Abstract
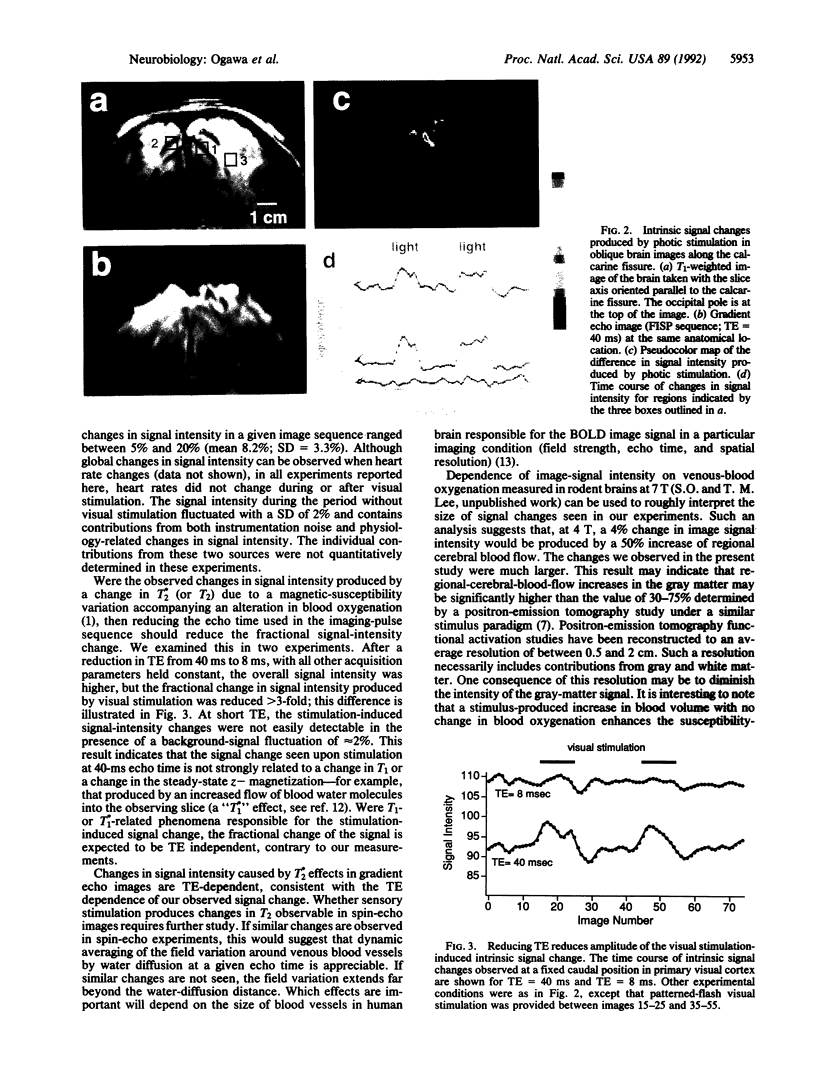
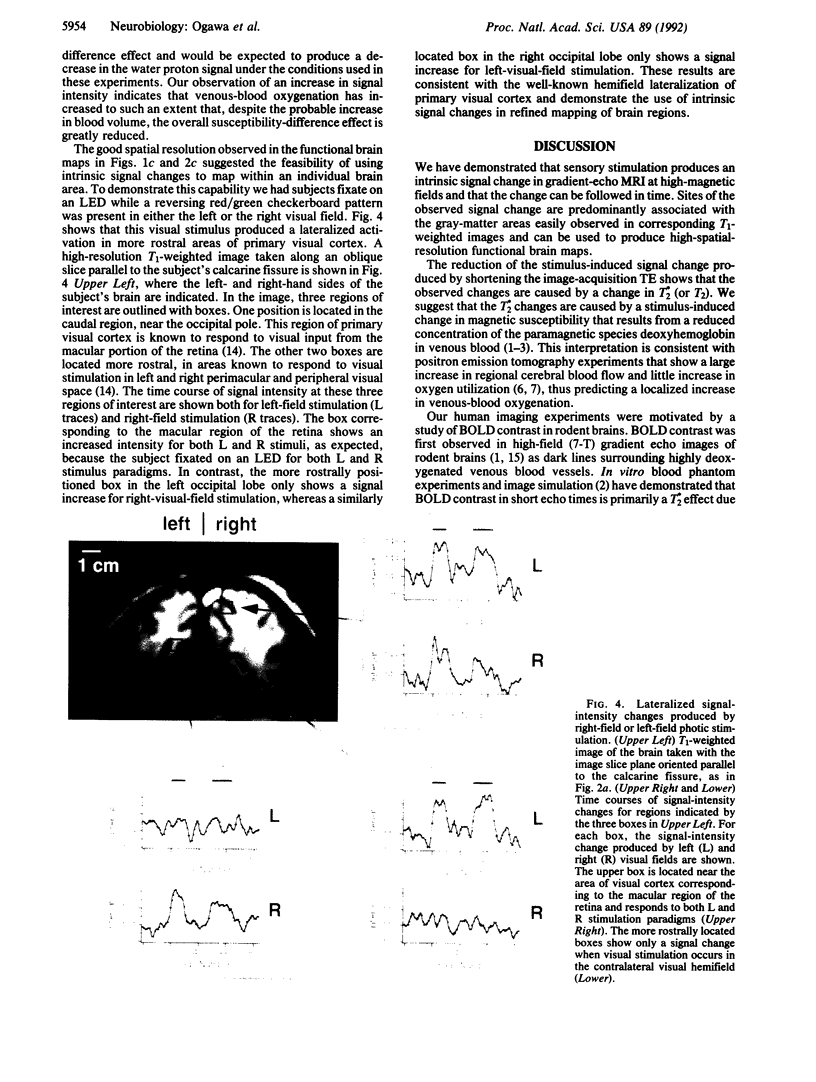
We report that visual stimulation produces an easily detectable (5-20%) transient increase in the intensity of water proton magnetic resonance signals in human primary visual cortex in gradient echo images at 4-T magnetic-field strength. The observed changes predominantly occur in areas containing gray matter and can be used to produce high-spatial-resolution functional brain maps in humans. Reducing the image-acquisition echo time from 40 msec to 8 msec reduces the amplitude of the fractional signal change, suggesting that it is produced by a change in apparent transverse relaxation time T*2. The amplitude, sign, and echo-time dependence of these intrinsic signal changes are consistent with the idea that neural activation increases regional cerebral blood flow and concomitantly increases venous-blood oxygenation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Brooks R. A., Di Chiro G. Magnetic resonance imaging of stationary blood: a review. Med Phys. 1987 Nov-Dec;14(6):903–913. doi: 10.1118/1.595994. [DOI] [PubMed] [Google Scholar]
- Fisel C. R., Ackerman J. L., Buxton R. B., Garrido L., Belliveau J. W., Rosen B. R., Brady T. J. MR contrast due to microscopically heterogeneous magnetic susceptibility: numerical simulations and applications to cerebral physiology. Magn Reson Med. 1991 Feb;17(2):336–347. doi: 10.1002/mrm.1910170206. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Spiller M., Lundbom N. Relaxometry of brain: why white matter appears bright in MRI. Magn Reson Med. 1990 Jun;14(3):482–495. doi: 10.1002/mrm.1910140306. [DOI] [PubMed] [Google Scholar]
- Menon R. S., Allen P. S. Application of continuous relaxation time distributions to the fitting of data from model systems and excised tissue. Magn Reson Med. 1991 Aug;20(2):214–227. doi: 10.1002/mrm.1910200205. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Kay A. R., Tank D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990 Oct;16(1):9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Stehling M. K., Turner R., Mansfield P. Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science. 1991 Oct 4;254(5028):43–50. doi: 10.1126/science.1925560. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Waterton J. C., Matthews P. M., Radda G. K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982 Feb 2;714(2):265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]