Abstract
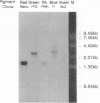
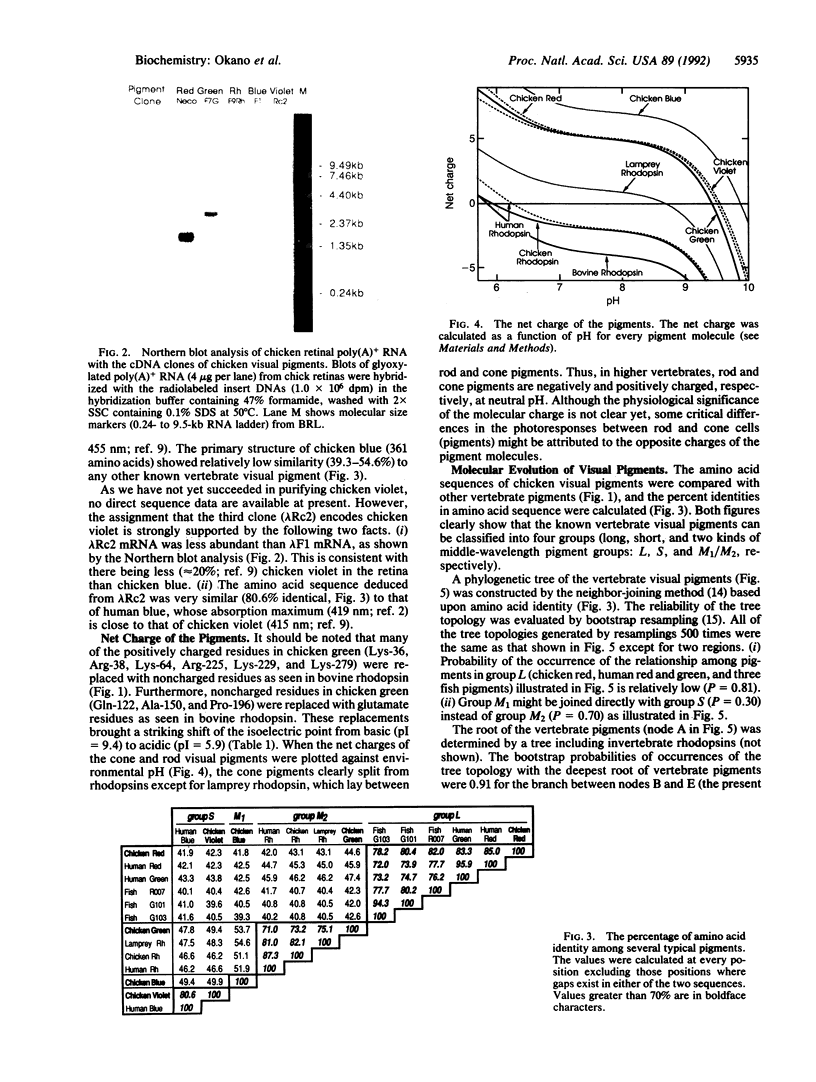
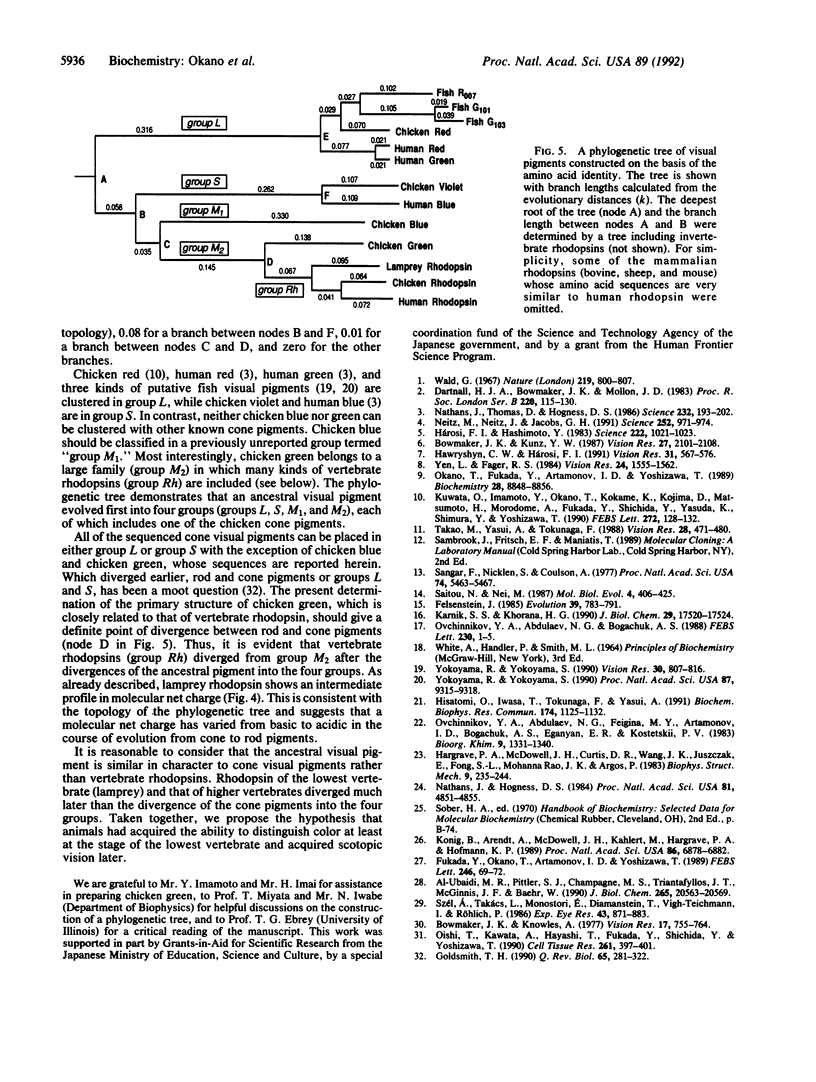
The chicken retina contains rhodopsin (a rod visual pigment) and four kinds of cone visual pigments. The primary structures of chicken red (iodopsin) and rhodopsin have been determined previously. Here we report isolation of three cDNA clones encoding additional pigments from a chicken retinal cDNA library. Based on the partial amino acid sequences of the purified chicken visual pigments together with their biochemical and spectral properties, we have identified these clones as encoding the chicken green, blue, and violet visual pigments. Chicken violet was very similar to human blue not only in absorption maximum (chicken violet, 415 nm; human blue, 419 nm) but also in amino acid sequence (80.6% identical). Interestingly, chicken green was more similar (71-75.1%) than any other known cone pigment (42.0-53.7%) to vertebrate rhodopsins. The fourth additional cone pigment, chicken blue, had relatively low similarity (39.3-54.6%) in amino acid sequence to those of the other vertebrate visual pigments. A phylogenetic tree of vertebrate visual pigments constructed on the basis of amino acid identity indicated that an ancestral visual pigment evolved first into four groups (groups L, S, M1, and M2), each of which includes one of the chicken cone pigments, and that group Rh including vertebrate rhodopsins diverged from group M2 later. Thus, it is suggested that the gene for scotopic vision (rhodopsin) has evolved out of that for photopic vision (cone pigments). The divergence of rhodopsin from cone pigments was accompanied by an increase in negative net charge of the pigment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowmaker J. K., Knowles A. The visual pigments and oil droplets of the chicken retina. Vision Res. 1977;17(7):755–764. doi: 10.1016/0042-6989(77)90117-1. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K., Kunz Y. W. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): age-dependent changes. Vision Res. 1987;27(12):2101–2108. doi: 10.1016/0042-6989(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Dartnall H. J., Bowmaker J. K., Mollon J. D. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Okano T., Artamonov I. D., Yoshizawa T. Chicken red-sensitive cone visual pigment retains a binding domain for transducin. FEBS Lett. 1989 Mar 27;246(1-2):69–72. doi: 10.1016/0014-5793(89)80255-8. [DOI] [PubMed] [Google Scholar]
- Goldsmith T. H. Optimization, constraint, and history in the evolution of eyes. Q Rev Biol. 1990 Sep;65(3):281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hawryshyn C. W., Harosi F. I. Ultraviolet photoreception in carp: microspectrophotometry and behaviorally determined action spectra. Vision Res. 1991;31(3):567–576. doi: 10.1016/0042-6989(91)90107-g. [DOI] [PubMed] [Google Scholar]
- Hisatomi O., Iwasa T., Tokunaga F., Yasui A. Isolation and characterization of lamprey rhodopsin cDNA. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1125–1132. doi: 10.1016/0006-291x(91)91537-m. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I., Hashimoto Y. Ultraviolet visual pigment in a vertebrate: a tetrachromatic cone system in the dace. Science. 1983 Dec 2;222(4627):1021–1023. doi: 10.1126/science.6648514. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Khorana H. G. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990 Oct 15;265(29):17520–17524. [PubMed] [Google Scholar]
- Kuwata O., Imamoto Y., Okano T., Kokame K., Kojima D., Matsumoto H., Morodome A., Fukada Y., Shichida Y., Yasuda K. The primary structure of iodopsin, a chicken red-sensitive cone pigment. FEBS Lett. 1990 Oct 15;272(1-2):128–132. doi: 10.1016/0014-5793(90)80465-u. [DOI] [PubMed] [Google Scholar]
- König B., Arendt A., McDowell J. H., Kahlert M., Hargrave P. A., Hofmann K. P. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neitz M., Neitz J., Jacobs G. H. Spectral tuning of pigments underlying red-green color vision. Science. 1991 May 17;252(5008):971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- Okano T., Fukada Y., Artamonov I. D., Yoshizawa T. Purification of cone visual pigments from chicken retina. Biochemistry. 1989 Oct 31;28(22):8848–8856. doi: 10.1021/bi00448a025. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Iu A., Abdulaev N. G., Feigina M. Iu, Artamonov I. D., Bogachuk A. S. Zritel'nyi rodopsin. III. Polnaia aminokislotnaia posledovatel'nost' i topografiia v membrane. Bioorg Khim. 1983 Oct;9(10):1331–1340. [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szél A., Takács L., Monostori E., Diamantstein T., Vigh-Teichmann I., Röhlich P. Monoclonal antibody-recognizing cone visual pigment. Exp Eye Res. 1986 Dec;43(6):871–883. doi: 10.1016/0014-4835(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Takao M., Yasui A., Tokunaga F. Isolation and sequence determination of the chicken rhodopsin gene. Vision Res. 1988;28(4):471–480. doi: 10.1016/0042-6989(88)90169-1. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Yen L., Fager R. S. Chromatographic resolution of the rod pigment from the four cone pigments of the chicken retina. Vision Res. 1984;24(11):1555–1562. doi: 10.1016/s0042-6989(84)80005-x. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yokoyama S. Convergent evolution of the red- and green-like visual pigment genes in fish, Astyanax fasciatus, and human. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9315–9318. doi: 10.1073/pnas.87.23.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R., Yokoyama S. Isolation, DNA sequence and evolution of a color visual pigment gene of the blind cave fish Astyanax fasciatus. Vision Res. 1990;30(6):807–816. doi: 10.1016/0042-6989(90)90049-q. [DOI] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Pittler S. J., Champagne M. S., Triantafyllos J. T., McGinnis J. F., Baehr W. Mouse opsin. Gene structure and molecular basis of multiple transcripts. J Biol Chem. 1990 Nov 25;265(33):20563–20569. [PubMed] [Google Scholar]