Abstract
AIM: To investigate the efficacy and safety of ulinastatin for patients with acute lung injury (ALI) and those with acute respiratory distress syndrome (ARDS).
METHODS: A systematic review of randomized controlled trials (RCTs) of ulinastatin for ALI/ARDS was conducted. Oxygenation index, mortality rate [intensive care unit (ICU) mortality rate, 28-d mortality rate] and length of ICU stay were compared between ulinastatin group and conventional therapy group. Meta-analysis was performed by using Rev Man 5.1.
RESULTS: Twenty-nine RCTs with 1726 participants were totally included, the basic conditions of which were similar. No studies discussed adverse effect. Oxygenation index was reported in twenty-six studies (1552 patients). Ulinastatin had a significant effect in improving oxygenation [standard mean difference (SMD) = 1.85, 95%CI: 1.42-2.29, P < 0.00001, I2 = 92%]. ICU mortality and 28-d mortality were respectively reported in eighteen studies (987 patients) and three studies (196 patients). We found that ulinastatin significantly decreased the ICU mortality [I2 = 0%, RR = 0.48, 95%CI: 0.38-0.59, number needed to treat (NNT) = 5.06, P < 0.00001], while the 28-d mortality was not significantly affected (I2 = 0%, RR = 0.78, 95%CI: 0.51-1.19, NNT = 12.66, P = 0.24). The length of ICU stay (six studies, 364 patients) in the ulinastatin group was significantly lower than that in the control group (SMD = -0.97, 95%CI: -1.20--0.75, P < 0.00001, I2 = 86%).
CONCLUSION: Ulinastatin seems to be effective for ALI and ARDS though most trials included were of poor quality and no information on safety was provided.
Keywords: Ulinastatin, Acute lung injury, Acute respiratory distress syndrome, Mortality, Oxygenation index
Core tip: Currently, many studies highlight the advantages of ulinastatin in lung protection, which is likely because acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) share a common pathogenesis with sepsis. We tried to provide more specific evidence on this practice by performing a meta-analysis. In our study (29 clinical trials included), we found that though all the studies were of low quality, ulinastatin might improve oxygenation and mortality and be truly effective in patients with ALI/ARDS.
INTRODUCTION
Ulinastatin, also known as human urinary trypsin inhibitor, can be found in urine, plasma and all organs[1]. It is a glycoprotein marketed as an experimental medication for acute pancreatitis and septic shock in Asia for its involvement in suppressing the systemic inflammation and proteolytic process[2-5]. Currently, many animal studies and clinical trials highlight its advantages in lung protection[6-38], which is likely because acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) share a common pathogenesis with sepsis, which is systemic inflammatory response syndrome. However, it remains uncertain whether ulinastatin can be recommended as a standard medication for ALI and ARDS. Without the support of large-scale, high-quality trials, it is difficult to draw a definite conclusion. Therefore, we perform a systematic review to evaluate the efficacy and safety of ulinastatin for ALI and ARDS to provide more specific evidence.
MATERIALS AND METHODS
Search strategy
We searched the published randomized controlled trials (RCTs) (from 1st January 2006 to 20th August 2012) from eight databases including Pubmed, Medline (Ovid SP), The Cochrane Library, Wanfang Database, China Biology Medicine Database, Chinese Periodical Database, China Knowledge Resource Integrated Database and Chinese Clinical Trial Registry with the following search terms: “Ulinastatin” or “Protease-Inhibitors” or “Glycoprotein” and “Acute Respiratory Distress Syndrome” or “ARDS” or “Acute Lung Injury” or “ALI”. There were no language restrictions on inclusive studies. All potentially relevant papers based on titles and abstracts were retrieved for full text screening. We also collected relevant articles by checking the references of the retrieved papers.
Study selection
Both the study selection (Leng YX, Song YF) and data extraction processes (Leng YX, Yang SG) were performed by two authors independently. Disagreements were resolved by group discussion. Figure 1 showed the flow chart of study selection process.
Figure 1.
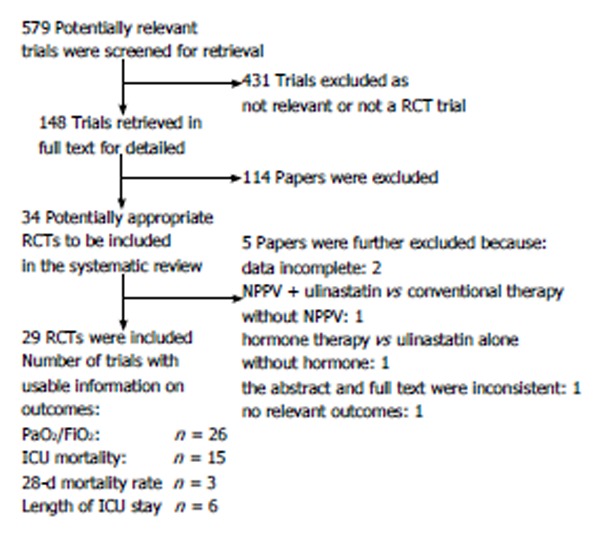
Flow chart of reviewed articles. RCT: Randomized controlled trial; NPPV: Noninvasive positive-pressure ventilation; ICU: Intensive care unit.
We included the RCT studies comparing ulinastatin plus routine treatment (treatment group) versus routine treatment alone or placebo plus routine treatment (control group) for ALI and ARDS. ALI and ARDS were diagnosed as: acute onset; pulmonary artery wedge pressure ≤ 18 mmHg or absence of clinical evidence of left atrial hypertension; bilateral infiltrates on chest radiography; ALI is present if PaO2/FiO2 ratio is ≤ 300; ARDS is present if PaO2/FiO2 ratio ≤ 200. Any dose and duration of ulinastatin were permitted. The outcomes included intensive care unit (ICU) mortality rate or PaO2/FiO2 ratio.
Data extraction and quality assessment
The following parameters were extracted from each inclusive study: (1) first author and year of the publication; (2) patients’ characteristics and study design; and (3) clinical outcomes (ICU mortality, 28-d mortality, PaO2/FiO2 ratio, length of ICU stay and adverse effect). The quality of all selected articles was evaluated according to the Jadad scale[39], which bases on the random assignment, double blinding, and flow of patients. The range of score is 0 (bad) to 5 (good).
Statistical analysis
Meta-analysis was conducted using RevMan 5.1 software. For dichotomous variables (ICU mortality, 28-d mortality) we estimated the pooled risk ratios (RRs) and 95%CI. For continuous variables (PaO2/FiO2 ratio and length of ICU stay), we calculated the estimation of standard mean difference (SMD). Heterogeneity was explored by the I2 test. If I2 < 50%, the fixed-effect model (Mantel-Haenszel) was employed, otherwise the random-effect model (DerSimonian and Laird) was used. The significance of pooled RR was determined by Z test. P < 0.05 was considered statistically significant. Funnel plots were used to detect the potential publication bias if more than ten studies were included. The sensitivity analysis was conducted by taking each single study away from the total and re-analyzing the remainder.
RESULTS
Study characteristics
After full text screening, 34 potentially relevant studies were identified. Among these studies, five were excluded because there were incomplete data (1 study), other interventions besides ulinastatin were included (2 studies), the abstract and full text were inconsistent (1 study), and no relative outcomes were reported (1 study) (Figure 1). Finally, 29 studies involving 1726 participants were included[10-38], the basic conditions of which were similar. The conventional therapy included mechanical ventilation, low dose hormone, nutritional support, treatment of primary diseases, etc. Of the included studies, no one discussed the adverse effect of ulinastatin. Oxygenation index was reported in 26 studies (1552 patients). Eighteen studies (987 patients) and three studies (196 patients) analyzed the ICU mortality and 28-d mortality, respectively. The length of ICU stay was reported in six studies (364 patients). Although all the trials announced the randomization, only four studies mentioned the allocation concealment without detailed description of mechanisms. Table 1 displays the quality and characteristics of these studies.
Table 1.
Quality and characteristics of all included studies
| Ref. | Yr | Jadad score | Design | Sample size | Gender (male/female) | Age (yr, mean or range ) | Dosage | Frequency | Duration (d) | Outcomes |
| Chen et al[10] | 2006 | 1 | NRCT | 70 | 40/30 | 36.6 | 200000 | bid | 2-7 | Oxygenation index |
| Gu et al[11] | 2011 | 1 | NRCT | 120 | 65/55 | 56.2 | 100000 | tid | 5 | Oxygenation index |
| Hu et al[12] | 2009 | 1 | NRCT | 54 | 39/15 | 41.2 | 300000 | tid | 7 | Oxygenation index Length of ICU stay 28-d mortality rate |
| Huang et al[13] | 2010 | 1 | NRCT | 80 | 41/39 | 49 | 100000 | tid | 5 | Oxygenation index Length of ICU stay ICU Mortality rate |
| Jiang et al[14] | 2006 | 1 | NRCT | 57 | 32/25 | 58.1 | 200000 | qd | 7-10 | Oxygenation index ICU Mortality rate |
| Liang et al[15] | 2011 | 1 | NRCT | 62 | 36/26 | 38.8 | 200000 | bid | 7 | Oxygenation index Length of ICU stay |
| Liang et al[16] | 2008 | 1 | NRCT | 76 | 42/34 | 57 | 200000 | bid | 6 | Oxygenation index ICU Mortality rate |
| Lu et al[17] | 2008 | 1 | NRCT | 60 | 42/18 | 39.7 | 50000 | qd | 3 | Oxygenation index |
| Ou et al[18] | 2008 | 1 | NRCT | 36 | 24/12 | 63.7 | 200000-300000 | bid | 5-7 | Oxygenation index ICU Mortality rate Incidence of MODS |
| Pi et al[19] | 2009 | 1 | NRCT | 40 | 25/15 | 37 | 200000- | bid | 5-7 | Incidence of MODS ICU Mortality rate |
| Qian et al[20] | 2009 | 1 | NRCT | 48 | 35/13 | 48 | 200000 | qid | 6 | Oxygenation index ICU Mortality rate Length of ICU stay |
| Qin[21] | 2007 | 1 | NRCT | 60 | 40/20 | 35 | 300000 | bid | 3 | Oxygenation index |
| Shang et al[22] | 2008 | 2 | RCT | 60 | 48/12 | 14-72 | 200000 | tid | 7 | Oxygenation index ICU Mortality rate |
| Shi et al[23] | 2011 | 1 | NRCT | 50 | 34/16 | 59.4 | 300000 | bid | 7-10 | Oxygenation index ICU Mortality rate |
| Wang et al[24] | 2011 | 1 | NRCT | 52 | 32/20 | 55.4 | 200000 | tid | 10 | ICU Mortality rate |
| Wang et al[25] | 2011 | 1 | NRCT | 60 | 44/16 | 18-60 | 200000 | bid | 5 | Oxygenation index |
| Xiang et al[26] | 2011 | 1 | NRCT | 72 | 46/26 | 46.8 | 200000 | tid | 7 | Oxygenation index |
| Xiong[27] | 2008 | 1 | NRCT | 50 | 28/22 | 35 | 300000 | bid | 7 | Oxygenation index |
| Yang et al[28] | 2011 | 1 | NRCT | 40 | NA | NA | 200000 | tid | 10 | Oxygenation index |
| Yang et al[29] | 2006 | 2 | NRCT | 80 | 58/22 | 14-72 | 300000 | bid | 7 | Oxygenation index ICU Mortality rate |
| Zhang et al[30] | 2009 | 1 | NRCT | 34 | 22/12 | 9-61 | 200000 | tid | 10 | Oxygenation index ICU Mortality rate |
| Zhang et al[31] | 2011 | 1 | NRCT | 82 | 43/39 | 18-65 | 200000 | bid | 7 | Oxygenation index 28-d mortality rate |
| Zhang[32] | 2010 | 2 | RCT | 60 | 45/15 | 43.3 | 300000 | bid | 7 | Oxygenation index |
| Zhang et al[33] | 2010 | 1 | RCT | 60 | 30/30 | 55.7 | 500000 | bid | 7 | Oxygenation index Length of ICU stay 28-d mortality rate |
| Zhang et al[34] | 2009 | 1 | NRCT | 61 | 54/7 | 61.9 | 200000 | bid | 7 | Oxygenation index |
| Zhao et al[35] | 2012 | 2 | RCT | 56 | 37/19 | 46.2 | 200000 | bid | 4 | Oxygenation index |
| Zhao et al[36] | 2007 | 1 | NRCT | 37 | 29/8 | 42.6 | 100000 | bid | 5 | Oxygenation index ICU Mortality rate |
| Zheng et al[37] | 2011 | 1 | NRCT | 60 | 42/18 | 40.2 | 50000 | qd | 3 | Oxygenation index ICU mortality rate Length of ICU stay |
| Zhou et al[38] | 2011 | 1 | NRCT | 40 | NA | 40.2 | 600000 | qid | 5 | Oxygenation index ICU Mortality rate |
NA: Not available; NRCT: Non-randomized controlled trial; RCT: Randomized controlled trial; ICU: Intensive care unit.
Oxygenation index
The basal oxygenation indexes in all studies were similar. After treatment with standard strategy or ulinastatin, the patients’ oxygenation indexes were improved in all studies. The effect of ulinastatin was more significant (Figure 2), which was confirmed by the meta-analysis (SMD = 1.85, 95%CI: 1.42-2.29, P < 0.00001, I2 = 92%, Figure 3A).
Figure 2.
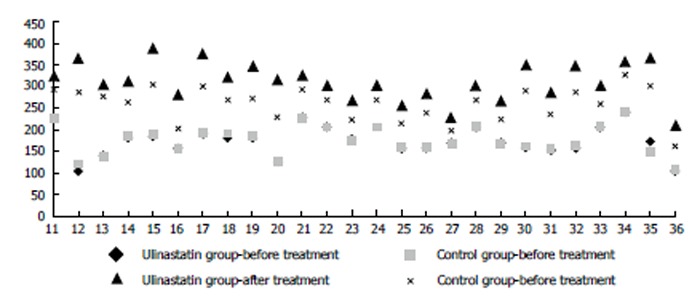
Oxygenation indexes of different groups before and after treatment. The horizontal axis, number of references.
Figure 3.
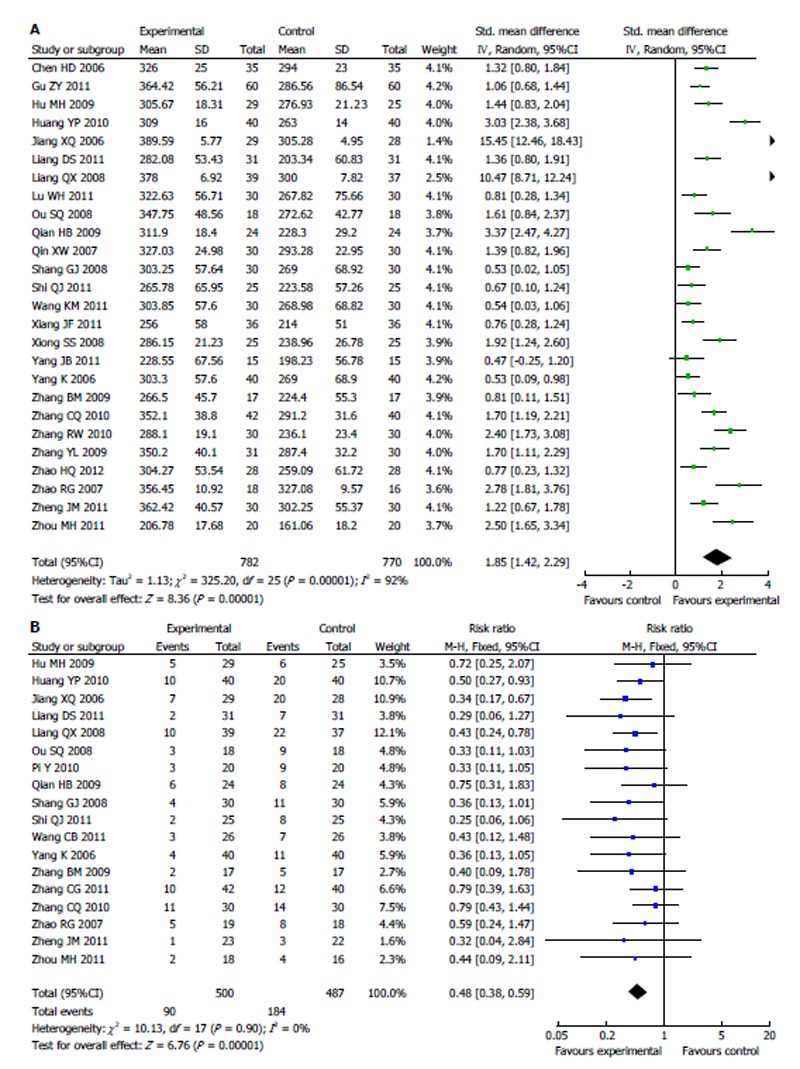
Meta-analysis of patients’ oxygenation index (A) and intensive care unit mortality rate (B) after treatment with conventional therapy vs with ulinastatin (random effects). A: Random effects model; B: Fixed effects model.
Mortality rate
Most studies (15/18) reported that the ICU mortality rate was not significantly different between ulinastatin treatment and conventional treatment. The 95%CI crossed 1.00. Nevertheless, the result of meta-analysis indicated that ulinastatin actually reduced the patients’ ICU mortality rate, and the pooled RR was 0.48 (95%CI: 0.38-0.59, I2 = 0%, Figure 3B). The number needed to treat (NNT) was 5.06. However, the 28-d mortality was not significantly different between the two groups (RR = 0.78, 95%CI: 0.51-1.19, I2 = 0%, Figure 4A), and the NNT was 12.66.
Figure 4.
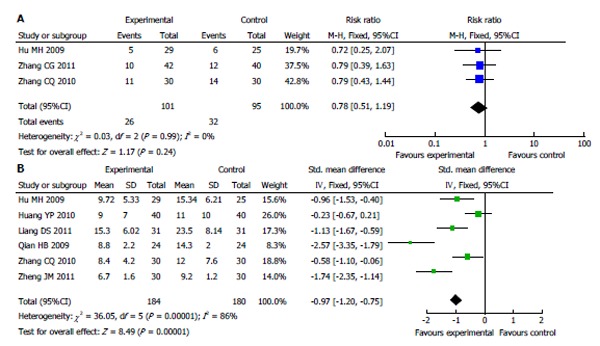
Meta-analysis of 28-d mortality rate (A) and length of intensive care unit stay (B) between treatment with conventional therapy and with ulinastatin. A: Fixed effects model; B: Random effects model.
Length of ICU stay
Five of the six studies reporting the length of ICU stay suggested that compared with conventional therapy, ulinastatin significantly decreased the length of ICU stay, which was confirmed by the result of meta-analysis (SMD = -0.97, 95%CI: -1.20--0.75, P < 0.00001, I2 = 86%, Figure 4B).
Publication bias and sensitivity analysis
Funnel plots of ICU mortality and oxygenation index are shown in Figure 5, which indicated that the publication bias did exist. The language bias may be the main bias because all the inclusive studies were written in Chinese. The sensitivity analysis showed that exclusion of any single study from the meta-analysis did not alter the overall conclusion. Though I2 of the oxygenation index and ICU stay were larger than 50%, we considered that those heterogeneities were probably related to great difference among studies.
Figure 5.
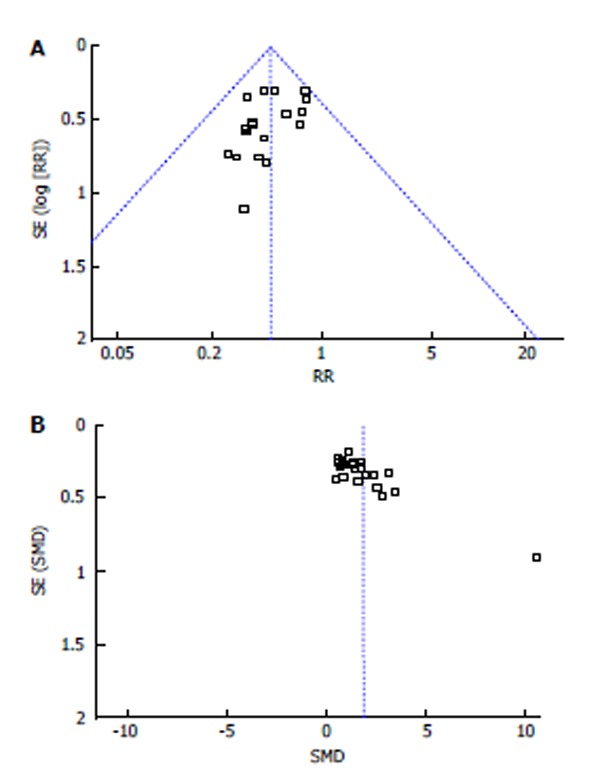
Funnel plots of intensive care unit mortality (A) and oxygenation index (B). SMD: Standard mean difference.
DISCUSSION
ARDS is a common severe lung complication with direct and indirect causes in ICU. In the past 20 years, the mortality rate decreased from 40%-70% to 30%-40%. This survival improvement is considered to be partly related with the better understanding and treatment of sepsis[40]. Since ulinastatin is marketed as an experimental medication for septic shock, the probable efficacy of ulinastatin for ALI and ARDS gains more and more attention.
It is reported that ulinastatin inhibits pathogenic changes in animal models of ALI/ARDS induced by many factors (including scald, seawater, LPS, phosgene)[6-9]. Immunoregulation and the mitigation of excessive inflammatory reaction might be involved. Downregulation of the human major histocompatibility complex class I chain-related antigen A (MICA), mitigation of lipid peroxidation and apoptosis may play important roles. Upregulation of MICA in scald induced lung injury can be ameliorated by ulinastatin[6]. Moreover, ulinastatin treatment can reduce the level of cytokines like serum E, P-selectin and VCAM-1, which are considered to be critical in the development of inflammatory responses[41]. Nevertheless, the effect of ulinastatin on pulmonary injury and the molecular mechanism(s) by which ulinastatin exerts its organ-protective activity remain obscurely studied. In addition, clinical trials also recommended application of ulinastatin for ALI/ARDS though no high quality evidence was reported. Only one meta-analysis on ulinastatin for ALI/ARDS was reported till now[42], in which only Chinese databases were detected. Accordingly, we yet have no enough evidence to support the recommendation of ulinastatin for ALI/ARDS. We performed this meta-analysis to evaluate the existing clinical trials objectively and to provide more specific evidence for ulinastatin selection for ALI/ARDS.
Our results seem to be inspiring. Compared with routine treatment alone, ulinastatin plus routine treatment significantly improved the oxygenation index (SMD = 1.85, 95%CI: 1.42-2.29, P < 0.00001) and reduced the ICU mortality rate (RR = 0.48, 95%CI: 0.38-0.59, NNT = 5.06, P <0.00001) and the length of ICU stay (SMD = -0.97, 95%CI: -1.20--0.75, P < 0.00001). Nevertheless, the validity of this meta-analysis to some extent is limited. No studies reported the adverse effect. Most of the clinical trials were of poor quality without description of randomization and allocation mechanisms. Meanwhile, the language bias is introduced in this review, because all the included trials were published in Chinese. Then, how should we interpret these clinical trials and the systematic review based on these trials Should the clinical practitioners consider ulinastatin as a first-line treatment Obviously, we can not draw a definite conclusion right now. Although ulinastatin seems to be effective for ALI/ARDS, high-quality RCTs discussing the efficacy and safety are needed in the future.
COMMENTS
Background
Ulinastatin is marketed as an experimental medication for septic shock in Asia for its involvement in suppressing the systemic inflammation and proteolytic process. Currently, many studies highlight its advantages in lung protection, which is because acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) share a common pathogenesis with sepsis. However, it remains uncertain whether ulinastatin can be recommended as a standard medication for ALI and ARDS.
Research frontiers
No large-scale randomized controlled trials (RCTs) studies or high quality meta-analysis on ulinastatin for ALI and ARDS were performed till now. Whether the application of ulinastatin in ALI and ARDS is appropriate remains unclear.
Innovations and breakthroughs
To provide more specific evidence for clinical practice, the authors performed a meta-analysis on ulinastatin for ALI and ARDS.
Applications
This study indicated that ulinastatin might be truly effective for ALI and ARDS though most RCT studies included were of poor quality.
Peer review
The authors conducted a systematic review and meta-analysis of the retrieved studies on the effects of ulinastatin on ALI and ARDS. The paper is essentially well written, and provides some information.
Footnotes
P- Reviewers: Chen HI, Pappas KT S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Liu SQ
References
- 1.Pugia MJ, Lott JA. Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med. 2005;43:1–16. doi: 10.1515/CCLM.2005.001. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi H, Kosuzume H, Ashida Y, Kato K, Honjo I. Effects of urinary trypsin inhibitor on pancreatic enzymes and experimental acute pancreatitis. Dig Dis Sci. 1984;29:26–32. doi: 10.1007/BF01296858. [DOI] [PubMed] [Google Scholar]
- 3.Uemura K, Murakami Y, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T. Randomized clinical trial to assess the efficacy of ulinastatin for postoperative pancreatitis following pancreaticoduodenectomy. J Surg Oncol. 2008;98:309–313. doi: 10.1002/jso.21098. [DOI] [PubMed] [Google Scholar]
- 4.Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX, Liu MH. Protective effect of Ulinastatin against murine models of sepsis: inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol. 2012;64:543–547. doi: 10.1016/j.etp.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Takano H. Urinary trypsin inhibitor as a therapeutic option for endotoxin-related inflammatory disorders. Expert Opin Investig Drugs. 2010;19:513–520. doi: 10.1517/13543781003649533. [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Liu Y, Ma L, Wang S. Protective effects of ulinastatin on pulmonary damage in rats following scald injury. Burns. 2012;38:1027–1034. doi: 10.1016/j.burns.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Shen J, Gan Z, Zhao J, Zhang L, Xu G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol Ind Health. 2012:Oct 16; Epub ahead of print. doi: 10.1177/0748233712463776. [DOI] [PubMed] [Google Scholar]
- 8.Rui M, Duan YY, Zhang XH, Wang HL, Wang DP. Urinary trypsin inhibitor attenuates seawater-induced acute lung injury by influencing the activities of nuclear factor-ĸB and its related inflammatory mediators. Respiration. 2012;83:335–343. doi: 10.1159/000333378. [DOI] [PubMed] [Google Scholar]
- 9.Fang Y, Xu P, Gu C, Wang Y, Fu XJ, Yu WR, Yao M. Ulinastatin improves pulmonary function in severe burn-induced acute lung injury by attenuating inflammatory response. J Trauma. 2011;71:1297–1304. doi: 10.1097/TA.0b013e3182127d48. [DOI] [PubMed] [Google Scholar]
- 10.Chen HD, Zhuo Y. The clinical effect of Ulinastatin intravenous for acute lung injury (ALI) Linchuang Feike Zazhi. 2006;11:252–253. [Google Scholar]
- 11.Gu ZY, Tian HY. The influence of Ulinastatin on plasma NO and endothelin-1 in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Zhonghua Linchuang Yishi Zazhi. 2011;5:190–191. [Google Scholar]
- 12.Hu MH, Xu XJ, Jin D, Ji CL, Chen YB, Zhang G. The influence of ulinastatin on endothelial permeability in patients with ARDS. Quanke Yixue Linchuang Yu Jiaoyu. 2009;7:229–231. [Google Scholar]
- 13.Huang YP, Xu XP, Wang XJ, Li MX, Pan RF. Clinical observation on the treatment of acute lung Injury with protective mechanical ventilation in combination with ulinastatin. Hebei Yixue. 2010;16:783–786. [Google Scholar]
- 14.Jiang XQ, Wang YS, Wang SJ, Hu JC, Song DB. The efficacy of ulinastatin on protective ventilated patients with acute lung injury. Zhongguo Jijiu Yixue. 2006;26:161–163. [Google Scholar]
- 15.Liang DS, Du ZL, Zeng H. Clinical studies of 31 cases on the treatment of ulinastatin for patients with acute lung injury. Guangxi Yike Daxue Xuebao. 2011;28:125–126. [Google Scholar]
- 16.Liang QX, Wei GX, Yan XM. The efficacy of ulinastatin with protective ventilation in patients with acute lung injury. Youjiang Yixue. 2008;36:7–9. [Google Scholar]
- 17.Lu WH, Xu XY, Tang ZZ, Chen ZQ, Cheng Q. The efficacy of ulinastatin in patients with traumatic acute lung injury. Linchuang Junyi Zazhi. 2008;36:863–865. [Google Scholar]
- 18.Ou SQ, Yu M, Wen YM, Tao Y. Clinical study on treatment of acute respiratory distress syndrome (ARDS) with ulinastatin. Chongqing Yixue. 2008;37:1336–1337. [Google Scholar]
- 19.Pi Y, Gui WF, Gu XL, Dai LX, Xiao ZY. Clinical study on treatment of ALI/ARDS induced by thoracic trauma with Ulinastatin. Shandong Yiyao. 2009;49:98–99. [Google Scholar]
- 20.Qian HB, Zheng ZQ, Lu JH, Guan GH, Pu QH. Clinical study on therapy of ARDS caused by pulmonary contusion with ulinastatin. Zhongguo Weizhongbing Jijiu Yixue. 2009;21:444–445. [Google Scholar]
- 21.Qin XW. Clinical study of therapy of acute lung injury with ulinastatin. Zhonghua Neike Zazhi. 2007;2:552–553. [Google Scholar]
- 22.Shang GJ, Nie ZX, Wang SZ. Clinical studies of 30 cases on the treatment of ulinastatin for patients with acute lung injury. Zhongwai Yiliao. 2008;30:117–118. [Google Scholar]
- 23.Shi QJ, Yang ZP, Ma SQ. Clinical study on therapy of acute lung injury (ALI) with ulinastatin in XiNing. Qinghai Yiyao Zazhi. 2011;41:5–7. [Google Scholar]
- 24.Wang CB, Tang Y, Li J, Xia CQ. Observation of the efficacy of ulinastatin on acute lung injury/acute respiratory distress syndrome. Zhongguo Yiyao Daobao. 2011;8:71–72. [Google Scholar]
- 25.Wang KM, SunYH , Hou YQ. The clinical observation of ulinastatin for the patients with acute lung injury. Zhongguo Shiyong Yiyao. 2011;6:11–12. [Google Scholar]
- 26.Xiang JF, Yang X, Gong JF. The influence of ulinastatin on respiratory mechanics and oxidative stress in ALI/ARDS patients. Shandong Yiyao. 2011;51:79–80. [Google Scholar]
- 27.Xiong SS. The efficacy of ulinastatin on patients with acute lung injury. Shiyong Linchuang Yixue. 2008;9:34–37. [Google Scholar]
- 28.Yang JB, Zhong ZL, Yang JY, Ye CL. The efficacy of ulinastatin on acute lung injury/acute respiratory distress. Neimenggu Zhongyiyao. 2011;5:82–83. [Google Scholar]
- 29.Yang K, Shen JS, Zhang QS. Clinical study of 40 cases on the treatment of ulinastatin for patients with acute lung injury induced by trauma. Zhongguo Jijiu Yixue. 2006;26:229–230. [Google Scholar]
- 30.Zhang BM, Sun Y, Xu JL, Pan LP. Ulinastatin for treatment of acute lung injury/acute respiratory distress syndrome: an analysis of 34 cases. Bengbu Yixueyuan Xuebao. 2009;34:1108–1110. [Google Scholar]
- 31.Zhang CG, Jiang X, Liu SG. The influence of ulinastatin on oxygenation index and mortality rate in patients with ARDS. Hainan Yixue. 2011;22:8–10. [Google Scholar]
- 32.Zhang RW. The effect of ulinastatin and dexamethasone on patients with traumatic acute lung injury. Zhejiang Chuangshang Waike. 2010;15:283–284. [Google Scholar]
- 33.Zhang CQ, Wang YY, Gao ZZ, Hong F, Nie WQ, Wang LM. The effect of ulinastatin on the prognosis of patiens with ARDS. Zhongguo Linchuang Shiyong Yixue. 2010;4:18–20. [Google Scholar]
- 34.Zhang YL, Pan LW, Zhuang R, Lin MX, Ying BY, Ruan HY. The influence of ulinastatin on matrix metalloproteinase-2 and c-reactive protein in patients wiith traumatic ARDS. Zhejiang Chuangshang Waike. 2009;14:6–8. [Google Scholar]
- 35.Zhao HQ, Lu K, Yuan KW, Dai ZD, Tian JH, Wang CY. The efficacy of ulinastatin on patients with acute cervical spinal cord injury accompanied acute lung injury. Shiyong Yiyao Zazhi. 2012;29:223–224. [Google Scholar]
- 36.Zhao RG, Lin H, Zhang MH. The influence of ulinastatin on the expression of platelet activating factor in patients with acute lung injury (ALI) Zhejiang Linchuang Yixue. 2007;9:439–440. [Google Scholar]
- 37.Zheng JM, Liu DL, Yang LJ. Clinical studies of 30 cases on ulinastatin for patients with acute lung injury. Shanxi Yixue Zazhi. 2011;40:1516–1518. [Google Scholar]
- 38.Zhou MH, Ren GL, Jiao FF. The clinical study of ulinastatin on patients with acute respiratory distress syndrome. Binzhou Yixueyuan Xuebao. 2011;34:122–124. [Google Scholar]
- 39.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 40.Eloise MH, Michael RP. Acute Respiratory Distress Syndrome. Medscape, 2012-03-19. Available from: http: //emedicine.medscape.com/article/165139-overview.
- 41.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32:925–932. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, Li P. Ulinastatin for Acute Lung Injury and Acute Respiratory Distress Syndrome: A Systematic Review. Herald of Med. 2009;28:302–304. [Google Scholar]


