 This model accounts for six risk factors including population (Finland, Japan, North American/other European countries), age, hypertension at baseline, prior history of subarachnoid hemorrhage (SAH), aneurysm size and aneurysm location. The PHASES model does not account for gender, presence of multiple aneurysms, and smoking status at baseline since these additional factors did not add value to the model for predicting rupture.9 The data were statistically analyzed (SAS Version 9.3, SAS Institute Inc., Cary, NC) to determine if the distribution of ARR differed between the MPO-positive and MPO-negative groups. Logistic regression was used to determine the association between ARR and MPO. We considered family history of SAH, irregular aneurysm shape, and documented aneurysm growth as additional risk factors for rupture and their association with MPO was analyzed using Fisher’s exact test. Effects associated with p<0.05 were considered statistically significant.
Results
From January 2008 to July 2013, 23 aneurysms from 19 patients (13 female; median age 54 years, age-range 29–75 years) were collected (Table I, on-line Data Supplement). The average aneurysm diameter was 8.0±1.0mm. Three were identified as ruptured, all of which were positive for MPO. Of the 20 UIAs, half were positive for MPO (Figure 1). MPO activity measurements in a small subset of aneurysms corroborated histological findings: In aneurysms stained negative for MPO, there was 55.2±4.6 U-MPO/mg-tissue (background level, n=2) that was approximately 4-fold lower than the MPO activity (200.5±17.8 U-MPO/mg-tissue, n=4) in aneurysms found to stain positive.
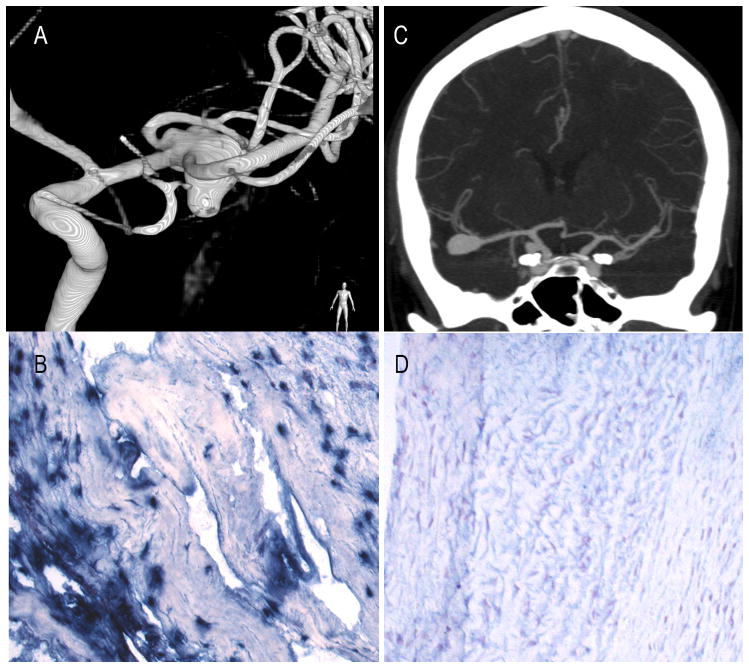
Figure 1.
Incidentally-found 10mm, left MCA aneurysm in a 46 year-old female (patient 17) is irregular with multiple blebs (A) and stains positive for MPO (B, 20×). A 29 year-old female (patient 16) with incidentally found right MCA and PComm artery aneurysms on CT-angiography (C). The 14mm MCA-aneurysm stains negative for MPO (D).
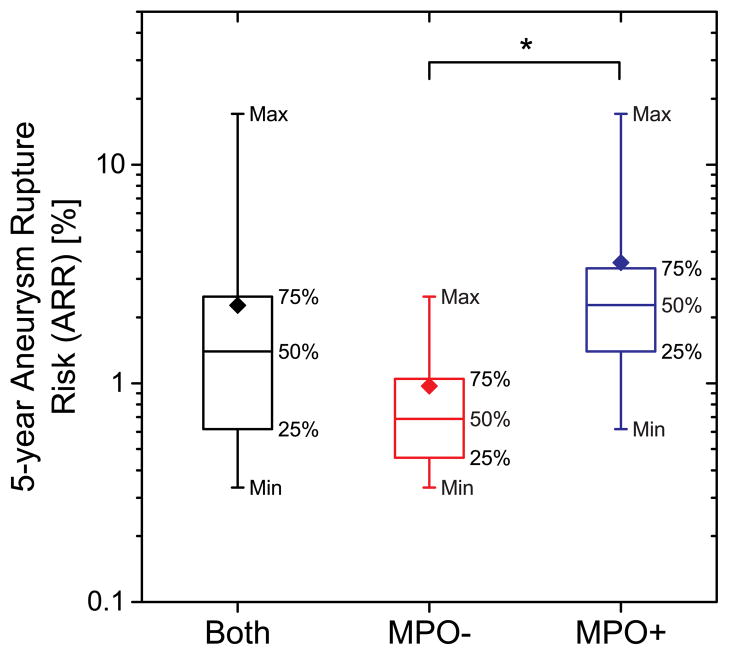
The ARR distribution did not satisfy the normality assumption (p<0.001, Shapiro-Wilks test). The median ARR (Figure 2) for all UIAs was 1.4% and was higher for the MPO-positive group (2.28%) than the MPO-negative group (0.69%). Wilcoxon-Mann-Whitney test indicated that the distributions of 5-year ARR were statistically different between MPO positive and negative groups (p<0.005). Logistic regression modeling ARR as a predictor of MPO was statistically significant (Likelihood Ratio χ2: 8.44, p=0.004) and satisfied the goodness-of-fit Hosmer-Lemeshow test (p=0.506). The likelihood for MPO-positive UIA was significantly associated (Wald χ2: 4.64, p=0.031) with ARR (Odds Ratio: 4.79; 95% confidence limits: 1.15–19.96). The receiver operating characteristic curve (Figure 3) had an area (AUC) of 0.86.
Figure 2.
Box-plot of the PHASES estimated 5-year aneurysm rupture risk for MPO-positive and MPO-negative groups. Diamonds represent the distribution mean. Asterisk (*) indicates statistically significant difference between the groups (p<0.005).
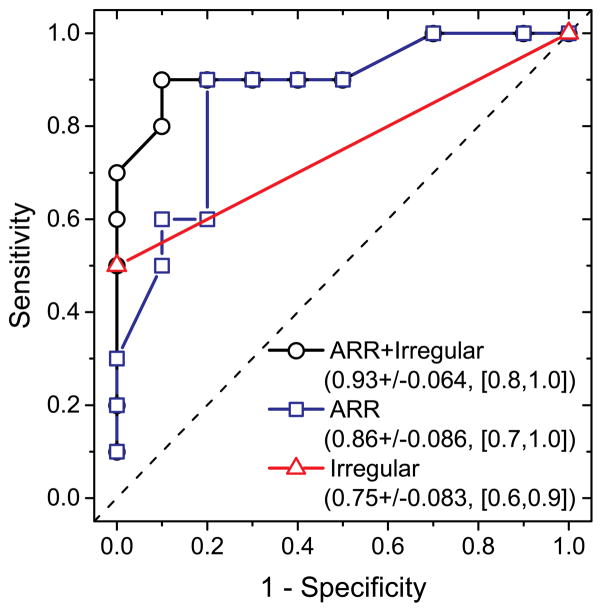
Figure 3.
ROC curves obtained by logistic regression modeling 5-year ARR and irregular aneurysm shape as predictors of MPO. The legend shows the predictors, AUC±standard error, and 95% Wald confidence limits.
Positive MPO staining also exhibited a statistically significant association with irregular aneurysm shape (Fisher’s exact test, p=0.033, 2-sided) but not with the other risk factors considered (p>0.068). Addition of irregular aneurysm shape to the logistic regression model (with Firth’s bias correction) resulted in a statistically significant model (Likelihood Ratio χ2: 8.78, p=0.012) and improved the AUC (Figure 3).
Since clinically acceptable thresholds for PHASES estimated ARR have not been established, an ordered logistic regression model was used to determine if MPO was predictive of higher ARR by grouping the ARR in terms of the distribution quartiles (ARR<0.62%:0; 0.62% ≤ ARR<1.4%:1; 1.4% ≤ ARR≥2.49%:2; ARR 2.49%:3). The overall model was statistically significant (Likelihood Ratio χ2: 10.62, p=0.001) and the proportional odds assumption could not be rejected (χ2: 1.615, p=0.446). MPO was a significant predictor (p=0.004) of 5-year ARR ordered in terms of the distribution quartiles (proportional odds: 23.32, 95% CI: 2.69–202.43).
Discussion
The role of neutrophilic MPO as a potential contributing factor to UIA rupture has not been extensively studied, but a confluence of evidence from studies aimed at determining risk factors for atherosclerosis and other cardiovascular diseases identified MPO as a key player in the progression of these diseases (reviewed in 10). More importantly, MPO levels have been shown to be correlated with the severity and outcome of vascular disease, making it a highly promising biomarker for diagnosis and staging.10,11 In addition to its role in inflammation, MPO mediates oxidative cell stress due to the production of reactive oxygen species. Chlorination of lipoproteins and nitrosylation of matrix proteins by MPO are thought to contribute to vessel wall damage.4,12
Inflammation is also a key element in the final stage of aneurysm development, i.e., rupture, and the infiltration of neutrophils has been found to be greater in ruptured versus unruptured aneurysms.13–15 The emerging picture suggests that both aberrant vascular remodeling and inflammation contribute to aneurysm formation and progression, but that a key factor which may predispose the wall to rupture is on-going inflammation mediated by an accumulation of neutrophils and monocytes/macrophages.13–15 Therefore, MPO could play a critical role in aneurysm progression to rupture. Our analysis of MPO in human UIAs indicates an association with risk of aneurysm rupture as predicted by the PHASES model, suggesting a potential role as a biomarker. It should be noted that our study design was limited by the requirement to obtain an aneurysm specimen at the time of surgery. We anticipate that with the future development of clinically acceptable non-invasive MPO imaging probes, the patient selection bias of the current report will be overcome.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institute on Aging grant 5R01AG034901-02 (PI: AAB).
Footnotes
Disclosures
None
References
- 1.Tulamo R, Frösen J, Hemesniemi MJ, Niemelä M. Inflammatory changes in the aneurysm wall: a review. J Neurointervent Surg. 2010;2:120–130. doi: 10.1136/jnis.2009.002055. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Penn DL, Witte SR, Komotar RJ, Sander Connolly E., Jr The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. J Clin Neurosci. 2014;21:28–32. doi: 10.1016/j.jocn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 5.Chen JW, Pham W, Weissleder R, Bogdanov A., Jr Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn Reson Med. 2004;52:1021–1028. doi: 10.1002/mrm.20270. [DOI] [PubMed] [Google Scholar]
- 6.Querol M, Chen JW, Weissleder R, Bogdanov A., Jr DTPA-bisamide-based MR sensor agents for peroxidase imaging. Org Lett. 2005;7:1719–1722. doi: 10.1021/ol050208v. [DOI] [PubMed] [Google Scholar]
- 7.Chen JW, Querol Sans M, Bogdanov A, Jr, Weissleder R. Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology. 2006;240:473–481. doi: 10.1148/radiol.2402050994. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo MJ, 3rd, Gounis MJ, Hong B, Ford JC, Wakhloo AK, Bogdanov AA., Jr Carotid artery brain aneurysm model: in vivo molecular enzyme-specific MR imaging of active inflammation in a pilot study. Radiology. 2009;252:696–703. doi: 10.1148/radiol.2523081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 10.Schindhelm RK, van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 11.Lim M, Bower RS, Wang Y, Sims L, Bower MR, Camara-Quintana J, et al. The predictive value of serum myeloperoxidase for vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neursurg Rev. 2012;35:413–419. doi: 10.1007/s10143-012-0375-4. [DOI] [PubMed] [Google Scholar]
- 12.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural Fragility and inflammatory response of ruptured cerebral aneurysms; a comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401. doi: 10.1161/01.str.30.7.1396. [DOI] [PubMed] [Google Scholar]
- 14.Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture; histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 15.Tulamo R, Frösen J, Junnikkala S, Paetau A, Pitkaniemi J, Kangasniemi M, et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59:1069–1076. doi: 10.1227/01.NEU.0000245598.84698.26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.