Abstract
Fluid therapy is perhaps the most common intervention received by acutely ill hospitalized patients; however, a number of critical questions on the efficacy and safety of the type and dose remain. In this review, recent insights derived from randomized trials in terms of fluid type, dose and toxicity are discussed. We contend that the prescription of fluid therapy is context-specific and that any fluid can be harmful if administered inappropriately. When contrasting ‘‘crystalloid vs colloid’’, differences in efficacy are modest but differences in safety are significant. Differences in chloride load and strong ion difference across solutions appear to be clinically important. Phases of fluid therapy in acutely ill patients are recognized, including acute resuscitation, maintaining homeostasis, and recovery phases. Quantitative toxicity (fluid overload) is associated with adverse outcomes and can be mitigated when fluid therapy based on functional hemodynamic parameters that predict volume responsiveness and minimization of non-essential fluid. Qualitative toxicity (fluid type), in particular for iatrogenic acute kidney injury and metabolic acidosis, remain a concern for synthetic colloids and isotonic saline, respectively. Physiologically balanced crystalloids may be the ‘‘default’’ fluid for acutely ill patients and the role for colloids, in particular hydroxyethyl starch, is increasingly unclear. We contend the prescription of fluid therapy is analogous to the prescription of any drug used in critically ill patients.
Keywords: Fluid therapy, Resuscitation, Critical illness, Peri-operative, Toxicity, Saline, Crystalloid, Colloid
Core tip: Fluid therapy is exceedingly common in acutely ill patients; however, numerous questions on the efficacy and safety of fluid therapy in terms of the type and dose remain. Fluid therapy prescription is context-specific and any fluid type can be harmful if administered inappropriately. When considering crystalloids versus colloids, differences in efficacy are modest but the risk of kidney toxicity and bleeding complications with hydroxyethyl starch appear more significant. The differences in chloride load across crystalloid solutions appears to have physiologic and clinically important effects, in particular for contributing to hyperchloremic metabolic acidosis, kidney injury and greater utilization of renal replacement therapy associated with 0.9% saline. Fluid therapy should be viewed as analogous to the prescription of any drug in acutely ill patients.
INTRODUCTION
Intravenous (iv) fluid therapy is one of the most common interventions administered to acutely hospitalized patients; however, a number of fundamental questions about its efficacy and safety remain.
The origins of the administration of iv fluids for acute resuscitation date back to the cholera epidemic of the early 19th century, when Dr. Thomas A Latta first administered a warmed iv solution of “two drachams of muriate, two scruples of carbonate of soda to sixty ounces of water” to combat the profound dehydration in six patients hospitalized at the Leith Infirmary in Scotland[1]. With this non-sterile hypotonic solution, he was able to spare a few moribund patients from refractory hypovolemic shock. Impressive volumes of fluid (over 12 liters in some cases) were required to restore hemodynamics, and as described resulted in “...an immediate return of the pulse, and improvement in the respiration¡ [and in] the appearance of the patient [were] the immediate effects”. Yet, even in 1832, an editorial subsequently published in the Lancet commented that “...the mass of the profession is unable to decide; and thus, instead of any uniform mode of treatment, every town and village has its different system or systems...” and that “...a suitable clinical investigation is required to resolve between such conflicting authorities¡”[2]. As such, after nearly two centuries of advancements in the modern medicine, this editorial seemed to be remarkably familiar in many respects to our current state of knowledge regarding the optimal prescription of fluid therapy for acutely ill patients.
Fluid used in acute resuscitation should be viewed in the same context as any other drug administered to patients. Their prescription is certainly analogous to how drugs are prescribed (Table 1). This is relevant when considering that the vast majority of hospitalized acutely ill patients, including children, will receive iv fluid therapy, usually as some combination of crystalloids, colloids and/or blood products.
Table 1.
Overview of the analogy of prescribing fluid therapy and prescribing a drug
| Steps for prescribing a drug | Prescribing an oral hypoglycemic medication | Prescribing fluid therapy |
| Define the clinical problem | Diabetes mellitus | Hypovolemia or other fluid responsive state |
| Specify the therapeutic objective | Lower blood glucose | Restore absolute/relative fluid deficit |
| Verify the suitability of the drug | Class of oral hypoglycemic agent | Crystalloid, colloid or blood product |
| Write a prescription to start the drug | Order written by MD, verified and dispensed by pharmacy | Order written by MD, verified by pharmacy, blood bank or RN, administered by RN |
| Monitor therapeutic response of the drug | Blood glucose or hemoglobin A1C, evidence of adverse effect/ toxicity | Monitor hemodynamic profile and end-organ perfusion, evidence of dose-response toxicity |
| Write an order to discontinue | Order written by MD, verified by pharmacy | Order written by MD, administered by RN |
Adapted from Raghunathan et al[58].
However, data have supported the notion that the form of fluid therapy prescribed is largely dependent on where medical care is provided (i.e., country, region, hospital, care unit) and on the specialty of the clinician (i.e., surgical, medical, anesthesia, emergency)[3,4]. There is wide variation in clinical practice with respect to the type and dose of fluid prescribed[3]. This variation in practice has historically been derived from a general lack of clarity in the literature on the principles of optimal fluid prescription (i.e., efficacy and safety)-the idea of prescribing fluid therapy for “the right patient, at the right time, and in the right context”. In the last few years, a number of large high-quality randomized trials have reported on the efficacy and safety intravenous fluid therapy for acute resuscitation in the critically ill[4-6]. These data are beginning to provide clarity to long-standing debates regarding fluid type and dose, during and following acute resuscitation and to better inform clinical practice to improve patient outcomes[7]. In this review, we discuss recent relevant evidence related to the type and dose of fluid therapy used in the resuscitation of critically ill patients.
DOSE OF FLUID THERAPY
As aforementioned, iv fluid therapy is one of the most common and certainly may be one of the most important initial interventions in the resuscitation of acute ill patients. A key concept for dosing fluid therapy in critically ill patients is to actively address ongoing losses coupled with constant reassessment of need for further hemodynamic support. The routine practice of providing “maintenance” or replacement of unmeasured fluid deficits such as “third space losses” for most patients is questionable and often contributes unnecessary fluid accumulation. The optimal target endpoints for fluid therapy during resuscitation remain controversial. Recent data suggest static metrics of resuscitation, such as thresholds in central venous pressure (CVP), as currently recommended by the Surviving Sepsis Campaign[8], may not accurately correlate with restoration of intravascular volume and improvement in tissue oxygen delivery and may be associated with worse outcome[9]. Additional measures such as achieving a normalized central venous oxygen saturation (> 65%-70%) and rapid serum lactate clearance (> 20% in 2 h) in response to fluid resuscitation (± additional hemodynamic support) have been recommended and correlate with improve outcome, both of these endpoints also have important caveats to consider[9,10]. Rather, functional hemodynamic measures such as stroke volume variation, pulse pressure variation[11], bedside ultrasonic interrogation of cardiac output or respiratory variation in inferior vena cava diameter and additional novel dynamic metrics such changes in cardiac output associated with passive leg raising, changes in end-tidal CO2 and end-expiratory endotracheal tube occlusion can better predict the hemodynamic response to fluid loading[12-15]. These dynamic measures are superior to blood pressure, CVP, and urine output targets. Importantly, critically ill patients are heterogeneous and may vary considerably with respect to baseline susceptibilities, admission diagnoses and response to fluid loading. When conventional blood pressure or urine output targets are used to guide fluid loading in critically ill patients, often large doses of fluids are administered, and in these circumstances, colloids such as hydroxyethyl starch (HES) are associated with toxicity[7]. The use of fluid boluses in critically ill patients without integrating functional hemodynamic parameters may be associated with cardiovascular decompensation and worse outcome[5]. These observations would strongly support the need for individualized resuscitation goals that integrate functional hemodynamic measures rather than use of generic resuscitation endpoints.
TYPE OF FLUID THERAPY
For a given dose of fluid administered, toxicity may depend on the type and composition of fluid being administered and on patient susceptibilities and physiology. Both patient-specific and context-specific differences should be considered when selecting the type of fluid therapy to be administered.
The debate regarding the relative risks and benefits of colloid and crystalloid solutions has raged on for years. Although various forms of crystalloid solutions have been used in humans since the 1830s, it was approximately 100 years more before the technology to isolate albumin from serum was available. In World War II, fractionated bovine albumin was first used on the battlefield as a resuscitation fluid. Synthetic colloids such as HES and gelatins have until recently been considered reasonable alternatives to albumin, due to their theoretical advantages such as mitigating the infectious risks of human blood products, improving blood rheology and microvascular flow, and modulating neutrophil aggregation. The choice of fluid type; however, has largely been a matter of individual clinician preference rather than being specifically directed by high-quality data from clinical trials.
In 1998, the crystalloid/colloid controversy came to a head with the publication of a systematic review that suggested that the use of human albumin was associated with one additional death for every 17 patients treated[16]. Despite this review having methodological misgivings, a political firestorm ensued when the Cochrane Injuries Group urged politicians to “take action” six weeks before the article was published by the BMJ. Unfortunately, the lay media reported the findings prior to peer review and publication, resulting in statistically-questionable, poorly-supported inflammatory news headlines such as “300 die as health chiefs dither”[17]. The director of the United Kingdom Cochrane Center went so far as to suggest that he would sue any doctor who gave him an infusion of albumin[15,16].
Due to this ongoing narrative, interest in the patterns of clinical use of crystalloid and colloid solutions for fluid resuscitation in the intensive care unit (ICU) has increased. An international study of 391 ICUs across 25 countries observed that colloid therapy was the primary fluid used in 48% of instances for acute resuscitation, whereas crystalloid and blood products were used in 33% and 28% of instances, respectively[3]. However, the variation in the type of fluid administered was six-fold different between countries. These data suggested that local factors, such as “unit protocols” and commercial marketing played an important role in guiding clinicians’ choice of fluid type for resuscitation. These data also recommended better evidence in the form of high-quality randomized trials were needed along with appropriate mechanisms to translate new knowledge from such data into bedside practice.
Several studies have repeated provided a physiological rationale for the preferential use of a colloid (with an emphasis on HES) over crystalloid therapy for resuscitation in septic shock and other in states of acute stress such as peri-operatively. HES solutions have been shown to attenuate the acute inflammatory response[18-21], mitigate endothelial barrier dysfunction and vascular leak[18,22], and preserve intestinal barrier function[17]. Small clinical trials have suggested superiority of HES solutions for resuscitation of the microcirculation in sepsis[22]. Small randomized clinical trials have also shown that early fluid resuscitation with HES solutions results in more rapid hemodynamic stabilization and shock reversal (i.e., greater efficacy) compared with crystalloids, and require significantly less fluid to restore intravascular volume[23,24].
Several more recent randomized trials have specifically evaluated the “colloid/crystalloid” hypothesis for fluid resuscitation in critically ill patients. The SAFE[25] (4% albumin in 0.9% saline vs 0.9% saline), CHEST[6] (6% HES in 0.9% saline vs 0.9% saline) and 6S[7] (6% HES in Ringer’s acetate vs Ringer’s acetate) trials were specifically designed to evaluate the effectiveness of colloids against corresponding crystalloids. These trials have shown that the efficacy of volume expansion of colloids over crystalloids (i.e., the ability to increase plasma volume) is greater for colloids (ratio 1.2-1.4:1 for crystalloid:colloid about 20%-40% enhanced effect with colloids); however, less than conventional teaching and evidence generated in experimental models[5-7,25]. This may be accounted for by the collapse of the classical “Starling model” based understanding of fluid movement across capillary membranes in critically ill states, where vascular endothelia is disrupted and hydrostatic (i.e., systemic venous hypertension/endothelial injury) and oncotic (i.e., hypoproteinemia) forces are deranged. Moreover, this also highlights that these issues are dynamic during the coarse of critical illness and that variable fluid types are expected to have heterogeneous effects that will depend upon: (1) the relative chloride load (i.e., strong ion difference); (2) the presence of colloid (i.e., HES or albumin); and (3) the underlying/evolving severity in patients pathophysiology.
The ideal electrolyte solution is yet undiscovered; however, for resuscitation may be one that reasonably parallels the plasma (chloride) and has a strong ion difference that is greater than zero (0.9% saline) but less than plasma during resuscitation[26]. Clinically important outcomes differ when comparing physiologically balanced crystalloids with isotonic saline solution. In the past year, a study at a single ICU[27] and another in patients undergoing major abdominal surgery[28], compared outcomes based on “chloride load”. Consistent with earlier preclinical and human studies[25-28], chloride restriction was found to be beneficial (Table 2). However, even large volume resuscitation with balanced crystalloid solutions is capable of inducing mild metabolic acidosis due to hemodilution of weak acids and relative changes in strong ion difference. The challenges with many balanced crystalloid solutions is that they contain small concentrations of calcium and additional electrolytes that theoretically increase the risk for precipitation or clot formation during co-administration with citrated blood products when compared with saline. However, 0.9% saline is non-physiologic and the high (chloride) and a lower strong ion difference compared to plasma (0.9% saline: 0 mmol/L vs plasma: 40 mmol/L), directly contributes to iatrogenic hyperchloremic metabolic acidosis. Indeed, the use of balanced crystalloid resuscitation in patients with diabetic ketoacidosis, despite the added (potassium) content [(K+) 5.0 mmol/L], was associated with more rapid correction of base deficit when compared to 0.9% saline[49]. Recent data have also clearly shown high (chloride) solutions contribute to renal vasoconstriction, decreased glomerular filtration, greater interstitial fluid accumulation[29-34] along with increased risk of acute kidney injury (AKI) and utilization of renal replacement therapy (RRT)[35].
Table 2.
Summary of studies comparing isotonic saline to balanced crystalloid solutions
| Study | Design | Population | Solutions | Outcome |
| McFarlane et al[59] | RCT | Elective hepatobiliary/pancreatic surgery | 0.9% saline vs PL-148 | Iatrogenic metabolic acidosis with 0.9% saline |
| Wilkes et al[47] | RCT | Major abdominal surgery | 0.9% saline vs Hartmann's (in HES) | Iatrogenic metabolic acidosis with 0.9% saline |
| O'Malley et al[48] | RCT | Kidney transplant recipients | 0.9% saline vs RL | Iatrogenic metabolic acidosis and hyperkalemia with 0.9% saline |
| Yunos et al[56] | Prospective before-and-after | Critically ill patients | Chloride-rich vs chloride-poor fluid strategy | More acidosis with chloride-rich; more alkalosis and reduced cost with chloride-poor |
| Chowdbury et al[26] | RCT (cross-over) | Healthy volunteers | 0.9% saline vs PL-148 (2 L infusion) | ↑ Δ [Cl-]; ↑ Strong ion difference; ↓ RBF; ↑ weight gain; ↑ extravascular volume; ↑ time to micturation |
| Chua et al[49] | Retrospective | Critically ill with DKA | 0.9% saline vs PL-148 | More rapid resolution of acidosis with PL-148 |
| Shaw et al[55] | Retrospective | Major abdominal surgery | 0.9% saline vs PL-148 | ↑ Major infection; ↑ composite of complications; ↑ blood transfusions; and ↑ RRT with 0.9% saline |
| Yunos et al[57] | Prospective before-and-after | Critically ill patients | Chloride-rich vs chloride-poor fluid strategy | ↑ AKI (KDIGO stage II/III); ↑ RRT with chloride-rich strategy |
Adapted from Raghunathan et al[58]. RCT: Randomized clinical trial; 0.9% saline: Normal saline; PL: Plasmalyte; RL: Ringers lactate; RBF: Renal blood flow; DKA: Diabetic ketoacidosis; AKI: Acute kidney injury; HES: Hydroxyethyl starch; RRT: Renal replacement therapy; KDIGO: Kidney disease improving global outcomes; FO: Fluid overload; FB: Fluid balance.
A CONCEPTUAL FRAMEWORK FOR FLUID MANAGEMENT
A novel conceptual framework for fluid management in critical illness introduces the idea of interrelated phases of fluid management differentiated according to the clinical status of the patient with evolving goals for fluid need[33] (Figure 1). The model proposed for the epidemiology of fluid balance in AKI may be extended across the spectrum of critical illness with caveats: (1) In the initial phase of acute resuscitation-the objective is restoration of effective circulating blood volume, organ perfusion and tissue oxygenation. Fluid accumulation and a positive fluid balance may be expected; (2) In the second phase of resuscitation-the goal is maintenance of intravascular volume homeostasis. The objective during this phase is to prevent excessive fluid accumulation and avoid unnecessary fluid loading; and (3) In the final stage, the objective centers around fluid removal and the concept of active “de-resuscitation” corresponding to a state of physiologic stabilization, organ injury recovery and convalescence. During this phase, unnecessary fluid accumulation may contribute to secondary organ injury and adverse events.
Figure 1.
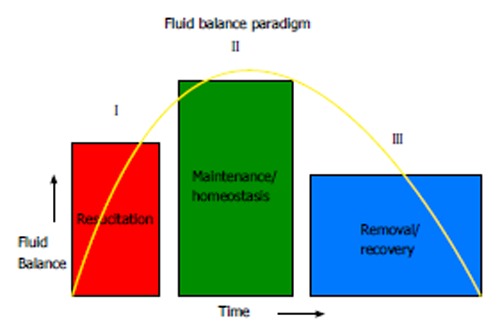
Fluid balance paradigm. The management of fluid therapy in critical illness can be conceptually viewed across three broad phases differentiated according to clinical status of the patient. During the “resuscitation” phase, the goal is restoration of effective intra-vascular volume, organ perfusion and tissue oxygenation. Fluid accumulation and a positive fluid balance may be expected. During the maintenance phase, the goal is maintenance of intravascular volume homeostasis. The broad aim here would be to mitigate excessive fluid accumulation and prevent unnecessary fluid loading. During the recovery phase, passive and/or active fluid removal would correspond to organ recovery.
Numerous studies in peri-operative and critical care settings support this concept of “ebb and flow” in fluid loading, fluid accumulation and removal. Indeed, these phases of resuscitation likely exist on a continuum and the observed variability in fluid balance is understood to be a dynamic process, does not necessarily follow a fixed temporal pattern or time scale and is likely highly individualized. For example, in septic patients with acute lung injury, the balance between early goal-directed therapy aimed at adequate initial fluid resuscitation coupled with downstream diuretic use and “de-resuscitation” (i.e., conservative late fluid management) can improve outcomes[37,38]. Similarly in pediatric septic shock, outcome improved with early appropriate fluid therapy[39]. Such phasic need for fluid and then need for active fluid removal has also been demonstrated in peri-operative settings[40]. Inappropriate fluid therapy, regardless of fluid type, may disrupt compensatory mechanisms and worsen outcome[5].
QUANTITATIVE TOXICITY
Fluid therapy is a critical aspect of initial acute resuscitation in critically ill patients. Following the acute resuscitative phase (i.e., achievement of immediate resuscitation goals and after hemodynamic stabilization), excessive fluid accumulation has been associated with worse clinical outcome, across a range in clinical settings, particularly in AKI[37] (Table 3). In patients with sepsis-associated AKI, continued fluid loading in the setting of apparent optimal systemic hemodynamics was shown not to improve kidney function, but worsen lung function and oxygenation[30]. Similar observational data in critically ill adults with sepsis-associated AKI has found fluid accumulation to be a predictor of 60-d mortality (HR = 1.21/L per 24 h, 95%CI: 1.13-1.28, P < 0.001)[37]. Additionally, although the FACCT trial did not demonstrate a mortality difference between a liberal and a more conservative fluid management strategy in the setting of acute lung injury, the conservative strategy was associated with improved lung function, reduced length of stay in ICU and a trend for lower utilization of RRT[36]. Increasing severity of fluid accumulation among both pediatric and adult patients with AKI at the time of initiation of RRT has been associated with higher mortality and reduced likelihood of recovery of kidney function[42-45]. For each 1% increase in percentage fluid overload (% FO, as calculated below) at RRT initiation, risk of death increased by 3%[31]. % FO = [(total fluid in-total fluid out)/admission body weight × 100].
Table 3.
Studies in critically ill patients describing the association with fluid overload and worse outcome
| Study | Design | Population | Exposures | Outcomes |
| Pediatric Studies | ||||
| Goldstein et al[33] | Retrospective | Pediatric critically ill starting CRRT | % FO | ↑ % FO associated with ↑ mortality |
| Foland et al[60] | Retrospective | Pediatric critically ill starting CRRT | % FO | ↑ % FO associated with ↑ organ dysfunction + mortality |
| Sutherland et al[31] | Retrospective | Pediatric critically ill starting CRRT | % FO | ↑ % FO associated with ↑ mortality |
| Arikan et al[30] | Retrospective | Pediatric critically ill starting CRRT | % FO | ↑ % FO associated with ↓ lung function |
| Adult Studies | ||||
| Payen et al[61] | Post-hoc prospective | Adult critically ill septic patients | FB | ↑ FB associated with ↑ mortality |
| Murphy et al[62] | Retrospective | Adult critically ill ALI patients | AIFR + CLFM | ↑ Survival for ↑ AIFR + ↑ CLFM |
| Bouchard et al[63] | Post-hoc prospective | Adult critically ill AKI patients | % FO > 10% | ↑ FB associated with ↑ mortality |
| Wiedemann et al[36] | RCT | Adult critically ill with ALI | Conservative vs liberal fluid management strategy | ↑ MV-free days; ↑ ICU-free days with conservative strategy |
| Fulop et al[64] | Retrospective | Adult critically ill starting CRRT | VRWG | ↑ VRWG associated with ↑ mortality |
| Boyd et al[65] | Post-hoc analysis from VASST | Adult critically ill septic patients | Quartiles of FB + CVP at 12 h and 4 d | ↑ FB at 12 h and 4 d associated with ↑ mortality; CVP < 8 at 12 h ↓ mortality |
| Grams et al[66] | Post-hoc FACCT | Adult critically ill with ALI + AKI | FB + diuretics | ↑ FB associated with ↑ mortality |
| Heung et al[67] | Retrospective | Adult critically ill starting CRRT | % FO | ↑ % FO associated with ↓ kidney recovery |
| Bellomo et al[68] | Post-hoc RENAL | Adult critically ill with AKI | FB | ↑ FB associated with ↑ mortality |
Adapted from Raghunathan et al[58]. ALI: Acute lung injury; AIFR: Adequate initial fluid resuscitation; CLFM: Conservative late fluid management; VRWG: Volume-related weight gain; AKI: Acute kidney injury; CVP: Central venous pressure; ICU: Intensive care unit; RCT: Randomized clinical trial.
Failure to appreciate these phases of fluid management following resuscitation may underscore the observed phenomenon of “fluid creep”, first identified in the burn literature in response to the overwhelming enthusiasm for aggressive and sustained fluid resuscitation[29,32]. These observations highlight the importance of monitoring fluid balance in critical illness, in particular after the initial phase of resuscitation, where obligatory fluid intake (i.e., medications, nutrition, blood products) may greatly exceed output (i.e., relative oliguria), leading to rapid fluid accumulation[34]. In these circumstances, there should be effort to minimize or avoid all non-essential fluid administration. However, data on fluid accumulation in critically ill patients is almost entirely post-hoc, associative and not causal. Very few prospective interventional studies, with the exception of the FACCT trial and selected studies of conservative peri-operative fluid regimens have informed on the optimal fluid management strategies for critically ill patients and evaluated their association with organ function, adverse events, and survival[35,36]. This represents an important knowledge gap in our understanding of how to optimally management fluid beyond the initial resuscitation for acutely ill patients.
QUALITATIVE TOXICITY
Colloid solutions
The saline vs albumin fluid evaluation (SAFE) trial, in which nearly 7000 critically ill patients were randomized to either 4% human albumin or saline for resuscitation was the first large scale high-quality trial to show no overall difference in mortality, ICU length of stay, need for mechanical ventilation or RRT, or hospital length of stay. However, subgroup analyses founds trends for higher mortality in trauma patients, predominantly with head injury (OR = 1.36, P = 0.06) and lower mortality in sepsis (OR = 0.87, P = 0.09)[37]. Subsequently, a post-hoc longer-term follow-up study of patients enrolled in the SAFE trial who had suffered traumatic brain injury was performed, confirming the initial trends to suggest a higher mortality in head-injured patients receiving albumin (OR = 1.88, P < 0.001)[38].
While HES solutions, including newer starches, appear equally or more efficacious (vs older starches or crystalloids in certain situations) for restoration of intravascular volume in acute resuscitation, data continues to accumulate to suggest harm in critical illness (Table 4). Small clinical trials have suggested HES solutions are also superior for resuscitation of the microcirculation in sepsis and contribute to more rapid hemodynamic stabilization and shock reversal, and require significantly less fluid to restore intravascular volume. There has been suggestion of an improved safety profile for HES solutions with a lower molecular weight and lower degree of molar substitution, in terms of bleeding complications and AKI; however, these findings have been inconsistent. Prior to VISEP, 6S and CHEST, the literature had largely been dominated by small lower quality randomized trials that precluded a clear appraisal of potential survival benefit and the risk of toxicity[39,40]. In addition, wide scale retractions have followed reporting of fraud in research evaluating the safety of HES[41,46]. Accrued data from large randomized trials have now raised serious concerns about potential for dose-associated kidney toxic effects of HES[5,6,42,43]. Experimental data have shown even newer generation HES solutions can still accumulate in tissues within hours of administration, including in the liver, kidney, lung, spleen and lymph nodes[69]. In the VISEP trial, pentastarch (10% HES 200/0.5) was compared to Ringer’s lactate for fluid resuscitation in ICU[42]. The trial was stopped early due to the increased incidence of AKI (34.9% vs 22.8%, P = 0.001) and a trend towards increased mortality (41% vs 33.8%, P = 0.09). These results were corroborated in the CHEST and 6S trials[5,6]. The CHEST trial evaluated the use of Voluven® (6% HES 130/0.4 in 0.9% saline) compared to 0.9% saline for acute resuscitation in ICU[5]. While there was no difference in mortality, there was an increase in the utilization of RRT (7.0% vs 5.8%, P = 0.04) in those receiving HES. In the 6S trial, Tetraspan® (6% HES 130/0.42 in Ringer’s acetate) was compared to Ringer’s lactate for acute resuscitation in severe sepsis[7]. Both the incidence of AKI (22% vs 16%, P = 0.04), and mortality (51% vs 43% at 90 d, P = 0.03) were significantly higher with HES. These data imply an increased risk for harm associated with HES solutions and have lead the European Society of Intensive Care Medicine to recommend against the use of HES in patients with severe sepsis or those at risk for AKI and has further suggested a moratorium on the use of HES except in the context of a clinical trial[49]. In addition, the United States Food and Drug Administration has recently issued black Boxed warning against their use in critically ill patients due to the increased risk of AKI and death[44]. However, there remains continued controversy on whether the use of HES in recent randomized trials was appropriate, such as only being used early and in limited volumes for the acute resuscitation of critically ill hypovolemic patients[45].
Table 4.
Summary of randomized trials of hydroxyethyl starch resuscitation in severe sepsis/septic shock and kidney outcomes
| Ref. | RCT type | n (HES/CON) | Population (n) | HES fluid | Control fluid | Kidney parameters | RRT (OR; 95%CI) |
| Schortgen et al[50] | Multi-centre | 129 (65/64) | Severe sepsis/ septic shock | 6% (200/0.62) | 3% gelatin | ↑ AKI ↑ oliguria, ↑ peak SCr | 1.20 (0.5-2.9) |
| Molnár et al[69] | Single centre | 30 (15/15) | Septic shock | 6% (200/0.60) | 3% gelatin | NR | NR |
| McIntyre et al[70] | Multi-centre | 40 (21/19) | Septic shock | 6% (200/0.50) | 0.9% NS | No difference | 3.00 (0.3-31.6) |
| Brunkhorst et al[42] | Multi-centre | 537 (262/275) | Severe sepsis/septic shock | 10% (200/0.5) | RL | ↑ AKI | 1.95 (1.3-2.9) |
| Guidet et al[23] | Multi-centre | 196 (100/96) | Severe sepsis/septic shock | 6% (130/0.4) | 0.9% NS | No difference | NR |
| Perner et al[6] | Multi-centre | 798 (398/400) | Severe sepsis/septic shock | 6% (130/0.42) | Ringer’s acetate | ↑ AKI | 1.35 (1.01-1.8) |
| Myburgh et al[5] | Multi-centre | 7000 (3315/3336) | Sepsis (27.4%) (1921/7000) | 6% (130/0.4) | 0.9% NS | ↑ RRT | 1.21 (1.00-1.45) |
RCT: Randomized clinical trial; HES: Hydroxyethyl starch; CON: Control; NS: Normal saline; RL: Ringer’s lactate; AKI: Acute kidney injury; RRT: Renal replacement therapy; NR: Not report.
All HES solutions are carried in crystalloid. In the 6S trial, both arms received balanced crystalloid solution (i.e., Ringer’s acetate); whereas in the CHEST trial, both groups received 0.9% saline. It is biologically plausible there may be considerable interaction between the adverse effects of HES and the chloride-rich 0.9% saline. When considering high chloride load is associated with adverse effects and worse outcome, it is therefore plausible that the harm associated with HES is exaggerated when used with 0.9% saline compared with a balanced crystalloid carrier.
In the 6S trial[6], patients were less likely to achieve shock reversal (i.e., failure to clear lactate); whereas, in the CHEST trial[5], shock was reversed (i.e., lactate cleared) with less total fluid administered in the HES group. These data imply that while HES may be more efficacious for shock resolution when compared to crystalloid; if there is delayed or failure to reverse shock, there may be greater toxicity and harm associated with HES; and this hazard may not be immediately apparent (i.e., risk of harm is delayed several days to weeks).
The use of hyperoncotic colloid solutions for acute resuscitation remains controversial. In a large multi-centre European study of 822 critically ill adults with shock receiving fluid resuscitation, use of hypertonic natural and synthetic colloids was associated with a several fold increased risk for AKI and death[50]. A recent systematic review found divergent findings for use of hyperoncotic colloids for resuscitation and subsequent risk of AKI[51]. In this meta-analysis of 7 trials including 1220 patients, hyperoncotic albumin was associated with reduced risk (OR = 0.24, P < 0.001); whereas hyperoncotic HES solutions were associated with increased risk for AKI (OR = 1.92; 95%CI: 1.31-2.81, P < 0.001). These data seem to further infer the kidney toxicity may be a class effect associated with HES solutions.
Crystalloid solutions
The iv solution used in 1832 by Dr Thomas Latta for the treatment of cholera would today be considered a balanced salt solution: 134 mmol/L Na+, 118 mmol/L Cl-, 16 mmol/L HCO3-[52]. Surprisingly, it was not until 1888 that a reference to normal or physiologic saline is found in the medical literature, and not until 1896 that 0.9% saline is described[53,54]. Despite the fact that 0.9% sodium chloride is not isotonic to serum, it is believed that in vitro experiments comparing the freezing points of various solutions to serum led to the belief that this solution was “physiologic”. Perhaps it was for simplicities’ sake that solutions containing mixtures of anions were avoided in favor of the addition of table salt to water.
However, data are accumulating to suggest chloride-rich solutions are problematic. As aforementioned, the high (chloride) and a lower strong ion difference compared to plasma (0.9% saline: 0 mmol/L vs plasma: 40 mmol/L), directly contribute to iatrogenic hyperchloremic metabolic acidosis, which may mask, simulate and/or precipitate adverse effects[55,56]. In a randomized crossover trial of healthy volunteers, renal blood flow and renal cortical perfusion decreased significantly following the bolus administration of 2 L of 0.9% saline compared to plasma-lyte 148[30]. The use of chloride-rich solutions in critically ill patients is not only associated with increased costs and laboratory utilization[57], but also increased incidence of AKI and RRT utilization[27].
These observations are supported from a recent interrogation of the Premier perspective comparative database of patients undergoing elective or emergent open general surgical operations evaluating the rate of adverse events associated with receiving either balanced or isotonic saline solutions on the day of surgery[28]. In this study, patients who received exclusively a calcium-free balanced salt solution (plasma-Lyte A or plasma-Lyte 148) were matched on a 3:1 basis with those receiving exclusively 0.9% saline. Although there were no statistically significant differences between the two groups at baseline, the differences in outcome were dramatic: significantly fewer postoperative infections (P = 0.006), less dialysis (P < 0.001), fewer blood transfusions (P < 0.001), fewer electrolyte disturbances (P = 0.046), fewer acidosis investigations (P < 0.001) and interventions (P = 0.02) were all associated with the use of balanced salt solutions compared with 0.9% saline. While these data are not a randomized comparison of balanced vs 0.9% saline solutions, randomized trials are ongoing.
CONCLUSION
Despite its ubiquitous use in critical care, further carefully performed, transparent research on fluid resuscitation in critical illness is desperately needed. Context appears to be crucial when prescribing fluid and any fluid can be harmful if dosed incorrectly. Differences in immediate efficacy between crystalloid and colloid solutions are modest at best, but the differences in longer-term safety appear more significant. Qualitative toxicity for colloids (even with newer lower molecular weight, less substituted HES solutions) and isotonic saline remain a concern. The observed differences in chloride load and strong ion difference in the various crystalloid solutions appear to be clinically important. We contend that physiologically balanced crystalloids may be the best “default” fluid for acutely ill patients, and that the role of colloids is unclear. Optimal dosing of any resuscitation fluid mandates an understanding the dynamic nature of fluid resuscitation, and future investigations will hopefully allow for the development of better tools to guide therapy.
Footnotes
P- Reviewers: Chang HT, Moschovi M S- Editor: Zhai HH L- Editor: A E- Editor: Liu SQ
References
- 1.Lewins R. Injection of saline solutions in extraordinary quantities into the veins in cases of malignant cholera. Lancet. 1832;18:243–244. [Google Scholar]
- 2.The Cases of Cholera Successfully Treated. Lancet. 1832;18:284. [Google Scholar]
- 3.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 5.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 6.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, Madsen KR, Møller MH, Elkjær JM, Poulsen LM, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 7.Myburgh JA. Fluid resuscitation in acute illness--time to reappraise the basics. N Engl J Med. 2011;364:2543–2544. doi: 10.1056/NEJMe1105490. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 12.Levitov A, Marik PE. Echocardiographic assessment of preload responsiveness in critically ill patients. Cardiol Res Pract. 2012;2012:819696. doi: 10.1155/2012/819696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monnet X, Teboul JL. End-tidal carbon dioxide and arterial pressure for predicting volume responsiveness by the passive leg raising test: reply to Piagnerelli and Biston. Intensive Care Med. 2013;39:1165. doi: 10.1007/s00134-013-2920-1. [DOI] [PubMed] [Google Scholar]
- 14.Monnet X, Bleibtreu A, Ferré A, Dres M, Gharbi R, Richard C, Teboul JL. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–157. doi: 10.1097/CCM.0b013e31822f08d7. [DOI] [PubMed] [Google Scholar]
- 15.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 16.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills H. 300 die as health chiefs dither. London: Observer; 1998. [Google Scholar]
- 18.Dieterich HJ, Weissmüller T, Rosenberger P, Eltzschig HK. Effect of hydroxyethyl starch on vascular leak syndrome and neutrophil accumulation during hypoxia. Crit Care Med. 2006;34:1775–1782. doi: 10.1097/01.CCM.0000218814.77568.BC. [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Hu Y, Ding J, Ge Y, Song J, Ai Q, Zhang Z, Xu J. Early treatment with hydroxyethyl starch 130/0.4 causes greater inhibition of pulmonary capillary leakage and inflammatory response than treatment instituted later in sepsis induced by cecal ligation and puncture in rats. Ann Clin Lab Sci. 2007;37:49–56. [PubMed] [Google Scholar]
- 20.Feng X, Liu J, Yu M, Zhu S, Xu J. Protective roles of hydroxyethyl starch 130/0.4 in intestinal inflammatory response and survival in rats challenged with polymicrobial sepsis. Clin Chim Acta. 2007;376:60–67. doi: 10.1016/j.cca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009–1016. doi: 10.1007/BF03018364. [DOI] [PubMed] [Google Scholar]
- 22.Marx G, Pedder S, Smith L, Swaraj S, Grime S, Stockdale H, Leuwer M. Resuscitation from septic shock with capillary leakage: hydroxyethyl starch (130 kd), but not Ringer’s solution maintains plasma volume and systemic oxygenation. Shock. 2004;21:336–341. doi: 10.1097/00024382-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, Forceville X, Feissel M, Hasselmann M, Heininger A, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care. 2012;16:R94. doi: 10.1186/cc11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magder S, Potter BJ, Varennes BD, Doucette S, Fergusson D. Fluids after cardiac surgery: a pilot study of the use of colloids versus crystalloids. Crit Care Med. 2010;38:2117–2124. doi: 10.1097/CCM.0b013e3181f3e08c. [DOI] [PubMed] [Google Scholar]
- 25.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152–F157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. 2007;28:382–395. doi: 10.1097/BCR.0B013E318053D3A1. [DOI] [PubMed] [Google Scholar]
- 30.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 32.Engrav LH, Colescott PL, Kemalyan N, Heimbach DM, Gibran NS, Solem LD, Dimick AR, Gamelli RL, Lentz CW. A biopsy of the use of the Baxter formula to resuscitate burns or do we do it like Charlie did it. J Burn Care Rehabil. 2000;21:91–95. doi: 10.1097/00004630-200021020-00002. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein SL. Fluid Management in Acute Kidney Injury. J Intensive Care Med. 2012:Nov 14; Epub ahead of print. doi: 10.1177/0885066612465816. [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 37.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 38.Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 39.Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S. Fluid resuscitation with 6 % hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;39:558–568. doi: 10.1007/s00134-013-2840-0. [DOI] [PubMed] [Google Scholar]
- 40.Hartog CS, Skupin H, Natanson C, Sun J, Reinhart K. Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses. Intensive Care Med. 2012;38:1258–1271. doi: 10.1007/s00134-012-2614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sessler DI, Kurz A. Departmental and institutional strategies for reducing fraud in clinical research. Anesth Analg. 2012;115:474–476. doi: 10.1213/ANE.0b013e3182580cbb. [DOI] [PubMed] [Google Scholar]
- 42.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 43.Raghunathan K, Shaw A. Hydroxyethyl starch or saline in intensive care. N Engl J Med. 2013;368:774–775. doi: 10.1056/NEJMc1215977. [DOI] [PubMed] [Google Scholar]
- 44.Food and Drug Administration. Hydroxyethyl Starch Solutions: FDA Safety Communication - Boxed Warning on Increased Mortality and Severe Renal Injury and Risk of Bleeding. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm358349.htm.
- 45.Meybohm P, Van Aken H, De Gasperi A, De Hert S, Della Rocca G, Girbes AR, Gombotz H, Guidet B, Hasibeder W, Hollmann MW, et al. Re-evaluating currently available data and suggestions for planning randomised controlled studies regarding the use of hydroxyethyl-starch in critically ill patients - a multidisciplinary statement. Crit Care. 2013;17:R166. doi: 10.1186/cc12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonelli M, Sandroni C. Hydroxyethyl starch for intravenous volume replacement: more harm than benefit. JAMA. 2013;309:723–724. doi: 10.1001/jama.2013.851. [DOI] [PubMed] [Google Scholar]
- 47.Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72:527–33; discussion 534. doi: 10.1016/s0003-4975(01)02745-x. [DOI] [PubMed] [Google Scholar]
- 48.O’Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, Bennett-Guerrero E. A randomized, double-blind comparison of lactated Ringer’s solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–124, table of contents. doi: 10.1213/01.ANE.0000150939.28904.81. [DOI] [PubMed] [Google Scholar]
- 49.Chua HR, Venkatesh B, Stachowski E, Schneider AG, Perkins K, Ladanyi S, Kruger P, Bellomo R. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27:138–145. doi: 10.1016/j.jcrc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Schortgen F, Girou E, Deye N, Brochard L. The risk associated with hyperoncotic colloids in patients with shock. Intensive Care Med. 2008;34:2157–2168. doi: 10.1007/s00134-008-1225-2. [DOI] [PubMed] [Google Scholar]
- 51.Wiedermann CJ, Dunzendorfer S, Gaioni LU, Zaraca F, Joannidis M. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care. 2010;14:R191. doi: 10.1186/cc9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Lazarus-Barlow WS. On the Initial Rate of Osmosis of Blood-Serum with reference to the Composition of “Physiological Saline Solution” in Mammals. J Physiol. 1896;20:145–157. doi: 10.1113/jphysiol.1896.sp000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Churton E. Leeds General Infirmary: A case of scirrhus of the pylorus, with excessive vomiting; repeated intravenous injections of saline solution; remarks. Lancet. 1888;132:620–621. [Google Scholar]
- 55.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, Kellum JA. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 56.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 57.Yunos NM, Kim IB, Bellomo R, Bailey M, Ho L, Story D, Gutteridge GA, Hart GK. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 58.Raghunathan K, Shaw AD, Bagshaw SM. Fluids are drugs: type, dose and toxicity. Curr Opin Crit Care. 2013;19:290–298. doi: 10.1097/MCC.0b013e3283632d77. [DOI] [PubMed] [Google Scholar]
- 59.McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia. 1994;49:779–781. doi: 10.1111/j.1365-2044.1994.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 60.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 61.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 64.Fülöp T, Pathak MB, Schmidt DW, Lengvárszky Z, Juncos JP, Lebrun CJ, Brar H, Juncos LA. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J. 2010;56:333–337. doi: 10.1097/MAT.0b013e3181de35e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 66.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27:956–961. doi: 10.1093/ndt/gfr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, Lo S, McArthur C, McGuiness S, Norton R, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med. 2012;40:1753–1760. doi: 10.1097/CCM.0b013e318246b9c6. [DOI] [PubMed] [Google Scholar]
- 69.Molnár Z, Mikor A, Leiner T, Szakmány T. Fluid resuscitation with colloids of different molecular weight in septic shock. Intensive Care Med. 2004;30:1356–1360. doi: 10.1007/s00134-004-2278-5. [DOI] [PubMed] [Google Scholar]
- 70.McIntyre LA, Fergusson D, Cook DJ, Rankin N, Dhingra V, Granton J, Magder S, Stiell I, Taljaard M, Hebert PC. Fluid resuscitation in the management of early septic shock (FINESS): a randomized controlled feasibility trial. Can J Anaesth. 2008;55:819–826. doi: 10.1007/BF03034053. [DOI] [PubMed] [Google Scholar]


