Abstract
The discoidin domain receptors, DDR1 and DDR2, are non-integrin collagen receptors that are members of the receptor tyrosine kinase family. Both DDRs bind a number of different collagen types and play important roles in embryo development. Dysregulated DDR function is associated with progression of various human diseases, including fibrosis, arthritis and cancer. By interacting with key components of the extracellular matrix and displaying distinct activation kinetics, the DDRs form a unique subfamily of receptor tyrosine kinases. DDR-facilitated cellular functions include cell migration, cell survival, proliferation and differentiation, as well as remodelling of extracellular matrices. This review summarises the current knowledge of DDR-ligand interactions, DDR-initiated signal pathways and the molecular mechanisms that regulate receptor function. Also discussed are the roles of DDRs in development and disease progression.
Keywords: transmembrane collagen receptor, receptor tyrosine kinase, cell-matrix interactions, cell signaling, receptor activation, therapeutic target
1. Introduction
The discoidin domain receptors, DDR1 and DDR2, are two closely related receptor tyrosine kinases (RTKs) that contain a discoidin homology (DS) domain in their extracellular regions. The DDRs were initially discovered by homology cloning based on their catalytic kinase domains and were orphan receptors until 1997 when two independent groups discovered that several different types of collagen are functional DDR ligands (Shrivastava et al., 1997; Vogel et al., 1997). RTKs are a large family (58 proteins in humans) of single-pass transmembrane receptors, characterised by structurally diverse extracellular ligand-binding regions and conserved cytosolic kinase domains. Based on their extracellular domain architecture, RTKs are divided into 20 subfamilies. RTK-dependent cellular signalling controls critical cellular processes, such as proliferation and differentiation, cell survival, cell migration and cell cycle control (Lemmon and Schlessinger, 2010). Typical RTKs (exemplified by the most studied receptors, members of the EGF and insulin receptor subfamilies) are activated by soluble peptide-like growth factors. It was therefore surprising that the DDRs are activated by collagens, major components of all types of extracellular matrix (ECM) (Kadler et al., 2007). Before this discovery, integrins were considered to be the only class of cell surface receptors that could transmit signals into cells by binding ECM components. Integrins are heterodimers of noncovalently associated α and β chains that constitute the main family of ECM receptors for cell adhesion (Hynes, 2002). Of the 24 distinct integrins in higher vertebrates, four serve as collagen-binding receptors (Leitinger, 2011).
The DDRs have a longer evolutionary history than the collagen-binding integrins: DDR homologues are found in invertebrates such as worms, insects and hydra, while collagen-binding integrins are restricted to vertebrates (Leitinger, 2011). A recent study defined a role for C. elegans DDRs as receptors that guide axons along major longitudinal tracts (Unsoeld et al., 2013). Like vertebrates, C. elegans has two ddr genes, but it is not clear whether the DDRs function as collagen receptors in C. elegans. Because the DDRs did not genetically interact with CLE-1, the only known collagen involved in axon guidance, it was concluded that CLE-1 is not a DDR ligand in this process (Unsoeld et al., 2013). However, it remains to be seen whether other C. elegans collagens interact with the DDRs in axon guidance.
RTKs transmit signals into cells by providing docking sites for effector molecules in the form of phosphorylated cytoplasmic tyrosines, a result of ligand-induced kinase activation and receptor autophosphorylation (Lemmon and Schlessinger, 2010). Upon collagen binding, the DDRs undergo autophosphorylation with very slow and sustained kinetics (Shrivastava et al., 1997; Vogel et al., 1997), a unique feature that distinguishes them from other RTKs. While we understand the molecular basis of the DDR-collagen interaction at the level of the isolated ligand-binding region, the biochemical and cellular mechanisms that control receptor activation on the surface of cells remain undefined. Like other RTKs, the DDRs regulate key cellular processes including cell migration, cell proliferation, cell differentiation, and cell survival. Additionally, the DDRs control remodelling of ECMs through the control of matrix metalloproteinase (MMP) expression and activity and have overlapping functions with collagen-binding integrins. This review provides an overview of the current knowledge of DDR structure and their tissue and developmental functions. I further discuss insights into the mechanism of receptor activation that have emerged from recent structural and functional studies and consider the interplay between DDRs and other cellular receptors such as integrins. Dysregulation of DDR expression and function is associated with a wide variety of human diseases; the review concludes with a discussion of the DDRs as potential therapeutic targets and their roles in disease progression.
2. Expression and Tissue Functions of DDRs
The DDRs are widely expressed in different tissues, both during development and in adult organisms. DDR1 mRNA is found in many tissues in mice and humans, with high levels in brain, lung, kidney, spleen, and placenta (Di Marco et al., 1993; Johnson et al., 1993; Laval et al., 1994; Perez et al., 1996; Perez et al., 1994). DDR2 mRNA is high in skeletal and heart muscle, kidney and lung (Karn et al., 1993; Lai and Lemke, 1994). Both DDRs are expressed in the developing nervous system (Lai and Lemke, 1994; Sanchez et al., 1994; Zerlin et al., 1993). DDR1 expression is predominant in epithelial cells, while DDR2 is found in cells of connective tissues that originate from embryonic mesoderm (Alves et al., 1995). The DDRs are also found in cells of the immune system (see below). However, no detailed or systematic analysis of the cellular distribution of DDR proteins in different tissues has been carried out.
While the DDRs play important roles in embryo development (as discussed in Section 5), their tissue functions in adults have not been established fully. Similarly, although we have some understanding of how dysregulated DDR function can lead to disease (discussed in Section 7), the normal functions of DDRs in regulating cellular behaviour are only incompletely understood. For example, both DDRs can control cell migration and adhesion in cell culture models, but, apart from roles in wound healing (DDR2) and immune responses (DDR1), it is not clear how these functions relate to physiological processes where DDR-mediated cell migration or adhesion is required in healthy adults. Our incomplete knowledge is partly due to the paucity of studies using tissue-specific knockouts of DDR expression. Overall, however, it is becoming increasingly accepted that the DDRs play important roles in tissue homeostasis and regeneration, as exemplified by the role of DDR2 on dermal fibroblasts in wound healing (Olaso et al., 2011b).
A key functional consequence of DDR binding to collagen may be their ability to up-regulate the expression and activity of MMPs. MMPs are a family of zinc-dependent proteases that degrade ECM components (Page-McCaw et al., 2007). MMP activity is tightly regulated by transcriptional control or proteolytic cleavage. DDR-mediated control of MMP activity has a direct influence on tissue remodelling through MMP-mediated degradation of matrix components, which likely facilitates cell migration and invasiveness in organ development or diseases such as atherosclerosis and cancer. Another consequence is the onset of matrix degeneration, a key event in the pathogenesis of osteoarthritis. The DDR2-mediated expression of MMP-13 in chondrocytes is further discussed below (Section 7.4). While we have some understanding of the consequences of dysregulated DDR-mediated MMP production in disease, relatively little is understood about the physiological roles of DDR-induced MMP expression or activation.
The available data show that both DDR1 and DDR2 promote MMP expression and/or activation. It was initially shown that DDR2 mediates the collagen-induced secretion of the collagenase MMP-1 in fibrosarcoma cells (Vogel et al., 1997). DDR1 mediates expression of the gelatinases MMP-2 and MMP-9 in murine vascular smooth muscle cells (SMCs), as demonstrated by reduced expression of these enzymes in Ddr1−/− cells (Hou et al., 2001; Hou et al., 2002). In human vascular SMCs, on the other hand, DDR1 seems to induce expression of MMP-1 (Ferri et al., 2004), an enzyme which does not have a murine orthologue. In human bronchial epithelium, DDR1 regulates expression of MMP-7 (matrilysin), which may contribute to epithelial repair (Roberts et al., 2011). Moreover, DDR1 induces expression of MMP-2 and MMP-9 in a number of malignant cells (for details see Valiathan et al., 2012). DDR2 has been found to regulate MMP-1, MMP-2 and the collagenases MMP-8 and MMP-13. MMP-1 secretion is promoted by DDR2 in human vascular SMCs (Ferri et al., 2004), NIH 3T3 cells and human synovial fibroblasts (Wang et al., 2002). DDR2 promotes expression and activity of MMP-2 in rat hepatic stellate cells, rat vascular SMCs and murine skin fibroblasts where it stimulates MMP-2 transcription (Olaso et al., 2001; Olaso et al., 2002; Olaso et al., 2011b; Shyu et al., 2009; Shyu et al., 2008). In human neutrophils, DDR2 activation stimulates the secretion of MMP-8 (Afonso et al., 2013). The DDR2-mediated induction of MMP-13, which occurs at the level of MMP-13 transcription (Su et al., 2009), is discussed under 7.4.
The DDRs have also been shown to be regulators of certain immunological functions. DDR1 is expressed in stimulated peripheral blood mononuclear cells (Kamohara et al., 2001) and on activated T cells (Chetoui et al., 2011; Hachehouche et al., 2010; Kamohara et al., 2001). DDR1 can mediate cell migration of monocytic cells and T cells in three-dimensional (3D) collagen matrices (Hachehouche et al., 2010; Kamohara et al., 2001). Integrins, which are the other key mediators of leukocyte interactions with tissue ECM molecules, are not involved in the migration of leukocytes in 3D collagen matrices (Friedl and Weigelin, 2008). A similar role was found for DDR2, which is expressed on circulating human neutrophils (Afonso et al., 2013). Neutrophil DDR2 is required for migration in 3D collagen matrices and promotes chemotaxis, by triggering MMP-8 activity and the generation of chemotactic collagen peptides (Afonso et al., 2013). Thus, the DDRs seem to be important players in immune responses, which depend on the effective migration of activated leukocytes into infectious or inflammatory tissue sites.
3. DDR structure and Ligand Interactions
3.1. Genomic structure and transcriptional regulation
The DDR cDNAs were isolated by several groups in the 1990s based on homolgy cloning with the intention to discover novel RTK gene products (Alves et al., 1995; Di Marco et al., 1993; Johnson et al., 1993; Karn et al., 1993; Lai and Lemke, 1994; Laval et al., 1994; Perez et al., 1996; Perez et al., 1994; Sanchez et al., 1994; Zerlin et al., 1993). While the kinase domain of the encoded proteins were noted to be about 45% identical to that of the neurotrophin receptor, TrkA, their extracellular regions were found to contain a novel protein domain not present in other RTKs. This domain is termed the discoidin domain based on homology to the lectin discoidin I, a protein secreted by the slime mold D. discoideum.
The human DDR1 gene maps to chromosome 6 (6p21.3), at the major histocompatibility complex locus, between the HLA-E and HLA-C genes (Edelhoff et al., 1995; Perez et al., 1994). The DDR1 gene spans ~24 kb and comprises 17 exons (Playford et al., 1996). The extracellular domain is encoded by exons 1-8, the transmembrane domain by exon 9. Exons 10-12 encode the cytosolic juxtamembrane (JM) domain, with the remaining exons predominantly coding for the catalytic domain. Alternative splicing yields five different gene products (see below). The two most abundant DDR1 isoforms, DDR1a and DDR1b, are the result of alternative splicing of exons 10-12, with DDR1b containing exons 10, 11 and 12 and DDR1a lacking exon 11 (Alves et al., 1995). DDR1c, on the other hand, contains an additional 18bp relative to DDR1b, which is due to the presence of an additional, cryptic splice acceptor site 5′ to the preferred splice site at the intron/exon boundary of exon 14 (Playford et al., 1996). The use of the cryptic splice site results in the addition of six amino acids to the kinase domain. Further alternative splicing generates two kinase-deficient variants: DDR1d lacks exons 11 and 12 while DDR1e misses the first half of exon 10 in addition to lacking exons 11 and 12 (Alves et al., 2001).
The DDR1 gene structure has not been explored in detail. The promoter region contains a functional consensus binding site for the tumour suppressor p53 (Ongusaha et al., 2003; Sakuma et al., 1996). Genotoxic stress, for example in the form of ionising radiation or chemotherapy, induces DDR1 expression in a p53-dependent manner (Das et al., 2006; Ongusaha et al., 2003; Sakuma et al., 1996). Likewise, the DNA repair protein XRCC3, which is induced by genotoxic stress, can up-regulate DDR1 expression (Martinez-Marignac et al., 2011). The DDR1 gene also contains a hnRNP A2 response element sequence, which may be involved in alternative splicing and nuclear export of DDR1 mRNA in oligodendrocytes (Roig et al., 2012). DDR1 expression is downregulated during induction of epithelial-mesenchymal transition (EMT). Consistent with this, the DDR1 promoter contains a putative binding site for the EMT-associated transcription factor Zeb1 (Taube et al., 2010). However, little information exists about how EMT-associated transcription factors regulate DDR1 expression. DDR1 expression can also be post-transcriptionally regulated by microRNAs: levels of microRNA-199a-5p and microRNA-199b-5p inversely correlate with DDR1 expression in human hepatocellular carcinoma cells and in acute myeloid leukaemia, respectively (Favreau et al., 2012; Shen et al., 2010).
The Ras/Raf/ERK signalling pathway is one of the signalling pathways that can regulate transcription of DDR1. For instance, the T cell receptor can induce DDR1 expression in human T cells through Ras/Raf/ERK and protein kinase C-dependent pathways (Chetoui et al., 2011). Moreover, in primary lung fibroblasts, DDR1 expression can be induced by collagen I, through DDR2 activation, in an ERK1/2-dependent manner (Ruiz and Jarai, 2011). In certain cell types, DDR1 activation can also positively regulate its own expression. For example, in MCF7 breast and HCT116 colon carcinoma cells, DDR1 activation results in Ras/Raf/ERK signalling, which induces further DDR1 expression (Ongusaha et al., 2003). However, in most cases, the upstream signals that regulate DDR1 transcription have not been defined yet.
Little research has been conducted on the genomic structure of the DDR2 gene. The human DDR2 gene locates to chromosome 1 (1q23.3) (Karn et al., 1993) and is composed of 19 exons, of which exons 4-19 are coding exons. The extracellular domain is encoded by exons 4-11, the transmembrane domain by exon 12. Exons 13 and 14 encode the cytosolic JM domain, with the remaining exons predominantly encoding the tyrosine kinase domain. No alternatively spliced isoforms of DDR2 have been described.
DDR2 expression is regulated by different transcription factors in a cell type-dependent manner. For example, in rat vascular SMCs, DDR2 expression can be increased by hypoxia or hyperbaric oxygen, which increases Myc-Max DNA binding activity in the DDR2 promoter (Chen et al., 2008; Shyu et al., 2009). During osteogenic differentiation, the ATF4 transcription factor binds to a CCAAT/enhancer binding site in the DDR2 promoter, which induces DDR2 transcription (Lin et al., 2010). DDR2 mRNA is also upregulated in hepatic stellate cells during liver injury (Olaso et al., 2001). In these cells, DDR2 mRNA can be downregulated by microRNA-29b, which targets collagen I, suggesting a relationship between collagen I expression and DDR2 expression (Sekiya et al., 2011).
3.2. Domain organisation and post-translational modifications
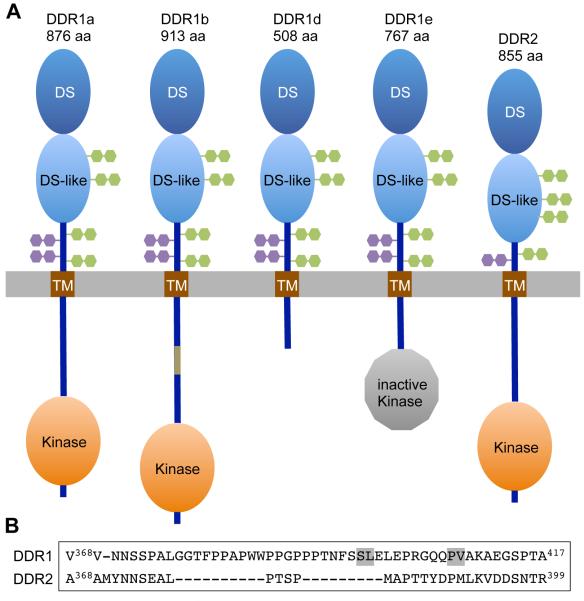
Like all RTKs the DDRs are single span type I transmembrane proteins with a C-terminal tyrosine kinase domain in their cytoplasmic regions. While many RTKs contain widely distributed structural motifs in their extracellular regions, such as immunoglobulin-like domains, fibronectin domains or cysteine-rich domains (Lemmon and Schlessinger, 2010), the DDRs have a unique structural arrangement of two globular domains: an N-terminal DS domain, tightly linked to a discoidin-like (DS-like) domain (Figs 1A and 2A) (Carafoli and Hohenester, 2013). DDR1 and DDR2 have a high degree of sequence identity in these globular domains (59% identity in DS domains, 51% identity in DS-like domains). Before the transmembrane domain, there are extracellular JM regions of about 50 (DDR1) or 30 (DDR2) amino acids. These regions are poorly conserved between DDR1 and DDR2 (Fig 1B) and are predicted to be unstructured (Carafoli and Hohenester, 2013; Fu et al., 2013). After the transmembrane domain, the DDRs contain unusually large cytosolic JM regions. These are up to 171 amino acids for DDR1, depending on the protein isoform, and 142 amino acids for DDR2. The catalytic kinase domains of about 300 amino acids are followed by very short C-terminal tails (8 amino acids for DDR1, 6 amino acids for DDR2) that do not contain any tyrosine residues.
Figure 1.
(A) Schematic structures of DDR1 and DDR2. The extracellular regions are composed of an N-terminal DS domain, followed by a DS-like domain and an extracellular JM region. The cytoplasmic regions contain a large JM region followed by the catalytic tyrosine kinase and a very short C-terminal tail. The plasma membrane is represented by a grey bar. Predicted N-glycosylation sites are depicted by green symbols, predicted O-glycosylation sites are indicated by purple symbols. Four isoforms are shown for DDR1: DDR1a, b, d and e. The cytoplasmic JM regions of DDR1b and DDR1c (not shown) contain additional 37 amino acids (shown in beige for DDR1b) compared with the DDR1a JM region. (B) Alignment of extracellular JM regions of human DDR1 and DDR2. MT1-MMP cleavage sites are highlighted in grey.
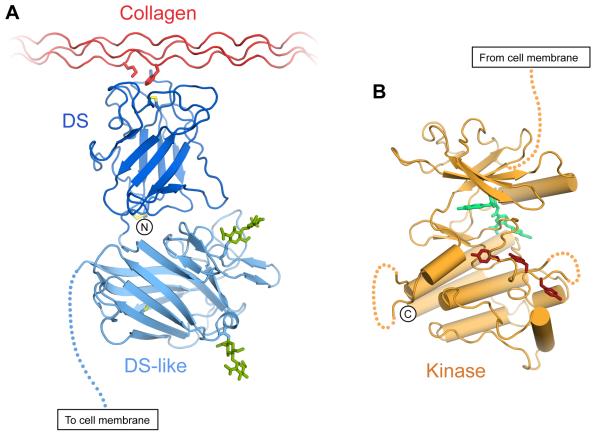
Figure 2.
Crystal structures of DDR1 globular domains. (A) Cartoon drawing of most of the DDR1 ectodomain bound to collagen, represented by a composite of the DDR2 DS domain bound to a collagen-like peptide (Carafoli et al., 2009) and the structure of the DS and DS-like domain of DDR1 (Carafoli et al., 2012). The side chains of key collagen residues (Met21 of the leading chain, Phe23 of the middle chain) are shown. Disulphide bonds are depicted in yellow. N-linked glycans in the DS-like domain are shown in green. The DDR1 N-terminus is indicated. The dashed line represents the extracellular JM region. (B) Structure of the DDR1 kinase domain in complex with type II inhibitor DDR1-IN-1 (Kim et al., 2013). The inhibitor is depicted in green, tyrosine residues of the activation loop (Tyr792, Tyr796 and Tyr797; DDR1b numbering) are shown in brown. Dashed lines represent loop regions with poor electron density. The dashed line towards the top of the Figure represents the cytoplasmic JM region. The DDR1 C-terminus (Val913) is indicated. The Figure was prepared using the coordinates of PDB entries 2WUH (Carafoli et al., 2009), 4AG4 (Carafoli et al., 2012) and 4BKI (Kim et al., 2013).
All DDR1 isoforms have in common the extracellular and transmembrane domains but differ in the cytoplasmic region. Of the five DDR1 isoforms, three (DDR1a, DDR1b, DDR1c) are functional receptors (Fig 1A). The longest isoform, termed DDR1c, contains 919 amino acids, while the most common isoforms, DDR1a and DDR1b, lack six amino acids in the kinase domain with respect to DDR1c. DDR1a additionally lacks 37 amino acids in the intracellular JM region (Fig 1A). The biological significance of the six amino acid insert in DDR1c remains unknown. DDR1d and DDR1e are truncated proteins with non-functional kinase domains, either lacking the entire kinase domain or parts of the JM domain and the ATP binding site. The inactive DDR1 isoforms DDR1d and DDR1e could modify DDR1-dependent signalling if co-expressed with full-length receptors, but evidence for such a mechanism is currently lacking.
Both DDRs contain several predicted N- and O-glycosylation sites (Fig 1A) and the mature proteins are N-glycosylated with complex glycosylated carbohydrates present in the DS-like domain and JM regions (Ali et al., 2010; Curat et al., 2001; Phan et al., 2013). Among the different N-glycoyslation sites, a conserved site, Asn211 in DDR1 and Asn213 in DDR2, seems to dominate (Phan et al., 2013). Whether the predicted O-glysoylation sites are modified is not clear.
3.3. Structures of the globular domains
The structure of the DDR DS and DS-like domains are known at atomic-level resolution from nuclear magnetic resonance (NMR) and X-ray crystallographic studies (Carafoli et al., 2009; Carafoli et al., 2012; Ichikawa et al., 2007). For a detailed discussion of available DDR ectodomain structures, the reader is referred to a recent review (Carafoli and Hohenester, 2013). DS domains are contained in a number of unrelated proteins in eukaryotes, and are found, as single domains or tandemly repeated, in secreted proteins (e.g. blood coagulation factors V and VIII) or in the extracellular regions of transmembrane proteins (e.g. neuropilin) (Baumgartner et al., 1998; Kiedzierska et al., 2007). These domains function as interaction modules and mediate a number of different biological functions (Baumgartner et al., 1998; Kiedzierska et al., 2007). DS domain ligands are diverse, ranging from carbohydrates to lipids and proteins.
The first DDR structure was obtained by NMR for the unliganded DDR2 DS domain (Ichikawa et al., 2007). Subsequently, crystal structures were determined for the DDR2 DS domain in complex with a collagen-derived peptide (Carafoli et al., 2009) and of a tandem of the DDR1 DS/DS-like domains in complex with an anti-DDR1 antibody fragment (Carafoli et al., 2012). The DDR DS domains comprise about 160 amino acids, and like all DS domains, adopt a β-barrel structure consisting of two anti-parallel sheets with a total of eight β-strands (Carafoli et al., 2009; Carafoli et al., 2012; Ichikawa et al., 2007) (Fig 2A). The top of the barrel contains five protruding loops. The bottom of the barrel forms a flat surface, and the structure is stabilised by two intramolecular disulphide bridges. A strictly conserved disulphide bridge between Cys31 (DDR1) or Cys30 (DDR2) at the DS domain N-terminus links to Cys185 near the C-terminus, and Cys74 (DDR1) or Cys73 (DDR2) forms a dilsulphide bond with Cys177, thereby linking loops 2 and 6.
The DDR1 DS-like domain contains about 180 amino acids and adopts an eight-stranded β-barrel fold similar to the preceding DS domain, despite very low sequence conservation (Carafoli et al., 2012). Compared with the DS domain, the DS-like domain contains five additional strands that protrude between the β1 and β2 strands (Carafoli et al., 2012). These contain a calcium binding site and are glycosylated at two asparagine residues, Asn211 and Asn260. Unlike the DDR DS domains, the DS-like domain lacks the disulphide bridge that connects the DS domain N- and C-termini, but contains a conserved disulphide bond that links the β4 and β7 strands. In addition, there is an unpaired cysteine (Cys287 in DDR1) that is buried.
Unlike the structurally diverse extracellular regions of RTKs, the structures of the conserved RTK kinases are very similar overall (Lemmon and Schlessinger, 2010). A crystal structure of the DDR1 kinase domain was recently determined in complex with a type II kinase inhibitor (type II kinase inhibitors bind to the inactive kinase conformation) (Kim et al., 2013). The DDR1 kinase domain is comprised of the typical N- and C-lobes and an ‘activation loop’ near the active site cleft, containing three tyrosine residue in DDRs (Fig 2B). Phosphorylation of tyrosines within the activation loop provides crucial regulatory control in most kinases. Interestingly, the C-terminal amino acids that are commonly assigned to a C-terminal ‘tail’ are actually part of an α helix in DDR1, which tightly interacts with the rest of the domain. It is therefore unlikely that the C-terminal amino acids play a regulatory role in DDR1.
3.4. Ligand specificity of DDRs and DDR binding sites in collagen
The DDRs are unique among RTKs in being activated by a component of the ECM, collagen. The 28 types of vertebrate collagens collectively are the most abundant proteins in the respective organisms (Ricard-Blum, 2011). Besides mediating cellular interactions, collagens have structural roles in ECMs and, together with other ECM molecules, define the biomechanical properties of tissues. All collagens are characterized by a triple-helical structure, whereby three polypeptide chains, termed α chains, coil around one another to give a right handed triple helix resembling a stiff cable. Collagen α chains are characterized by repeating glycine-X-X’ sequences, whereby amino acids in positions X and X’ are often proline and 4-hydroxyproline (O), respectively. Some collagens are composed of three identical α chains (homotrimeric collagens), whereas others are made up of two or three distinct α chains (heterotrimeric collagens). In tissues, most collagens form supramolecular assemblies in which the individual collagen triple helices form higher order structures such as fibres or sheet-like networks. The major collagen families are the fibrillar collagens (collagen types I-III) and the network forming collagens (e.g. the basement membrane collagen type IV).
The DDRs bind only to native, triple-helical collagens, and not to heat-denatured collagens such as gelatin (Leitinger, 2003; Shrivastava et al., 1997; Vogel et al., 1997). Both receptors display a broad ligand specificity and are activated by a number of different collagen types. While fibrillar collagens are ligands for both DDRs (Shrivastava et al., 1997; Vogel et al., 1997), non-fibrillar collagens are recognised with distinct preferences. For example, DDR1, but not DDR2, binds the basement membrane collagen type IV (Shrivastava et al., 1997; Vogel et al., 1997), while DDR2 seems to preferentially bind collagen type II (Leitinger et al., 2004) and type X (Leitinger and Kwan, 2006). DDR1 can also bind to collagen type VIII (Hou et al., 2001), but DDR2 binding to this collagen has not been tested.
DDR binding sites in fibrillar collagens have been mapped and some binding motifs have been characterised in detail. Like collagen binding integrins, the DDRs recognise distinct amino acid motifs in collagens rather than general features of the triple helix. Mapping binding sites in fribrillar collagens is challenging due to their large size and insolubility. An initial study used atomic force microscopy to visualise DDR2 binding sites in collagen type I but did not identify specific binding motifs (Agarwal et al., 2002). Early mapping data came from a study with recombinant variants of collagen II, which localised a DDR2 binding motif to the second quarter of the collagen II collagenous (COL) domain (amino acids 235-468 of the triple helix) (Leitinger et al., 2004). More detailed studies used libraries of overlapping triple-helical peptides, the so-called Collagen Toolkits, that are derived from the COL domains of the homotrimeric collagens type II and type III and collectively cover their entire COL domains (Farndale et al., 2008). These studies identified a six amino acid motif, GVMGFO (O is hydroxyproline), as a high-affinity binding motif for both DDRs (Konitsiotis et al., 2008; Xu et al., 2011a). DDR2 has additional binding sites in collagens II and III, but their exact amino acid sequences are as yet undetermined (Konitsiotis et al., 2008; Xu et al., 2011a). GVMGFO is present in the fibrillar collagens I-III but not in collagen IV, indicating that DDR1 binds a different type of motif in non-fibrillar collagens. Interestingly, carboxy-methylation of collagen I at lysine residues, with on overall methylation efficiency of 5%, interfered with DDR2 binding to collagen, indicating that lysine residues in the vicinity of the DDR2 binding site are highly susceptible to chemical modification (Khosravi et al., 2014). These observations may help understand the effect of m-periodate-treated collagen, which fails to activate DDR2 (Vogel et al., 1997). Periodate can be used to deglycosylate proteins, and the inability of periodate-treated collagen to activate DDR2 was ascribed to this effect (Vogel et al., 1997). However, m-periodate will also oxidise unsubstituted amino acids, in particular hydroxylysines (Aronson et al., 1967), which are abundant in collagens. Since unglycosylated triple-helical peptides that encompass the DDR binding site are able to bind to and activate the DDRs (Konitsiotis et al., 2008; Xu et al., 2011a), it is unlikely that glycosylation of collagen is important for DDR binding, and periodate is likely to cause loss of DDR binding activity through oxidation of amino acids rather than deglycosylation.
3.5. Molecular basis of collagen binding
The collagen binding sites of DDRs are entirely contained in their DS domains, as shown by in vitro experiments with recombinant extracellular proteins that recapitulate the receptors’ binding specificity (Ichikawa et al., 2007; Leitinger, 2003). Initial mapping studies bymutagenesis defined three spatially adjacent surface-exposed loops in the DS domains that are highly conserved between DDR1 and DDR2 as critical for the DDR-collagen interaction (Abdulhussein et al., 2004; Leitinger, 2003). The solution structure of the unliganded DDR2 DS domain was subsequently determined by NMR; the collagen binding site was mapped by transferred cross-saturation experiments and verified by mutagenesis (Ichikawa et al., 2007). These experiments defined a collagen binding trench that is created by the five loops protruding from the “top” of the DS domain. With the identification of the GVMGFO sequence as a DDR binding motif, a triple-helical peptide encompassing this motif could be synthesised that was suitable for co-crystallisation with the DS domain. The crystal structure of the DDR2 DS domain in complex with this peptide confirmed the previously defined collagen footprint (Ichikawa et al., 2007) and revealed an amphiphilic binding pocket for the apolar GVMGFO motif (Carafoli et al., 2009). The floor and one wall of the binding trench are characterised by apolar residues (Trp52, Thr56, Asn175 and Cys73-Cys177), while the other wall contains a salt bridge (Arg105 – Glu113) and Asp69. These main collagen binding residues are strictly conserved in DDR1, which is consistent with both receptors binding to fibrillar collagens. However, several amino acids at the periphery of the GVMGFO peptide-binding interface are not conserved in DDR1 and are responsible for the distinct collagen binding preferences of the DDRs. Substituting these residues in DDR2 for those of DDR1 created a DDR2 protein that was able to bind collagen IV (Xu et al., 2011a). Therefore, specific regions in the DS domains help discriminate between fibrillar and non-fibrillar collagen types.
4. Regulation of DDR Activity
4.1. Mechanism of receptor activation
The fist step in transmembrane signal transduction of RTKs manifests itself as autophosphorylation of cytoplasmic tyrosine residues. A requirement for this is the generation of receptor dimers (Lemmon and Schlessinger, 2010). In the absence of ligand, typical RTKs are thought to exist as monomers or be in equilibrium with a small amount of inactive dimers. Ligand binding to RTKs induces dimer formation and the resulting conformational changes in the dimer bring the kinase domains into close proximity allowing the phosphorylation of tyrosine residues in the JM and kinase domains (Lemmon and Schlessinger, 2010). An exception is the insulin receptor, which forms disulphide linked dimers and is activated by conformational changes within a dimer. The DDRs are unusual RTKs in that they form ligand-independent stable dimers that are non-covalently linked (Mihai et al., 2009; Noordeen et al., 2006). Therefore, the paradigm of ligand-induced receptor dimerisation does not apply to the DDRs. DDR dimers likely form during biosynthesis (Noordeen et al., 2006) and exist on the cell surface prior to ligand binding (Mihai et al., 2009; Noordeen et al., 2006). DDR dimerisation seems to involve multiple contacts in the extracellular, transmembrane and intracellular regions, with the transmembrane domain being a key region for dimerisation (Noordeen et al., 2006). Noordeen et al., using a bacterial TOXCAT reporter assay (Russ and Engelman, 1999), showed that the isolated DDR1 transmembrane region mediates very strong helix association, via the action of a leucine-based sequence motif (Noordeen et al., 2006). A later study that compared the propensity of all RTK transmembrane domains for self-association confirmed the very strong self-association potential of the DDR1 and DDR2 transmembrane domains, which were found to give the strongest signals of all RTKs in the TOXCAT assay (Finger et al., 2009).
Like all RTKs, the DDRs undergo ligand-induced receptor autophosphorylation, but this process is unusually slow. While typical RTKs are activated within seconds to minutes, maximal DDR activation (phosphorylation) is often achieved only hours after stimulation with collagen and can remain detectable for up to several days post-stimulation (Shrivastava et al., 1997; Vogel et al., 1997). There are cell type-dependent differences in activation kinetics. For example, DDR1 in human embryonic kidney cells is maximally phosphorylated 60-90 minutes after collagen stimulation, whereas it takes several hours for a strong phosphorylation signal to appear in certain cancer cell lines (L’Hote et al., 2002; Shrivastava et al., 1997; Vogel et al., 1997). Intriguingly, two independent studies showed that maximal DDR2 phosphorylation is dependent on the tyrosine kinase Src (Ikeda et al., 2002; Yang et al., 2005). Based on these studies a model emerged that suggests that ligand binding promotes Src to phosphorylate tyrosines in the DDR2 activation loop, which in turn stimulates intramolecular autophosphorylation of additional tyrosine residues. These phosphorylated tyrosines then promote DDR2 binding to cytoplasmic signalling partners. Similar to the situation with DDR2, the phosphorylation of DDR1 by Src seems to be required for full phosphorylation of DDR1 (Dejmek et al., 2003; Lu et al., 2011). DDR-Src interactions may thus play crucial roles in initiating DDR signalling.
Currently, the molecular and cellular mechanisms behind the slow DDR phosphorylation rate are unknown. Since conformational changes within the receptor, or cytosolic association and dissociation events, per se are unlikely to account for the slow DDR activation kinetics, a cellular process is likely to contribute to the delay in receptor autophosphorylation. An early study by L’Hote et al. proposed differences in ligand-induced DDR1 phosphorylation between adherent cells and cells in suspension, with faster phosphorylation kinetics in non-adherent cells (L’Hote et al., 2002), but no other experimental evidence has confirmed these findings. The authors further suggested that an inhibitory protein, potentially a phosphatase, may be associated with DDRs in the inactive state (L’Hote et al., 2002). While it is conceivable that such a protein forms a complex with inactive DDRs, it is not clear what kind of trigger would induce the release of this potential inhibitory protein to allow intracellular phosphorylation to occur.
The redistribution in the cell membrane of DDRs, or of another molecule essential for DDR activation, has been suggested as a potential mechanism underlying the slow DDR activation (Carafoli and Hohenester, 2013). DDR1b fused to yellow fluorescent protein at the C-terminus was shown to redistribute into an aggregated state within minutes of collagen addition (Mihai et al., 2009), but this has not been confirmed for untagged DDR1. It is possible that DDR activation is accompanied by a change in receptor oligomeric state, but the stoichiometry of the receptor on the cell surface, and whether ligand binding induces higher-order oligomers, are currently not known. It will be important to establish whether the active form of DDRs are signalling dimers or higher-order receptor clusters.
Since collagens form supramolecular assemblies that contain multiple interaction sites for cellular receptors, one could speculate that DDR activation occurs by clustering by their multivalent ligands. However, such a mechanism is not supported experimentally. For DDR activation experiments, fibrillar collagens are commonly added as isolated triple helices to cells that are grown as monolayers; the triple helices may produce fibrils during the incubation period in cell culture medium at 37°C, and it is therefore difficult to know what form of collagen (fibril or single triple helices) activates the DDRs. However, it is clear that DDR activation does not require collagen to be in its fibrillar state. This conclusion comes from experiments in which triple-helical collagen-mimetic peptides encompassing the DDR-binding motif, GVMGFO, caused DDR phosphorylation with the same kinetics as full-length tissue-derived collagen I (Konitsiotis et al., 2008; Xu et al., 2011a). Therefore, ligand multivalency and clustering by the ligand are not essential for DDR activation. It will be important to establish whether DDRs can be activated by native collagen fibrils. In this regard it is interesting that certain DDR2-dependent effects, such as cell cycle arrest of cancer cell lines or downregulation of focal adhesion kinase in vascular smooth vessel cells, were only obtained with polymerised collagen I but not with collagen I in the form of isolated triple helices (Bhadriraju et al., 2009; Wall et al., 2005). If single triple helices and collagen fibrils indeed transmit different signals into cells, DDR activity could be regulated by collagen remodelling in tissues.
Little is known about the molecular mechanism of DDR transmembrane signalling and we currently lack understanding about how collagen binding is translated to activation of the kinase domain. The crystal structures of the unliganded DDR1 DS domain (Carafoli et al., 2012) and the collagen-bound DDR2 DS domain (Carafoli et al., 2009) are very similar, with no significant differences in the conformation of the collagen-binding loops (Carafoli et al., 2012). This contrasts with large-scale conformational changes as a result of collagen binding to the α2 integrin I domain, which are linked to the process of transmembrane signalling (Luo et al., 2007). A series of anti-DDR1 monoclonal antibodies was generated that block collagen-induced DDR1 phosphorylation (Carafoli et al., 2012). These antibodies block signalling allosterically, i.e. without interfering with collagen binding, and their epitopes are located on the DS-like domain (Carafoli et al., 2012). The antibodies likely stabilise the inactive conformation of DDR1 and are believed to inhibit DDR1 activation by sterically blocking a ligand-induced conformational change or oligomeric association that is necessary for transmembrane signalling. Apart from the ligand-binding loops, a highly conserved patch on the surface of the DS domain is essential for DDR activation (Carafoli et al., 2012). This patch is distant from the collagen-binding site and may be required for receptor-receptor contacts in the active receptor or alternatively provide a low affinity secondary binding site for collagen. Given the unusually long, and presumably unstructured, extracellular and cytosolic JM regions, it is difficult to envisage tight coupling between ligand binding and the conformation of the cytosolic kinase domain. Cytosolic JM domains of other RTKs can contribute to autoinhibition (Hubbard, 2004) or activation of the kinase domain (Jura et al., 2009). It remains to be established whether the DDR JM regions are involved in autoinhibition or activation of the kinase domains.
4.2. Regulation of DDR1 signalling – endocytosis
Receptor-mediated signalling can be regulated in many ways. Attenuation of RTK signalling is commonly achieved by receptor downregulation through endocytosis. Many RTKs undergo endocytosis following ligand binding, leading to degradation of the ligand-receptor complex in lysosomes or recycling of the receptor to the plasma membrane. It used to be thought that RTK endocytosis would simply terminate signalling by degradation of activated receptor complexes after internalisation from the cell surface. However, RTK signalling can also continue from endosomes (Murphy et al., 2009; von Zastrow and Sorkin, 2007). As discussed above, ligand-induced DDR phosphorylation occurs with much slower kinetics than that of typical RTKs and in many cell types the presence of phosphorylated DDRs is sustained over prolonged periods (24 hours or longer). Downregulation by rapid endocytosis and lysosomal degradation of the receptors therefore does not appear to be a major mechanism by which DDR activity is attenuated. Very little information exists about DDR endocytosis, which was so far only addressed in a single study. Mihai et al used DDR1b fused to yellow fluorescent protein and found collagen-induced aggregation and internalisation of this fusion protein and what appeared to be recycling of the receptor to the cell surface (Mihai et al., 2009). Based on the internalisation kinetics, they proposed a model in which collagen binding would induce rapid DDR1b internalisation and endosomal signalling activation. In this model, DDR1b becomes phosphorylated on cytoplasmic vesicles, before being recycled to the plasma membrane (Mihai et al., 2009). However, untagged wild-type DDR1 was not studied, and experimental evidence that DDR1 phosphorylation occurs in an intracellular compartment, rather than on the cell surface, was not provided. More studies are required to clarify whether DDR1 indeed undergoes collagen-induced endocytosis and is able to signal from intracellular compartments. Moreover, the nature of the endocytic pathway is currently unknown. DDR1b and DDR1c contain a tyrosine-based sorting signal, a so-called NPXY motif, in their intracellular JM region. Cytosolic NPXY motifs direct membrane proteins to clathrin-coated pits, the first step in clathrin-mediated endocytosis (Bonifacino and Traub, 2003). DDR1a lacks the NPXY motif. It is thus possible that different isoforms of DDR1 take different intracellular trafficking routes but no experimental data are available on DDR1a internalisation. It is also not known whether DDR2 (which also lacks a cytosolic NPXY motif) undergoes ligand-induced endocytosis. Whether and how DDR signalling is regulated by endocytosis and how this process regulates receptor function in the presence and absence of ligand is currently an underexplored topic that should be addressed experimentally.
4.3. Regulation of DDR1 signalling - cleavage of ectodomain
Ectodomain “shedding”, the proteolytic release of extracellular domains of membrane-anchored proteins, provides a key regulatory mechanism of the signalling capacity of cell surface receptors (Arribas and Borroto, 2002; van Kilsdonk et al., 2010). In this process, the cleavage of the receptor proteins at an extracellular site occurs at or near the plasma membrane and is usually mediated by a protease present at the membrane. Ectodomain shedding can regulate the pool of receptors that are available to bind to ligand. Alternatively, it can create biologically functional ectodomain fragments that are active in the pericellular space, or lead to the creation of cleavage fragments of the remnant receptor that can translocate to the nucleus and modify transcription (Higashiyama et al., 2011).
Constitutive DDR1 shedding was first noticed by Alves et al. (Alves et al., 1995). Subsequent work by Vogel et al. suggested that collagen binding to DDR1 promotes restricted proteolysis of the DDR1 ectodomain via a zinc-dependent metalloprotease of the disintegrin family (Vogel, 2002), but this study was limited to the detection of the membrane-anchored C-terminal DDR1 fragment and did not demonstrate the generation of the shed N-terminal ectodomain fragment. Further work by Slack et al. showed delayed appearance of the N-terminal soluble DDR1 ectodomain in conditioned medium, when cells were incubated with collagen (Slack et al., 2006). This study confirmed collagen-induced DDR1 shedding to be mediated by a zinc-dependent metalloproteinase of either the MMP or the A Disintegrin And Metalloproteinase (ADAM) family but the nature of the specific protease was not identified. Recent work by Fu et al. analysed constitutive DDR1 shedding (Fu et al., 2013). The authors found that shedding was specifically mediated by transmembrane MMPs (MT1-MMP, MT2-MMP, MT3-MMP) but not by secreted MMPs. Two cleavage sites within the extracellular JM region were identified: Ser397-Leu398 and Pro407-Val408, 9 and 19 amino acid residues away from the transmembrane domain, respectively (Fig 1B). Because DDR1 shedding occurred independently of receptor activation by ligand, it was postulated that the membrane-tethered collagenases might regulate DDR1 activation by clearing DDR1 from the cell surface, independent of collagen stimulation (Fu et al., 2013). However, MT-MMPs might also cleave DDR1 that has been activated by ligand and thus modify DDR1-dependent signalling. The physiological significance of constitutive and collagen-induced DDR1 ectodomain shedding is unknown. The release of the soluble DDR1 ectodomain could abrogate collagen-dependent DDR1 functions by reducing the receptor pool that can bind collagen. The shed ectodomain might also have paracrine functions, in particular since the isolated DDR1 ectodomain retains the collagen-binding function (Leitinger, 2003). Biological functions of the shed ectodomain might include modification of collagen fibrillogenesis (Agarwal et al., 2007; Flynn et al., 2010) and blocking of the function of full-length DDR1 on cells (Abbonante et al., 2013; Hachehouche et al., 2010). Further studies are required to clarify the fate of the soluble ectodomain and the remnant receptor fragment, in order to understand how DDR1 ectodomain shedding regulates cellular responses to collagen. The extracellular JM regions of the DDRs are not conserved, and DDR2 lacks residues equivalent to the DDR1 cleavage sites Ser397-Leu398 and Pro407-Val408 (Fig 1B). In keeping with these facts, MT1-MMP-mediated shedding of DDR2 has not been detected (Fu et al., 2013), indicating differential regulation of DDR activation by MT-MMPs.
5. DDR Functions During Development
Both DDRs play key roles in development, with DDR1 important in organogenesis and DDR2 in bone growth. As mentioned above, DDR1 expression is mainly found in epithelial cells, in particular in the kidney, lung, gastrointestinal tract and brain, while DDR2 is found in cells of connective tissue (Alves et al., 1995), including fibroblasts of different origins and bone cells such as chondrocytes and osteoblasts.
5.1. DDR1 functions in organogenesis
Knockout mice lacking DDR1 are characterised by small stature, with the females displaying multiple reproductive defects, including impaired blastocyst implantation, which makes a large percentage of knockout females infertile (Vogel et al., 2001). The most pronounced defect are mammary gland branching abnormalities, with the epithelium failing to secrete milk. Female mice are thus unable to nourish their pups, which have to be fed by wild-type foster mothers shortly after birth. DDR1 is expressed throughout all stages of mammary development in wild-type mice (Barker et al., 1995). In Ddr1−/− mice, the mammary glands of late-stage pregnant females showed a condensed alveolar structure, with the fat pad filled with ducts (Vogel et al., 2001). During development, ductal growth is delayed in puberty, resulting in enlarged terminal end buds and abnormal secondary branching (Vogel et al., 2001). Additionally, the epithelium is hyper-proliferative, and enhanced ECM deposition is found in the stroma. Transplantation experiments with knockout tissue in wild-type recipients showed that the defects in Ddr1−/− mice are cell-autonomous and restricted to the mammary epithelium (Faraci-Orf et al., 2006). Interestingly, Wnt5a, which is required for mammary gland branching development, can regulate collagen-induced phosphorylation of DDR1 in mammary glands and isolated mammary cells (Dejmek et al., 2003; Jonsson and Andersson, 2001; Roarty and Serra, 2007). DDR1 was shown to participate in the signalling cascade leading to lactation by controlling Stat5 phosphorylation and transcription (Faraci-Orf et al., 2006). DDR1 thus appears to play an important role in differentiation, cell motility, collagen synthesis and signalling. The severe defect in mammary gland development and the complete absence of lactation in the Ddr1−/− mice are in contrast to the much milder defect in mice lacking the collagen-binding integrin α2β1. Mice lacking α2β1 integrin have mild branching abnormalities that do not affect lactation (Chen et al., 2002). It thus appears that DDR1 and α2β1 integrin regulate distinct aspects of the branching process.
The lack of DDR1 in mice further leads to defects in kidney and inner ear architecture, two organs that share certain morphological and ultrastructural features and are linked in many genetic disorders (Torban and Goodyer, 2009). Ddr1−/− mice display progressive morphological alterations and severely decreased auditory function; hence DDR1 functions as a key regulator in the maintenance of tissue architecture of the inner ear (Meyer zum Gottesberge et al., 2008). In the kidney, DDR1 is expressed in glomerular epithelial cells (Gross et al., 2010; Kerroch et al., 2012). While no apparent gross abnormalities of kidneys are observed in Ddr1−/− mice, these mice display an altered glomerular basement membrane structure with localised matrix overproduction and develop mild proteinuria, but no chronic renal disease (Gross et al., 2004). A very similar defect is seen in mice lacking α2β1 (Girgert et al., 2010), indicating that α2β1 and DDR1 play similar roles in maintaining glomerular architecture. Since renal defects in mice lacking either DDR1 or α2β1 are subtle, it is likely that the two collagen receptors can compensate for each other functionally.
5.2. DDR2 functions in bone growth – mice
Targeted deletion of DDR2 in mice resulted in knockout mice that exhibit dwarfism with short long bones and a shorter snout, due to reduced chondrocyte proliferation (Labrador et al., 2001). A spontaneous, autosomal-recessive mutation in a mouse colony was found to result from deletion of most of the Ddr2 gene (Kano et al., 2008). These mice, termed slie, show sterility in addition to dwarfism, which led the authors to conclude that DDR2 controls gonadal functions, as all slie females were anovulatory and most slie males lacked spermatogenesis (Kano et al., 2008). However, infertility has not been reported for Ddr2−/− mice, and it is therefore possible that additional defects are responsible for the observed infertility of slie mice.
DDR2 is a key regulator of bone growth that controls several aspects of the process. It participates in endochondrial ossification by regulating chondrocyte maturation (Zhang et al., 2011) and helps regulate intramembranous ossification by controlling osteoblast differentiation via phosphorylation of Runx2, a master transcription factor in skeletal development (Lin et al., 2010; Zhang et al., 2011). However, a detailed understanding of which collagen ligand(s) activates DDR2 to control endochondral proliferation and ossification is missing. In osteoblasts, DDR2-collagen interactions also mediate the secretion of lysyl oxidase (Khosravi et al., 2014), an enzyme that catalyses cross-linking of collagen fibers, a modification that is essential for bone strength.
Transgenic mice with DDR2 overexpression display altered body size, which is the only significantly different parameter compared with their normal littermates (Kawai et al., 2014). In particular, transgenic mice have increased body length but decreased body weight, resulting in a lower body mass index, presumably arising from increased leptin production which in turn results in decreased epididymal fat pads (Kawai et al., 2014). These observations suggest that DDR2 may control fat metabolism, in addition to skeletogenesis, but more detailed studies are required in order to elucidate whether DDR2 directly controls leptin secretion.
5.3. DDR2 functions in bone growth - humans
DDR2-dependent functions in human skeletal growth were uncovered through the analysis of a rare human genetic disorder, a chondrodysplasia termed spondylo-meta-epiphyseal dysplasia with short limbs and abnormal calcifications (SMED-SL). This autosomal recessive disorder is characterised by disproportionate short stature, short limbs, broad fingers, bone abnormalities and premature calcifications (Borochowitz et al., 1993). Bargal et al. discovered that SMED-SL is caused by DDR2 mutations and identified three missense mutations and one splice site mutation in eight patients from seven different consanguineous families (Bargal et al., 2009). An additional missense mutation in DDR2 was subsequently identified in a further patient (Ali et al., 2010). The cellular and biochemical mechanisms leading to SMED-SL from missense mutations were analysed in human cell lines. While three of the mutations resulted in trafficking defects with DDR2 protein retained in the endoplasmic reticulum, the fourth mutation resulted in DDR2 that was correctly trafficked to the cell surface but failed to bind collagen due to a mutation in a key residue of the collagen-binding site (Ali et al., 2010). Therefore SMED-SL can result from at least two different loss-of-function mechanisms, and loss of DDR2’s ability to interact with collagen is sufficient to cause severe skeletal abnormalities, reinforcing an essential role for DDR2 in human skeletal growth.
6. Signalling by DDRs
6.1. Signalling pathways activated by DDRs
Ligand binding to RTKs leads to phosphorylation of distinct cytoplasmic tyrosine residues, which serve as docking sites for the assembly of downstream signalling molecules that are recruited to the receptor (Lemmon and Schlessinger, 2010). DDR1b and DDR1c have 15 tyrosine residues in their cytosolic domain (Fig 3), while DDR1a has 13 and DDR2 14. All of these tyrosines could function as potential ligand-induced phosphotyrosine sites that act as docking sites for signalling adaptors. However, detailed information about which tyrosines become phosphorylated upon collagen binding is lacking. A proteomic study that used pervanadate-activated DDR1b (pervanadate inhibits protein tyrosine phosphatases) identified Tyr484, Tyr513 and Tyr520 in the JM region as phosphosites (Lemeer et al., 2012) (Fig 3). In a recent phosphoproteomic study, collagen-induced DDR2 phosphorylation was detected on two sites in the kinase domain (Tyr684 and Tyr813) (Iwai et al., 2013b). Interestingly, a site in the JM domain (Tyr481) was found to be constitutively phosphorylated, but the phosphorylation of Tyr471, which was shown to be a docking site for the adaptor ShcA (Src homology 2 domain containing transforming protein A) (Ikeda et al., 2002), was not detectable using anti-phosphotyrosine immunoprecipitation and peptide identification following protein digestion (Iwai et al., 2013b).
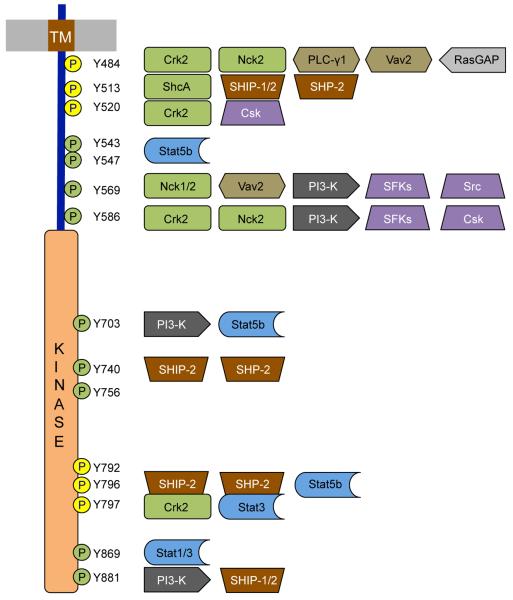
Figure 3.
Interaction map of phosphotyrosine-based DDR1 interactions. The indicated amino acid numbers refer to the DDR1b isoform; DDR1a lacks Tyr513 and Tyr520. Biochemically verified phosphorylation sites in the JM region and the activation loop of the kinase domain are shown in yellow. The Figure summarizes results obtained mainly from phosphotyrosine peptide pulldowns in human placenta tissue (Lemeer et al., 2012). Only proteins with SH2 or PTB domains are shown. Crk2, adaptor protein Crk2; Nck1/2, adaptor protein Nck1/2; PLC-γ1, phospholipase C γ1; Vav2, guanine nucleotide exchange factor Vav2; RasGAP, negative regulator of Ras; ShcA, SH2 containing transforming protein A; SHIP1/2, SH2 containing inositol polyphosphate 5-phosphatase 1/2; SHP-2, SH2 containing protein tyrosine phosphatase 2; Csk, C-terminal Src kinase; Stat1/3/5b, signal transducer and activator of transcription 1/3/5b; PI3-K, phosphoinositide 3-kinase; SFKs, Src family tyrosine kinases (Yes, Lyn, Fyn).
Several adaptor molecules have been identified that are recruited to phosphorylated sites on the DDR cytosolic regions. However, our knowledge about how these signalling molecules are linked to specific cell regulatory functions remains limited. A key adaptor molecule that binds to DDR1b, but not to DDR1a, is ShcA (Vogel et al., 1997). Nck2 (non-catalytic region of tyrosine kinase adaptor protein 2), a Src homology 2 (SH2) domain-containing protein, is another adaptor protein recruited to activated DDR1 (Koo et al., 2006). Other molecules known to interact with activated DDR1 include the protein tyrosine phosphatases SHP-1 (Abbonante et al., 2013) and SHP-2 (Koo et al., 2006; Wang et al., 2006), the p85 catalytic subunit of the phosphatidylinositol (PI-3) kinase (Dejmek et al., 2003; L’Hote et al., 2002; Suh and Han, 2011), C-terminal Src kinase (Csk) (Yang et al., 2009) and members of the Signal Transducers and Activators of Transcription (Stat) family (Faraci-Orf et al., 2006; Wang et al., 2006). Using pervanadate-activated DDR1b as a bait, ShcA and another adaptor, Grb2, were identified in DDR1b immunoprecipitates (Lemeer et al., 2012). Further studies using a library of phosphorylated peptides comprising all 15 potential tyrosine phosphorylation sites of DDR1 identified over 30 proteins as potential DDR1 interactors (Lemeer et al., 2012) (Fig 3). Most of these proteins contain either a SH2 or a phosphotyrosine binding (PTB) domain. Apart from previously identified DDR1 signalling partners, novel interactors included RasGAP, a negative regulator of Ras, and the guanine nucleotide exchange factors Vav2 and Vav3. However, it remains to be seen whether the newly identified molecules functionally interact with ligand-activated DDR1 in cells.
Our knowledge of intracellular signalling partners for DDR2 is very limited. ShcA is recruited to collagen-activated DDR2 (Ikeda et al., 2002) but no other adaptor molecules have been shown to directly interact with collagen-activated DDR2 in cells. Several potential downstream effectors of DDR2 signalling, including SHP-2, Nck1, the Src family kinase Lyn, PLCL2 (phospholipase C-like 2), and PIK3C2A (phosphatidylinositol-4-phosphate 3-kinase) were identified using phosphoproteomics (Iwai et al., 2013b). However, additional experimental validation is needed to verify whether these candidate effectors interact directly with specific phosphotyrosine sites on DDR2.
The signalling pathways activated by DDRs are not completely understood. Different cellular outcomes can arise in a context and cell-type dependent manner (Fig 4). For example, DDRs can activate the mitogen-activated protein (MAP) kinase pathway via distinct MAP kinase family members. DDR1-dependent extracellular signal-regulated kinase (ERK)1/2 activation occurs in smooth muscle, mammary epithelial and transfected embryonic kidney cells, as well as in megakaryocytes (Abbonante et al., 2013; Hilton et al., 2008; Lu et al., 2011; Ongusaha et al., 2003), while in mesangial cells the function of DDR1 may be to repress ERK1/2 (Curat and Vogel, 2002). DDR1 signalling can also occur via c-jun N-terminal kinase (JNK), as shown in pancreatic cancer cells (Shintani et al., 2008) and in adipose stromal cells from human tissue, where DDR1 transduces biomechanical signals that trigger aromatase transcription (Ghosh et al., 2013). DDR2 activates ERK2 in breast cancer cells (Zhang et al., 2013) and uses ERK1/2 and p38, but not JNK, to activate MMP-13 expression in chondrocytes (Xu et al., 2007; Xu et al., 2005), while it uses p38 and JNK, but not ERK1/2, to induce IL-12 production (Poudel et al., 2013). Conflicting reports exist whether DDR2 uses p38 MAP kinase (Lin et al., 2010) or ERK1/2 (Zhang et al., 2011) for activation of the transcription factor Runx 2 during osteoblast differentiation. The observed differences may be the result of different protocols utilised for osteoblast differentiation. DDR1 activation is further linked to the PI-3 kinase/Akt signalling pathway in a variety of human normal and cancer cell lines and mouse embryonic stem cells (Ongusaha et al., 2003; Suh and Han, 2011).
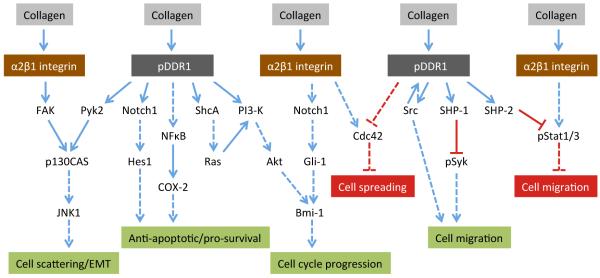
Figure 4.
Selected intracellular events induced by DDR1 binding to collagen. Cellular outcomes depend on the cellular context. In pancreatic cancer cells or mouse embryonic stem cells, DDR1 cooperates with α2β1 in signal transduction, leading to cell scattering/EMT or cell cycle progression, respectively. In MDCK cells, DDR1 antagonises α2β1 functions such as cell spreading and cell migration. In other cell types, such as megakaryocytes or smooth muscle cells, DDR1 promotes cell migration. Solid lines depict direct signalling effectors, dashed blue lines indicate indirect steps. Cellular outcomes in green are processes that are promoted by DDR1 activation, while cellular outcomes in red are suppressed by DDR1 activation.
DDR1 activation in breast and colon carcinoma cell lines triggers pro-survival signals under conditions of genotoxic stress (Ongusaha et al., 2003) and leads to increased chemoresistance of breast cancer cells via the nuclear factor κB (NFκB) pathway (Das et al., 2006). In colon carcinoma cells, DDR1-dependent prosurvival effects are mediated through Notch 1 signalling by generating a γ-secretase-cleaved Notch 1 intracellular domain, which translocates to the nucleus to regulate pro-survival genes (Kim et al., 2011). In addition to cell type-dependent differences in biological outcome, cellular responses may also depend on specific DDR ligands. For example, in human embryonic kidney cells, collagen I does not activate DDR1b-dependent MAP kinase signalling whereas stimulation with collagen IV leads to sustained DDR1b-induced MAP kinase activation (Ongusaha et al., 2003). Moreover, while collagen I triggers DDR1-induced Pyk2 phosphorylation in pancreatic cancer cells (Shintani et al., 2008), the non-fibrillar collagen XV seems to inhibit DDR1-dependent Pyk2 phosphorylation in the same cells (Clementz et al., 2013).
6.2 Cooperation with other pathways, integrins
RTK-triggered signalling pathways are highly interconnected with signalling pathways induced by other RTKs or different classes of cell surface receptors (Comoglio et al., 2003; Natarajan and Berk, 2006; Soung et al., 2010). In this way, different receptor systems cooperate to effect a particular signalling outcome. While little evidence exists for cooperation of DDRs with other RTKs, a recent phosphoproteomic study showed that the insulin signalling pathway promotes collagen-induced DDR2 phosphorylation (Iwai et al., 2013a); the mechanism by which this is achieved has not been explored. In this context it is also interesting that the RTK EphA2 was co-immunoprecipitated with pervanadate-activated DDR1 (Lemeer et al., 2012). However, this interaction has not been validated in other systems and it is not clear whether the two receptors interact in a functional way.
It is clear that DDR activation occurs independently of β1 integrins (Vogel et al., 2000), but integrins and DDRs have been shown to modulate each other’s function. In particular, cross-talk between DDR1 and collagen-binding integrins was observed in several experimental systems. Four integrins of the β1 integrin subfamily share collagen binding specificity with the DDRs (Leitinger, 2011). Integrins and DDRs can either cooperate with one another in signal transduction and cell adhesion or inhibit each other’s function, with the outcome seemingly dependent on the cell type. In MDCK cells, several studies demonstrated a negative regulation of α2β1 integrin function by DDR1 (Fig 4). Thus, MDCK cell interactions with collagen I resulted in DDR1-mediated suppression of α2β1-induced cell migration (Wang et al., 2005; Wang et al., 2006), with the molecular mechanism involving DDR1 binding to the phosphatase SHP-2, thereby suppressing α2β1-mediated collagen-induced phosphorylation of the transcriptional activators Stat1 and Stat3 (Wang et al., 2006). In MDCK cells, DDR1 also inhibited α2β1-dependent cell spreading, through suppression of the activity of the small Rho-family GTPase Cdc42 (Yeh et al., 2009). Opposing effects of DDR1 and β1 integrins have also been found in human adipose stromal cells in 3D collagen matrices: while DDR1 activates transcription of stromal aromatase, β1 integrins have an inhibitory effect (Ghosh et al., 2013). However, in these cells, the two receptor systems seem to act independently from one another, rather than intersecting at a common downstream signalling molecule. In contrast to the negative regulation of α2β1 by DDR1 in MDCK cells, cooperation of the two receptors is required in pancreatic cancer cells, which undergo EMT upon interaction with collagen I. In these cells, both DDR1 and α2β1 coordinate to signal activation of JNK, with in turn upregulates N-cadherin expression and promotes cell scattering (Shintani et al., 2008) (Fig 4). Similar cooperation between DDR1 and α2β1 promotes the self renewal of mouse embryonic stem cells through cell cylce regulation (Suh and Han, 2011). Here, α2β1 and DDR1 pathways converge at the gene regulatory polycomb protein Bmi-1 (Fig 4).
In contrast to the negative regulation of cell migration in MDCK cells, DDR1 has been shown to be pro-migratory in many other cell types, including leukocytes (see Section 2) and cancer cells (see Section 7.6), but the downstream signalling pathways involved in DDR1-mediated cell migration are only poorly characterised. In megakaryocytes, DDR1 activation stimulates migration by recruiting the phosphatase SHP1, which in turn leads to dephosphorylation of the tyrosine kinase Syk, preventing Syk-mediated inhibition of megakaryocyte migration on collagen (Abbonante et al., 2013) (Fig 4). Similarly, in epithelial cells DDR1 enables migration by blocking Syk-mediated inhibition of migration (Neuhaus et al., 2011). However, whether DDR1 cooperates with integrins to overcome Syk-mediated migration inhibition is not known.
As mentioned above, DDR1 inhibits α2β1-dependent cellular functions in MDCK cells, including cell adhesion (Wang et al., 2006). However, in many other cell types, cell adhesion seems to be promoted by DDR1 (e.g. (Curat and Vogel, 2002; Hou et al., 2001; Kamohara et al., 2001; Ram et al., 2006; Yoshida and Teramoto, 2007)). In keeping with these findings, Xu et al. found that overexpressioin of DDR1 or DDR2 in human embryonic kidney cells promoted integrin-mediated cell adhesion to collagen I (Xu et al., 2012). Although the molecular mechanism by which this is accomplished has not been explored, DDR-mediated signalling enhanced integrin activation of α1β1 and α2β1 without regulating the surface expression levels of these collagen-binding integrins, suggesting that DDR signalling can regulate the affinity of β1 integrins (Xu et al., 2012). A subsequent study by Staudinger et al. also found DDR1-mediated enhancement of β1 integrin function. In NIH 3T3 cells, overexpression of DDR1 resulted in enhanced α2β1 and α5β1 (fibronectin-binding integrin) activation in focal adhesions, with increased focal adhesion maturation (Staudinger et al., 2013). In contrast to the study by Xu et al. which observed no DDR-regulated effect on integrin surface levels (Xu et al., 2012), Staudinger et al. found DDR1 overexpression to result in increased surface expression of α2β1 and α5β1 (Staudinger et al., 2013), indicating that DDR1 may regulate integrin trafficking to the cell surface.
In conclusion, the DDRs can positively and negatively regulate integrin-mediated cellular functions in several ways: DDR-induced signalling can converge with integrin-triggered pathways to regulate certain cellular functions, whereby each receptor engages its own downstream pathway; in addition, DDR-mediated signalling can directly affect the activity of integrins.
6.4 Collagen-independent functions for DDR1
Although DDR1 clearly functions as a collagen receptor, collagen-independent signalling functions have also been described, raising the intriguing possibility that DDR1 has additional, as yet unidentified, ligands or that it can be transactivated by membrane-embedded receptors or sensors. In human adipose stromal cells that are grown in 3D collagen matrices, DDR1 induces aromatase production seemingly independently of its collagen-binding function (Ghosh et al., 2013). In this experimental set-up, DDR1 activation may occur via a putative sensor for matrix compliance (Ghosh et al., 2013). In carcinoma cells with wild-type p53, collagen-independent DDR1 activation (autophosphorylation) is triggered in response to p53-dependent DNA damage or ionising radiation, demonstrating that DDR1 becomes activated by genotoxic stress (Ongusaha et al., 2003). In cells with mature adherens junctions, DDR1 localises to cell-cell junctions rather than to the apical or basal membrane. This was observed in confluent MDCK cells, a number of other epithelial cell lines, and A431 squamous carcinoma cells (Hidalgo-Carcedo et al., 2011; Wang et al., 2009; Yeh et al., 2011). Localisation of DDR1 to cell-cell contacts requires E-cadherin function (Hidalgo-Carcedo et al., 2011; Wang et al., 2009). Once at cell-cell junctions DDR1 does not interact with collagen, as it is sequestered away from apical or basal membranes where it could interact with collagen (Wang et al., 2009). Depletion of E-cadherin restores collagen-induced DDR1 activity by redistribution of DDR1 away from the lateral membrane to apical and basal sites (Wang et al., 2009). In carcinoma cells, DDR1 is required for collective cell migration and invasion through its interaction with the cell polarity regulator proteins Par3 and Par6 at cell-cell contacts, which results in decreased actomyosin contractility (Hidalgo-Carcedo et al., 2011). Intriguingly, neither DDR1 kinase activity nor its collagen-binding function are required to regulate actomyosin contractility.
7. DDRs as potential Therapeutic Targets in Disease
Both DDRs have been linked to a wide variety of human disorders, ranging from fibrotic disorders of different organs, atherosclerosis, arthritis and many types of cancers. Targeted deletion of DDRs in mice and the use of a number of mouse models of chronic human diseases have helped to unravel DDR functions in disease progression. The DDRs usually play positive roles in pathologies, and the use of DDR inhibitors is therefore an attractive therapeutic approach, in particular for diseases that currently have limited treatment options.
That the DDRs are considered promising targets for drug discovery is reflected by a sharp increase in research in this area. Several small molecule kinase inhibitors that were originally developed to target the activity of the Breakpoint Cluster Region-Abelson kinase (BCR-ABL) for the use in myelogenous leukemia, namely imatinib, nilotinib and dasatinib, also potently inhibit DDR activity (Day et al., 2008; Rix et al., 2007). However, these drugs have a broad specificity and are also active against a number of additional kinases. Recently, two groups reported optimised orally bioavailable DDR1 kinase inhibitors, with selectivity over DDR2 (Gao et al., 2013; Kim et al., 2013). For DDR2, there are currently no such compounds described but two naturally occurring products, actinomycin D and a product from a marine-derived Bacillus Hunanensis strain, are potent DDR2 inhibitors (Hu et al., 2013; Siddiqui et al., 2009). However, whether these compounds bind directly to DDR2, and if so, to which structural region, as well as other details of their mechanism of action are currently unknown.
7.1. Atherosclerosis models – DDR1
Atherosclerosis is characterised by thickened neointimal lesions in the vessel wall to which SMCs contribute by increased proliferation and migration, as well as MMP and ECM synthesis. Initial studies by Bendeck and colleagues demonstrated that SMC DDR1 plays an important role in the thickening of the intimal layer after vascular injury (Hou et al., 2001). Mice lacking DDR1 were protected from intimal thickening after mechanical carotid injury and showed decreased SMC proliferation, MMP production and ECM synthesis. Furthermore, DDR1 was shown to mediate collagen-dependent SMC migration (Hou et al., 2002) and SMC collagen remodelling in vitro (Ferri et al., 2004), reinforcing a potential role for DDR1 in atherosclerosis. Mice lacking the low-density lipoprotein (LDL) receptor (Ldlr−/− mice) represent a more complex model of human atherosclerosis, taking into account inflammation and lipid infiltration, as observed by progressive accumulation of macrophages in addition to SMSc and lipid-laden foam cells in the intimal areas, thus resembling human atherosclerotic plaques. Studies using the Ldlr−/− mouse model showed DDR1 to be critical in atherosclerotic plaque development by promoting both inflammation and fibrosis in early plaque formation (Franco et al., 2009; Franco et al., 2008) and to play a role in atherosclerotic plaque progression and complications such as calcifications (Ahmad et al., 2009; Franco et al., 2010). In contrast to such a prominent and multifaceted role in atherosclerosis for DDR1, DDR2 does not appear to be involved in the disease pathology, as suggested from in vitro experiments that found no role for DDR2 in SMC proliferation, migration or matrix remodelling (Hou et al., 2012). However, DDR2 is found in atherosclerotic plaques (Ferri et al., 2004) and at present it cannot be ruled out that DDR2 has a role in atherosclerosis in vivo.
7.2. Kidney disease models – DDR1
In keeping with its role in maintaining glomerular architecture, DDR1 has been shown to be a regulator of kidney disease. Hypertension-induced renal disease often leads to chronic renal failure. Using a mouse model, Flamant et al. showed that Ddr1−/− mice are protected against hypertension-induced kidney disease and that DDR1 mediates both inflammation and fibrosis in this pathology (Flamant et al., 2006). A similar pathophysiological role for DDR1 was found in another kidney disorder, Alport syndrome, a hereditary collagen IV disorder that leads to progressive kidney fibrosis and end stage renal failure. Loss of DDR1 expression in Col4a3−/− mice, which serve as a model for progressive renal scarring, led to improved lifespan, kidney function and reduced inflammation and fibrosis (Gross et al., 2010). Since DDR1 is expressed in glomerular epithelial cells, the interaction of DDR1 with glomerular basement membrane collagen IV could be important in the progression of kidney fibrosis in Alport syndrome. However, this mechanism has not yet been demonstrated experimentally.
Consistent with a pathogenic role for DDR1 in renal disease, Ddr1−/− mice were protected from crescentic glomerulonephritis (Kerroch et al., 2012) and obstructive nephropathy (Guerrot et al., 2011). In both pathologies, DDR1 mediated inflammatory responses and fibrosis. In humans, certain single nucleotide polymorphisms of DDR1 are associated with susceptibility and disease progression of childhood nephropathy (Hahn et al., 2010), suggesting a role for DDR1 in the development and progression of this disorder. In summary, several studies have highlighted DDR1 as a key mediator of the initiation and progression of inflammatory renal disorders. Since DDR1 function is not required for normal renal physiology, pharmacological targeting of DDR1 in nephropathies with inflammatory responses has therapeutic potential.
7.3. Fibrotic diseases – DDR1 and DDR2
Both DDRs are thought to play pathogenic roles in organ fibrosis, in particular DDR1 in lung fibrosis and DDR2 in liver fibrosis, disorders for which effective drug treatment options are currently lacking. Bleomoycin-induced lung fibrosis is a widely used mouse model for human idiopathic pulmonary fibrosis. Ddr1−/− mice were largely protected from bleomycin-induced lung injury, and similar to the situation with chronic renal disease, DDR1 mediated both inflammation and fibrosis in this model (Avivi-Green et al., 2006). DDR1 is expressed in bronchial epithelium in human lung tissue, and in vitro experiments are consistent with a role for DDR1 in epithelial repair (Roberts et al., 2011). Thus DDR1 may be important in modulating idiopathic pulmonary fibrosis, which is characterised by persistent epithelial injury. DDR2 may also contribute to lung fibrosis, given that collagen I, derived from lung epithelial cells in response to bleomycin injury, was shown to activate fibroblast expressed DDR2 (Yang et al., 2013).
The role of DDR2 in liver disease is more complex. In contrast to Ddr1−/− mice, which are largely protected from chronic fibrosis of the lung, vascular or renal tissue, Ddr2−/− mice are not protected but more susceptible to inflammation and fibrosis, compared with wild-type mice, under conditions of chronic liver injury (Olaso et al., 2011a). This suggests that DDR2 protects the liver from fibrosis in chronic injury. On the other hand, in acute liver injury, DDR2 mediates hepatic stellate cell activation, proliferation and invasion, processes which are deemed to be a profibrotic response (Olaso et al., 2001). In rats, DDR2 expression is enhanced in experimental alcoholic liver fibrosis and associated with enhanced collagen deposition and matrix remodelling (Luo et al., 2013; Zhang et al., 2010). Silencing DDR2 expression decreased alcohol-induced liver injury and fibrosis in a model for early stage alcoholic liver disease (Luo et al., 2013). The collective findings suggest that DDR2 may play a pathogenic role in early stages of liver fibrosis, and a different, if not opposing role, in later stages of chronic liver disease.
7.4. Arthritis models and patient studies – DDR2
Osteoarthritis (OA), is a condition for which currently only ineffective drug regimes exist that provide pain relief but do not modify disease progression (Pulsatelli et al., 2013). DDR2 may be a promising new drug target for OA. Evidence supporting this notion comes from studies using mouse models of the disease, as well as from human patient material. Several studies by Li and co-workers defined upregulation of DDR2 expression in chonodrocytes, which leads to increased expression of MMP-13, a key event linked to early OA pathogenesis. MMP-13 is a critical enzyme that contributes to the degeneration of cartilage in OA by cleaving collagen and aggrecan. Increased DDR2 and MMP-13 expression were found in the joints of different OA mouse models, including models for genetic forms of OA and a model of surgically induced OA (Hu et al., 2006; Xu et al., 2007; Xu et al., 2005). Furthermore, increased DDR2 and MMP-13 expression were also observed in cartilage from patients with OA (Sunk et al., 2007; Xu et al., 2007). These findings are backed up by a study from another lab: Holt et al. found similar early changes, namely upregulation of DDR2 and MMP-13, in a different mouse model of OA, the heterozygous sedc mouse (Holt et al., 2012). Thus, it appears that DDR2 overexpression is one of the key early changes that is common to many different forms of OA. Because reduction of DDR2 expression in mouse models of OA attenuates cartilage degeneration (Xu et al., 2010), DDR2 is considered an attractive target in OA treatment.
What triggers DDR2 upregulation in OA in the first place is not entirely clear, but it is hypothesised that during the early phases of OA chondrocytes interact with native collagen II, thereby activating DDR2 signalling which in turn results in both DDR2 overexpression and MMP-13 induction (Xu et al., 2011b). In normal articular cartilage, chondrocytes are surrounded by the pericellular matrix, whereas collagen II fibrils are located in the territorial and interterritorial matrices and thus do not directly interact with chondrocytes. Indeed, conditional overexpression of DDR2 in mature mouse articular cartilage did not lead to MMP-13 expression or OA-like changes, unless surgery was used to destabilise the medial meniscus, in which case accelerated progression to OA was observed, which was linked to DDR2 activation (Xu et al., 2011b). An independent study using in vitro cultures of primary chondrocytes (cells without pericellular matrix) and chondrons (chondrocytes with intact pericellular matrix) showed that the presence of the pericellular matrix prevents collagen-stimulated MMP-13 activation (Vonk et al., 2011). Taken together, these results suggest that DDR2 accelerates progression of cartilage degeneration and hence OA but cannot initiate these changes unless the pericellular matrix is disrupted.
Less is known about the involvement of DDR2 in rheumatoid arthritis (RA). However, DDR2 is highly expressed in synovial fibroblasts from patients with RA, and increased DDR2 expression is linked to increased MMP-13 expression in human RA synovium (Su et al., 2009), indicating that DDR2 may play a similar role in RA progression as in OA pathogenesis.
7.5. Cancer – DDR1 and DDR2
Many cancers are characterised by dysregulated expression and activities of one or more RTKs, whose altered functions can directly contribute to cancer progression. Hence, there are a number of therapeutic strategies that target RTKs (Gschwind et al., 2004). Like many other RTKs, the DDRs play a key role in cancer progression, in part by regulating the interaction of cancer cells with collagens. The reader is directed to a recent comprehensive review that discusses the roles of the DDRs in cancer (Valiathan et al., 2012). In brief, both DDRs are overexpressed in a large number of different types of cancer, ranging from lung, breast, brain, oesophagus, head and neck, liver and prostate cancers to lymphomas and leukaemias (Valiathan et al., 2012). Dysregulated DDR expression has been shown in a number of studies to correlate with unfavourable outcomes for patients, and altered functions of DDR1 and DDR2 likely contribute to tumourigenesis. Moreover, DDR1 can confer resistance to chemotherapy and mediate pro-survival signals in breast cancer and lymphoma cell lines (Cader et al., 2013; Ongusaha et al., 2003) and may be involved in the recurrence of certain types of cancer (Jian et al., 2012). However, the molecular mechanisms underlying the roles of the DDRs in various steps of cancer progression are largely undefined. Below, I discuss the roles of DDRs in lung cancer and a recent study that identified the molecular mechanism leading to DDR2-mediated breast cancer invasion and metastasis.
For lung cancer, there is good evidence that the DDRs play a key role in cancer progression and metastasis, in particular for non-small cell lung carcinomas (NSCLCs) where dysregulated DDR expression, altered phosphorylation and mutations have been reported (Valiathan et al., 2012). DDR1 and DDR2 were both found to be amongst the most highly phosphorylated RTKs in a set of 150 NSCLC tumour samples (Rikova et al., 2007), but the relevance of these findings to NSCLC patients is not clear at present. Overexpression of DDR1 in tumour relative to normal lung tissue has been found in several studies, and all but one of these reports found this to be associated with poor NSCLC prognosis (Ford et al., 2007; Miao et al., 2013; Valencia et al., 2012; Yang et al., 2010). DDR1 mediated cell migration and invasion of NSCLS cell lines in in vitro experiments (Miao et al., 2013; Yang et al., 2010), as well as cell survival, homing and colonisation in a mouse model of human lung cancer metastasis to bone (Valencia et al., 2012). DDR1 is thus a promising molecular target for NSCLC patients with bone metastasis, where its inhibition, in combination with chemotherapy, might provide clinical benefits.
A comprehensive study that analysed the entire tyrosine kinome in squamous cell carcinoma of the lung found somatic DDR2 mutations in a small proportion of tumour samples and cell lines (Hammerman et al., 2011). The mutations were found throughout the entire coding region, located both in the globular domains and the JM regions. Some of the DDR2 missense mutations resulted in an oncogenic gain of function phenotype in in vitro experiments using NIH-3T3 fibroblasts. Squamous cell lines harbouring these mutations were selectively killed by DDR2 knockdown or by treatments with the multi-kinase inhibitor dasatinib (Hammerman et al., 2011). These results indicate that the mutations are potential ‘driver’ mutations and led to the conclusion that DDR2 may provide the first targetable mutations in lung squamous carcinoma. However, the contribution of wild-type DDR2 in these assays and cell lines has not yet been established, and further studies are required to elucidate the oncogenic role of DDR2 in lung squamous carcinoma. Another issue is that the DDR2 mutations identified so far affect so many different amino acids, rather than being confined to a few recurring locations, and the oncogenic potential of most of the mutations is not clear.
A recent study by Zhang et al., on the role of DDR2 in breast cancer, has revealed a correlation between amplified copy number of the DDR2 gene in invasive breast tumours, which occurred in 5% of tumours, and poor patient survival (Zhang et al., 2013). These data are in agreement with another study that found an association between increased DDR2 expression in breast tumours, relative to matched normal tissue from each patient, and a particularly aggressive subset of breast cancers, as manifested by high degree of lymph node metastases, distant metastases and higher TNM (Tumour, Node, Metastasis) cancer staging (Ren et al., 2013). Zhang et al. were able to identify the molecular mechanism by which DDR2 facilitates breast cancer metastasis: collagen I activates DDR2, which in turn leads to increased ERK2 activation, leading to phosphorylation of the transcription factor SNAIL1, an inducer of EMT. Phosphorylated SNAIL1 accumulates in the nucleus where it is protected from ubiquitylation and subsequent proteasomal degradation. Therefore, DDR2 stabilises SNAIL1, which is critical for breast cancer invasion and migration, as shown by in vitro and in vivo assays (Zhang et al., 2013). In conclusion, the DDRs can regulate different steps of cancer progression, contribute to chemoresistance and cell survival, regulate metastasis of some aggressive forms of cancers and are potentially involved in tumour recurrence. Since dysregulated DDR functions are associated with particularly aggressive types of cancers for which no targeted treatments exist, DDR-selective inhibitors, used in combination therapy, may be a promising avenue in rational cancer therapy. However, since kinase-independent functions have been described for DDR1, in particular in collective cancer cell migration and invasion, which depends on DDR1 (Hidalgo-Carcedo et al., 2011), small molecule inhibitors that block DDR kinase functions may not be effective against all DDR-mediated steps in cancer progression.
8. Conclusions
Since collagens were first identified as ligands for the DDRs, we have gained a good understanding of the structural basis of ligand recognition. We also have gained many insights into the in vivo functions of DDRs and the roles they play in development and disease. However, many mysteries remain about some of the most fundamental DDR characteristics, and compared with most RTK families the DDRs remain under-researched. As outlined above, both DDRs are potential drug targets for a number of human diseases with currently limited treatment options. Successful drug development will require a much deeper understanding of basic DDR biology. Unresolved issues start with a poor understanding of the nature of the DDR ligand in vivo. Can collagen fibers, which are abundant in many tissues, activate the DDRs, or is DDR activation only triggered by isolated collagen triple helices such as might be present when tissues undergo remodelling and repair? Do different ligands (or the physical state of collagen) induce different signalling pathways? If collagen fibres trigger DDR activation, how is unwanted DDR activation controlled? Furthermore, despite crystallographic characterisation of the DDR-collagen interaction, we lack insight into the molecular mechanism of transmembrane signalling. How is the information of ligand binding by the DS domains transmitted across the cell membrane to induce kinase activation? What is the receptor stoichiometry on cellular membranes? Major questions also remain about the mechanism underlying the slow activation kinetics and how cells interpret sustained receptor activation. How is DDR signalling switched off? Are the receptors phosphorylated for sustained periods in vivo?
Our knowledge of DDR-induced signalling pathways still is fragmentary. In particular, we do not know which signalling effectors interact with the phosphorylated receptors and how different effectors are linked to the control of specific cellular functions. The DDRs are at the interface between RTKs and ECM receptors and DDR signalling intersects with signalling pathways triggered by other receptor systems, but again, we have little understanding about the pathways involved and how cells use this receptor cross-talk to fine-tune cellular outcome. Since collagen-independent functions have emerged for DDR1, it is likely that other receptors, perhaps other RTKs, can induce DDR activation, but collagen-independent DDR functions are as yet poorly characterised. Collagen-induced DDR activation is required for normal embryo development and tissue homeostasis, but we have little understanding about how DDRs control matrix remodelling. It is likely that their roles go beyond simply controlling MMP expression and that their physical interaction with collagen may be required to control not only the size or diameter of collagen fibers but also their orientation and alignment (Flynn et al., 2010; Zhang et al., 2013).
New roles for DDRs in disease progression have emerged in recent studies, but the validation of the DDRs as drug targets is still incomplete. In particular, it is not clear for which diseases the DDR kinase activity is essential. Common drugs in targeted RTK therapies use small molecule kinase inhibitors, which would be useless in diseases that are not DDR kinase dependent. Furthermore, since all RTK kinases (and non-receptor kinases) share similar structures, it is very difficult to obtain drugs with high specificity and selectivity. Successful anti-RTK therapies based on monoclonal antibodies against RTK ectodomains are in clinical use (e.g. herceptin/trastuzumab for HER2 positive metastatic breast cancer). Future anti-DDR therapies may involve the generation of blocking antibodies, such as were developed for DDR1 (Carafoli et al., 2012). However, it remains to be seen whether blocking DDR1 antibodies are effective in halting or reversing disease progression in animal models. Another avenue may be the development of therapies that block DDR expression, such as targeted delivery of RNAi-based therapeutics.
Acknowledgments
I thank Erhard Hohenester for critical reading of this manuscript and for providing Figure 2. I acknowledge funding from the Medical Research Council UK (grant G0701121) and the Biotechnology and Biological Sciences Research Council UK (grant BB/I011226/1).
References
- Abbonante V, Gruppi C, Rubel D, Gross O, Moratti R, Balduini A. Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte-collagen interactions. J Biol Chem. 2013;288:16738–16746. doi: 10.1074/jbc.M112.431528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- Afonso PV, McCann CP, Kapnick SM, Parent CA. Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood. 2013;121:1644–1650. doi: 10.1182/blood-2012-08-451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal G, Kovac L, Radziejewski C, Samuelsson SJ. Binding of discoidin domain receptor 2 to collagen I: an atomic force microscopy investigation. Biochemistry. 2002;41:11091–11098. doi: 10.1021/bi020087w. [DOI] [PubMed] [Google Scholar]
- Agarwal G, Mihai C, Iscru DF. Interaction of discoidin domain receptor 1 with collagen type 1. J Mol Biol. 2007;367:443–455. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Ahmad PJ, Trcka D, Xue S, Franco C, Speer MY, Giachelli CM, Bendeck MP. Discoidin domain receptor-1 deficiency attenuates atherosclerotic calcification and smooth muscle cell-mediated mineralization. Am J Pathol. 2009;175:2686–2696. doi: 10.2353/ajpath.2009.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali BR, Xu H, Akawi NA, John A, Karuvantevida NS, Langer R, Al-Gazali L, Leitinger B. Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients. Hum Mol Genet. 2010;19:2239–2250. doi: 10.1093/hmg/ddq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. Faseb J. 2001;15:1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- Aronson RB, Sinex FM, Franzblau C, Van Slyke DD. The oxidation of protein-bound hydroxylysine by periodate. J Biol Chem. 1967;242:809–812. [PubMed] [Google Scholar]
- Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174:420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen DH, Smithson SF, Borochowitz Z, Belostotsky R, Raas-Rothschild A. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet. 2009;84:80–84. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, Dale TC, Gusterson BA, Crompton MR. Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumours. Oncogene. 1995;10:569–575. [PubMed] [Google Scholar]
- Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Sci. 1998;7:1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadriraju K, Chung KH, Spurlin TA, Haynes RJ, Elliott JT, Plant AL. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials. 2009;30:6687–6694. doi: 10.1016/j.biomaterials.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Borochowitz Z, Langer LO, Jr., Gruber HE, Lachman R, Katznelson MB, Rimoin DL. Spondylo-meta-epiphyseal dysplasia (SMED), short limb-hand type: a congenital familial skeletal dysplasia with distinctive features and histopathology. Am J Med Genet. 1993;45:320–326. doi: 10.1002/ajmg.1320450308. [DOI] [PubMed] [Google Scholar]
- Cader FZ, Vockerodt M, Bose S, Nagy E, Brundler MA, Kearns P, Murray PG. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood. 2013;122:4237–4245. doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, Leitinger B, Hohenester E. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17:1573–1581. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli F, Hohenester E. Collagen recognition and transmembrane signalling by discoidin domain receptors. Biochim Biophys Acta. 2013;1834:2187–2194. doi: 10.1016/j.bbapap.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli F, Mayer MC, Shiraishi K, Pecheva MA, Chan LY, Nan R, Leitinger B, Hohenester E. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;20:688–697. doi: 10.1016/j.str.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The a2 integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Wang BW, Wang DL, Shyu KG. Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochem Biophys Res Commun. 2008;374:662–667. doi: 10.1016/j.bbrc.2008.07.092. [DOI] [PubMed] [Google Scholar]
- Chetoui N, El Azreq MA, Boisvert M, Bergeron ME, Aoudjit F. Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. J Cell Biochem. 2011;112:3666–3674. doi: 10.1002/jcb.23300. [DOI] [PubMed] [Google Scholar]
- Clementz AG, Mutolo MJ, Leir SH, Morris KJ, Kucybala K, Harris H, Harris A. Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS ONE. 2013;8:e72250. doi: 10.1371/journal.pone.0072250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Curat CA, Eck M, Dervillez X, Vogel WF. Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding. J Biol Chem. 2001;276:45952–45958. doi: 10.1074/jbc.M104360200. [DOI] [PubMed] [Google Scholar]
- Curat CA, Vogel WF. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J Am Soc Nephrol. 2002;13:2648–2656. doi: 10.1097/01.asn.0000032419.13208.0c. [DOI] [PubMed] [Google Scholar]
- Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin Domain Receptor 1 Receptor Tyrosine Kinase Induces Cyclooxygenase-2 and Promotes Chemoresistance through Nuclear Factor-{kappa}B Pathway Activation. Cancer Res. 2006;66:8123–8130. doi: 10.1158/0008-5472.CAN-06-1215. [DOI] [PubMed] [Google Scholar]
- Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol. 2008;599:44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103:344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- Di Marco E, Cutuli N, Guerra L, Cancedda R, De Luca M. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J Biol Chem. 1993;268:24290–24295. [PubMed] [Google Scholar]
- Edelhoff S, Sweetser DA, Disteche CM. Mapping of the NEP receptor tyrosine kinase gene to human chromosome 6p21.3 and mouse chromosome 17C. Genomics. 1995;25:309–311. doi: 10.1016/0888-7543(95)80144-b. [DOI] [PubMed] [Google Scholar]
- Faraci-Orf E, McFadden C, Vogel WF. DDR1 signaling is essential to sustain Stat5 function during lactogenesis. J Cell Biochem. 2006;97:109–121. doi: 10.1002/jcb.20618. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Lisman T, Bihan D, Hamaia S, Smerling CS, Pugh N, Konitsiotis A, Leitinger B, de Groot PG, Jarvis GE, Raynal N. Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem Soc Trans. 2008;36:241–250. doi: 10.1042/BST0360241. [DOI] [PubMed] [Google Scholar]
- Favreau AJ, Cross EL, Sathyanarayana P. miR-199b-5p directly targets PODXL and DDR1 and decreased levels of miR-199b-5p correlate with elevated expressions of PODXL and DDR1 in acute myeloid leukemia. Am J Hematol. 2012;87:442–446. doi: 10.1002/ajh.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164:1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger C, Escher C, Schneider D. The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Sci Signal. 2009;2:ra56. doi: 10.1126/scisignal.2000547. [DOI] [PubMed] [Google Scholar]
- Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, Dussaule JC. Discoidin Domain Receptor 1 Null Mice Are Protected against Hypertension-Induced Renal Disease. J Am Soc Nephrol. 2006;17:3374–3381. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- Flynn LA, Blissett AR, Calomeni EP, Agarwal G. Inhibition of collagen fibrillogenesis by cells expressing soluble extracellular domains of DDR1 and DDR2. J Mol Biol. 2010;395:533–543. doi: 10.1016/j.jmb.2009.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96:808–814. doi: 10.1038/sj.bjc.6603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco C, Ahmad PJ, Hou G, Wong E, Bendeck MP. Increased cell and matrix accumulation during atherogenesis in mice with vessel wall-specific deletion of discoidin domain receptor 1. Circ Res. 2010;106:1775–1783. doi: 10.1161/CIRCRESAHA.109.213637. [DOI] [PubMed] [Google Scholar]
- Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circ Res. 2009;105:1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- Franco C, Hou G, Ahmad PJ, Fu EY, Koh L, Vogel WF, Bendeck MP. Discoidin domain receptor 1 (ddr1) deletion decreases atherosclerosis by accelerating matrix accumulation and reducing inflammation in low-density lipoprotein receptor-deficient mice. Circ Res. 2008;102:1202–1211. doi: 10.1161/CIRCRESAHA.107.170662. [DOI] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Fu HL, Sohail A, Valiathan RR, Wasinski BD, Kumarasiri M, Mahasenan KV, Bernardo MM, Tokmina-Roszyk D, Fields GB, Mobashery S, Fridman R. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J Biol Chem. 2013;288:12114–12129. doi: 10.1074/jbc.M112.409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Duan L, Luo J, Zhang L, Lu X, Zhang Y, Zhang Z, Tu Z, Xu Y, Ren X, Ding K. Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem. 2013;56:3281–3295. doi: 10.1021/jm301824k. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ashcraft K, Jahid MJ, April C, Ghajar CM, Ruan J, Wang H, Foster M, Hughes DC, Ramirez AG, Huang T, Fan JB, Hu Y, Li R. Regulation of adipose oestrogen output by mechanical stress. Nat Commun. 2013;4:1821. doi: 10.1038/ncomms2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Muller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179:83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachehouche LN, Chetoui N, Aoudjit F. Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Mol Immunol. 2010;47:1866–1869. doi: 10.1016/j.molimm.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Hahn WH, Suh JS, Cho BS, Kim SD. Linkage and association study of discoidin domain receptor 1 as a novel susceptibility gene for childhood IgA nephropathy. Int J Mol Med. 2010;25:785–791. doi: 10.3892/ijmm_00000405. [DOI] [PubMed] [Google Scholar]
- Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, Brace LE, Woods BA, Lin W, Zhang J, Deng X, Lim SM, Heynck S, Peifer M, Simard JR, Lawrence MS, Onofrio RC, Salvesen HB, Seidel D, Zander T, Heuckmann JM, Soltermann A, Moch H, Koker M, Leenders F, Gabler F, Querings S, Ansen S, Brambilla E, Brambilla C, Lorimier P, Brustugun OT, Helland A, Petersen I, Clement JH, Groen H, Timens W, Sietsma H, Stoelben E, Wolf J, Beer DG, Tsao MS, Hanna M, Hatton C, Eck MJ, Janne PA, Johnson BE, Winckler W, Greulich H, Bass AJ, Cho J, Rauh D, Gray NS, Wong KK, Haura EB, Thomas RK, Meyerson M. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Nanba D, Nakayama H, Inoue H, Fukuda S. Ectodomain shedding and remnant peptide signalling of EGFRs and their ligands. J Biochem. 2011;150:15–22. doi: 10.1093/jb/mvr068. [DOI] [PubMed] [Google Scholar]
- Hilton HN, Stanford PM, Harris J, Oakes SR, Kaplan W, Daly RJ, Ormandy CJ. KIBRA interacts with discoidin domain receptor 1 to modulate collagen-induced signalling. Biochim Biophys Acta. 2008;1783:383–393. doi: 10.1016/j.bbamcr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Holt DW, Henderson ML, Stockdale CE, Farrell JT, Kooyman DL, Bridgewater LC, Seegmiller RE. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis Cartilage. 2012;20:430–439. doi: 10.1016/j.joca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- Hou G, Wang D, Bendeck MP. Deletion of discoidin domain receptor 2 does not affect smooth muscle cell adhesion, migration, or proliferation in response to type I collagen. Cardiovasc Pathol. 2012;21:214–218. doi: 10.1016/j.carpath.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54:2891–2900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- Hu Y, Potts MB, Colosimo D, Herrera-Herrera ML, Legako AG, Yousufuddin M, White MA, Macmillan JB. Discoipyrroles A-D: Isolation, Structure Determination, and Synthesis of Potent Migration Inhibitors from Bacillus hunanensis. J Am Chem Soc. 2013;135:13387–13392. doi: 10.1021/ja403412y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:464–471. doi: 10.1038/nrm1399. [DOI] [PubMed] [Google Scholar]
- Hynes R. Integrins. Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I. Structural basis of the collagen-binding mode of discoidin domain receptor 2. Embo J. 2007;26:4168–4176. doi: 10.1038/sj.emboj.7601833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, Friedman SL, Lin HC. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 2002;277:19206–19212. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- Iwai LK, Chang F, Huang PH. Phosphoproteomic analysis identifies insulin enhancement of discoidin domain receptor 2 phosphorylation. Cell Adh Migr. 2013a;7:161–164. doi: 10.4161/cam.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai LK, Payne LS, Luczynski MT, Chang F, Xu H, Clinton RW, Paul A, Esposito EA, Gridley S, Leitinger B, Naegle KM, Huang PH. Phosphoproteomics of collagen receptor networks reveals SHP-2 phosphorylation downstream of wild-type DDR2 and its lung cancer mutants. Biochem J. 2013b;454:501–513. doi: 10.1042/BJ20121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian ZX, Sun J, Chen W, Jin HS, Zheng JH, Wu YL. Involvement of discoidin domain 1 receptor in recurrence of hepatocellular carcinoma by genome-wide analysis. Med Oncol. 2012;29:3077–3082. doi: 10.1007/s12032-012-0277-x. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A. 1993;90:5677–5681. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001;114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1a) promotes migration of leukocytes in three-dimensional collagen lattices. Faseb J. 2001;15:2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- Kano K, Marin de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol. 2008;22:1866–1880. doi: 10.1210/me.2007-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, Strebhardt K. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993;8:3433–3440. [PubMed] [Google Scholar]
- Kawai I, Matsumura H, Fujii W, Naito K, Kusakabe K, Kiso Y, Kano K. Discoidin domain receptor 2 (DDR2) regulates body size and fat metabolism in mice. Transgenic Res. 2014;23:165–175. doi: 10.1007/s11248-013-9751-2. [DOI] [PubMed] [Google Scholar]
- Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, Rondeau E, Ronco P, Boffa JJ, Chatziantoniou C, Dussaule JC. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J. 2012;26:4079–4091. doi: 10.1096/fj.11-194902. [DOI] [PubMed] [Google Scholar]
- Khosravi R, Sodek KL, Faibish M, Trackman PC. Collagen Advanced Glycation Inhibits Its Discoidin Domain Receptor 2 (DDR2)-Mediated Induction of Lysyl Oxidase in Osteoblasts. Bone. 2014;58:33–41. doi: 10.1016/j.bone.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedzierska A, Smietana K, Czepczynska H, Otlewski J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta. 2007;1774:1069–1078. doi: 10.1016/j.bbapap.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Kim HG, Hwang SY, Aaronson SA, Mandinova A, Lee SW. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem. 2011;286:17672–17681. doi: 10.1074/jbc.M111.236612. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim HG, Tan L, Weisberg EL, Liu F, Canning P, Choi HG, Ezell SA, Wu H, Zhao Z, Wang J, Mandinova A, Griffin JD, Bullock AN, Liu Q, Lee SW, Gray NS. Discovery of a Potent and Selective DDR1 Receptor Tyrosine Kinase Inhibitor. ACS Chem Biol. 2013;8:2145–2150. doi: 10.1021/cb400430t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283:6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580:15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- L’Hote C,G, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. Faseb J. 2002;16:234–236. doi: 10.1096/fj.01-0414fje. [DOI] [PubMed] [Google Scholar]
- Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez AC, Klein R. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lemke G. Structure and expression of the Tyro 10 receptor tyrosine kinase. Oncogene. 1994;9:877–883. [PubMed] [Google Scholar]
- Laval S, Butler R, Shelling AN, Hanby AM, Poulsom R, Ganesan TS. Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell Growth Differ. 1994;5:1173–1183. [PubMed] [Google Scholar]
- Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25:355–364. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344:993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, Kuster B. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics. 2012;75:3465–3477. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KL, Chou CH, Hsieh SC, Hwa SY, Lee MT, Wang FF. Transcriptional upregulation of DDR2 by ATF4 facilitates osteoblastic differentiation through p38 MAPK-mediated Runx2 activation. J Bone Miner Res. 2010;25:2489–2503. doi: 10.1002/jbmr.159. [DOI] [PubMed] [Google Scholar]
- Lu KK, Trcka D, Bendeck MP. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc Pathol. 2011;20:71–76. doi: 10.1016/j.carpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Liu H, Sun X, Guo R, Cui R, Ma X, Yan M. RNA interference against discoidin domain receptor 2 ameliorates alcoholic liver disease in rats. PLoS ONE. 2013;8:e55860. doi: 10.1371/journal.pone.0055860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Marignac VL, Rodrigue A, Davidson D, Couillard M, Al-Moustafa AE, Abramovitz M, Foulkes WD, Masson JY, Aloyz R. The effect of a DNA repair gene on cellular invasiveness: XRCC3 over-expression in breast cancer cells. PLoS ONE. 2011;6:e16394. doi: 10.1371/journal.pone.0016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Gross O, Becker-Lendzian U, Massing T, Vogel WF. Inner ear defects and hearing loss in mice lacking the collagen receptor DDR1. Lab Invest. 2008;88:27–37. doi: 10.1038/labinvest.3700692. [DOI] [PubMed] [Google Scholar]
- Miao L, Zhu S, Wang Y, Li Y, Ding J, Dai J, Cai H, Zhang D, Song Y. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol. 2013;30:626. doi: 10.1007/s12032-013-0626-4. [DOI] [PubMed] [Google Scholar]
- Mihai C, Chotani M, Elton TS, Agarwal G. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol. 2009;385:432–445. doi: 10.1016/j.jmb.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Berk BC. Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- Neuhaus B, Buhren S, Bock B, Alves F, Vogel WF, Kiefer F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol Life Sci. 2011;68:3757–3770. doi: 10.1007/s00018-011-0676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281:22744–22751. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of Discoidin Domain Receptor 2 Promotes Hepatic Fibrosis after Chronic Carbon Tetrachloride through Altered Paracrine Interactions between Hepatic Stellate Cells and Liver-Associated Macrophages. Am J Pathol. 2011a;179:2894–2904. doi: 10.1016/j.ajpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277:3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- Olaso E, Lin HC, Wang LH, Friedman SL. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair. 2011b;4:5. doi: 10.1186/1755-1536-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, Lee SW. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. Embo J. 2003;22:1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JL, Jing SQ, Wong TW. Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines. Oncogene. 1996;12:1469–1477. [PubMed] [Google Scholar]
- Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, Copeland NG, Wong TW. Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain. Oncogene. 1994;9:211–219. [PubMed] [Google Scholar]
- Phan TN, Wong EL, Sun X, Kim G, Jung SH, Yoon CN, Yang BS. Low Stability and a Conserved N-Glycosylation Site Are Associated with Regulation of the Discoidin Domain Receptor Family by Glucose via Post-Translational N-Glycosylation. Biosci Biotechnol Biochem. 2013a;77:1907–1916. doi: 10.1271/bbb.130351. [DOI] [PubMed] [Google Scholar]
- Playford MP, Butler RJ, Wang XC, Katso RM, Cooke IE, Ganesan TS. The genomic structure of discoidin receptor tyrosine kinase. Genome Res. 1996;6:620–627. doi: 10.1101/gr.6.7.620. [DOI] [PubMed] [Google Scholar]
- Poudel B, Ki HH, Lee YM, Kim DK. Induction of IL-12 production by the activation of discoidin domain receptor 2 via NF-kappaB and JNK pathway. Biochem Biophys Res Commun. 2013;434:584–588. doi: 10.1016/j.bbrc.2013.03.118. [DOI] [PubMed] [Google Scholar]
- Pulsatelli L, Addimanda O, Brusi V, Pavloska B, Meliconi R. New findings in osteoarthritis pathogenesis: therapeutic implications. Ther Adv Chronic Dis. 2013;4:23–43. doi: 10.1177/2040622312462734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin Domain Receptor-1a (DDR1a) Promotes Glioma Cell Invasion and Adhesion in Association with Matrix Metalloproteinase-2. J Neurooncol. 2006;76:239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- Ren T, Zhang J, Liu X, Yao L. Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol. 2013;30:397. doi: 10.1007/s12032-012-0397-3. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Magowan L, Hall IP, Johnson SR. Discoidin domain receptor 1 regulates bronchial epithelial repair and matrix metalloproteinase production. Eur Respir J. 2011;37:1482–1493. doi: 10.1183/09031936.00039710. [DOI] [PubMed] [Google Scholar]
- Roig B, Moyano S, Martorell L, Costas J, Vilella E. The Discoidin domain receptor 1 gene has a functional A2RE sequence. J Neurochem. 2012;120:408–418. doi: 10.1111/j.1471-4159.2011.07580.x. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Jarai G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem. 2011;286:12912–12923. doi: 10.1074/jbc.M110.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ WP, Engelman DM. TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc Natl Acad Sci U S A. 1999;96:863–868. doi: 10.1073/pnas.96.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S, Saya H, Tada M, Nakao M, Fujiwara T, Roth JA, Sawamura Y, Shinohe Y, Abe H. Receptor protein tyrosine kinase DDR is up-regulated by p53 protein. FEBS Lett. 1996;398:165–169. doi: 10.1016/s0014-5793(96)01234-3. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Tapley P, Saini SS, He B, Pulido D, Barbacid M. Multiple tyrosine protein kinases in rat hippocampal neurons: isolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proc Natl Acad Sci U S A. 1994;91:1819–1823. doi: 10.1073/pnas.91.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, Beckebaum S. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang BW, Chang H. Hyperbaric oxygen activates discoidin domain receptor 2 via tumour necrosis factor-alpha and the p38 MAPK pathway to increase vascular smooth muscle cell migration through matrix metalloproteinase 2. Clin Sci. 2009;116:575–583. doi: 10.1042/CS20080215. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang BW, Kuan P, Chang H. RNA interference for discoidin domain receptor 2 attenuates neointimal formation in balloon injured rat carotid artery. Arterioscler Thromb Vasc Biol. 2008;28:1447–1453. doi: 10.1161/ATVBAHA.108.165993. [DOI] [PubMed] [Google Scholar]
- Siddiqui K, Kim GW, Lee DH, Shin HR, Yang EG, Lee NT, Yang BS. Actinomycin D Identified as an Inhibitor of Discoidin Domain Receptor 2 Interaction with Collagen through an Insect Cell Based Screening of a Drug Compound Library. Biol Pharm Bull. 2009;32:136–141. doi: 10.1248/bpb.32.136. [DOI] [PubMed] [Google Scholar]
- Slack BE, Siniaia MS, Blusztajn JK. Collagen type I selectively activates ectodomain shedding of the discoidin domain receptor 1: involvement of Src tyrosine kinase. J Cell Biochem. 2006;98:672–684. doi: 10.1002/jcb.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung YH, Clifford JL, Chung J. Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep. 2010;43:311–318. doi: 10.5483/bmbrep.2010.43.5.311. [DOI] [PubMed] [Google Scholar]
- Staudinger LA, Spano SJ, Lee W, Coelho N, Rajshankar D, Bendeck MP, Moriarty T, McCulloch CA. Interactions between the discoidin domain receptor 1 and b1 integrin regulate attachment to collagen. Biology Open. 2013;2:1148–1159. doi: 10.1242/bio.20135090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Yu J, Ren T, Zhang W, Zhang Y, Liu X, Sun T, Lu H, Miyazawa K, Yao L. Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Mol Cell Biochem. 2009;330:141–152. doi: 10.1007/s11010-009-0127-0. [DOI] [PubMed] [Google Scholar]
- Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226:3422–3432. doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, Xu L, Li Y. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56:3685–3692. doi: 10.1002/art.22970. [DOI] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, Hollier BG, Ram PT, Lander ES, Rosen JM, Weinberg RA, Mani SA. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsoeld T, Park JO, Hutter H. Discoidin domain receptors guide axons along longitudinal tracts in C. elegans. Dev Biol. 2013;374:142–152. doi: 10.1016/j.ydbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Torban E, Goodyer P. The kidney and ear: emerging parallel functions. Annu Rev Med. 2009;60:339–353. doi: 10.1146/annurev.med.60.052307.120752. [DOI] [PubMed] [Google Scholar]
- Valencia K, Ormazabal C, Zandueta C, Luis-Ravelo D, Anton I, Pajares MJ, Agorreta J, Montuenga LM, Martinez-Canarias S, Leitinger B, Lecanda F. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18:969–980. doi: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kilsdonk JW, van Kempen LC, van Muijen GN, Ruiter DJ, Swart GW. Soluble adhesion molecules in human cancers: sources and fates. Eur J Cell Biol. 2010;89:415–427. doi: 10.1016/j.ejcb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Vogel W, Brakebusch C, Fassler R, Alves F, Ruggiero F, Pawson T. Discoidin domain receptor 1 is activated independently of b1 integrin. J Biol Chem. 2000;275:5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514:175–180. doi: 10.1016/s0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology. 2011;50:463–472. doi: 10.1093/rheumatology/keq305. [DOI] [PubMed] [Google Scholar]
- Wall SJ, Werner E, Werb Z, DeClerck YA. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280:40187–40194. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Hsu YM, Tang MJ. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J Cell Physiol. 2005;203:295–304. doi: 10.1002/jcp.20227. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A Discoidin Domain Receptor 1/SHP-2 Signaling Complex Inhibits {alpha}2beta1-Integrin-mediated Signal Transducers and Activators of Transcription 1/3 Activation and Cell Migration. Mol Biol Cell. 2006;17:2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yeh YC, Tang MJ. DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading. Am J Physiol Cell Physiol. 2009;297:C419–429. doi: 10.1152/ajpcell.00101.2009. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu H, Liu X, Deng Y, Sun T, Li F, Ji S, Nie X, Yao L. Functional analysis of discoidin domain receptor 2 in synovial fibroblasts in rheumatoid arthritis. J Autoimmun. 2002;19:161–168. doi: 10.1006/jaut.2002.0606. [DOI] [PubMed] [Google Scholar]
- Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B. Discoidin domain receptors promote alpha1beta1- and alpha2beta1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS ONE. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011a;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol. 2011b;179:1338–1346. doi: 10.1016/j.ajpath.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, Li Y. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62:2736–2744. doi: 10.1002/art.27582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Li Q, Ren S, Lu X, Fang L, Zhou W, Zhang F, Xu F, Zhang Z, Zeng R, Lottspeich F, Chen Z. Proteomic, functional and motif-based analysis of C-terminal Src kinase-interacting proteins. Proteomics. 2009;9:4944–4961. doi: 10.1002/pmic.200800762. [DOI] [PubMed] [Google Scholar]
- Yang J, Wheeler SE, Velikoff M, Kleaveland KR, Lafemina MJ, Frank JA, Chapman HA, Christensen PJ, Kim KK. Activated Alveolar Epithelial Cells Initiate Fibrosis through Secretion of Mesenchymal Proteins. Am J Pathol. 2013;183:1559–1570. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J Biol Chem. 2005;280:39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS, Chung MJ. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24:311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J Cell Physiol. 2009;218:146–156. doi: 10.1002/jcp.21578. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Wu CC, Wang YK, Tang MJ. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell. 2011;22:940–953. doi: 10.1091/mbc.E10-08-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida D, Teramoto A. Enhancement of pituitary adenoma cell invasion and adhesion is mediated by discoidin domain receptor-1. J Neurooncol. 2007;82:29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8:2731–2739. [PubMed] [Google Scholar]
- Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell BIol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Yan M, Liu L, Wu TJ, Ma LL, Wang LX. Expression of discoidin domain receptors (DDR2) in alcoholic liver fibrosis in rats. Arch Med Res. 2010;41:586–592. doi: 10.1016/j.arcmed.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su J, Yu J, Bu X, Ren T, Liu X, Yao L. An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. J Bone Miner Res. 2011;26:604–617. doi: 10.1002/jbmr.225. [DOI] [PubMed] [Google Scholar]