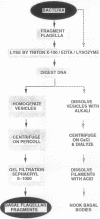
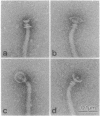
Abstract
We have used electron microscopy to examine freshly isolated Salmonella typhimurium and Escherichia coli basal flagellar fragments, purified without resort to extremes of pH or ionic strength. Such fragments contain the large bell-like basal structures visualized recently in freeze-substituted or fixed preparations. We have found mot (non-motile) mutants produced by lesions in fli genes (G, M, N) in which the bell structures do not coisolate with the flagellar basal body. The coisolation of the bell with the flagellar basal body was unaffected in strains lacking the genes for the motility-associated Mot proteins or for the Che family of proteins, which are necessary for chemotaxis. Proper assembly and interaction of the cytoplasmically located bell with the membrane-associated flagellar basal structures appears to be necessary for motor function. The FliG, FliM, and FliN proteins are thought to form a structural complex responsible for energization and switching of the flagellar motor. Our findings are consistent with the existence of such a complex and imply that it forms part of the flagellar bell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Dean G. E., Jones C. J., Macnab R. M., Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S. Y., Parkinson J. S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988 Jan 15;239(4837):276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G. E., Aizawa S. I., Macnab R. M. flaAII (motC, cheV) of Salmonella typhimurium is a structural gene involved in energization and switching of the flagellar motor. J Bacteriol. 1983 Apr;154(1):84–91. doi: 10.1128/jb.154.1.84-91.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks A., DeRosier D. J. Additional structures associated with bacterial flagellar basal body. J Mol Biol. 1990 Feb 20;211(4):669–672. doi: 10.1016/0022-2836(90)90063-R. [DOI] [PubMed] [Google Scholar]
- Francis N. R., Irikura V. M., Yamaguchi S., DeRosier D. J., Macnab R. M. Localization of the Salmonella typhimurium flagellar switch protein FliG to the cytoplasmic M-ring face of the basal body. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6304–6308. doi: 10.1073/pnas.89.14.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grütter M. G., Hawkes R. B., Matthews B. W. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature. 1979 Feb 22;277(5698):667–669. doi: 10.1038/277667a0. [DOI] [PubMed] [Google Scholar]
- Hjorth R., Pertoft H. Removal of percoll from microsomal vesicles by gel filtration on sephacryl-S-1000 superfine. Biochim Biophys Acta. 1982 May 21;688(1):1–4. doi: 10.1016/0005-2736(82)90570-3. [DOI] [PubMed] [Google Scholar]
- Iino T., Komeda Y., Kutsukake K., Macnab R. M., Matsumura P., Parkinson J. S., Simon M. I., Yamaguchi S. New unified nomenclature for the flagellar genes of Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1988 Dec;52(4):533–535. doi: 10.1128/mr.52.4.533-535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Dapice M., Humayun I. Energy transduction in the bacterial flagellar motor. Effects of load and pH. Biophys J. 1990 Apr;57(4):779–796. doi: 10.1016/S0006-3495(90)82598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Dapice M., Reese T. S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988 Aug 5;202(3):575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Becktel W. J., Matthews B. W. Hydrophobic stabilization in T4 lysozyme determined directly by multiple substitutions of Ile 3. Nature. 1988 Aug 4;334(6181):406–410. doi: 10.1038/334406a0. [DOI] [PubMed] [Google Scholar]
- Meister M., Caplan S. R., Berg H. C. Dynamics of a tightly coupled mechanism for flagellar rotation. Bacterial motility, chemiosmotic coupling, protonmotive force. Biophys J. 1989 May;55(5):905–914. doi: 10.1016/S0006-3495(89)82889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978 Jul;135(1):45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R., Talbert P. B., Houts S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. J Bacteriol. 1983 Jul;155(1):265–274. doi: 10.1128/jb.155.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Sheetz M. P. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991 Dec;115(5):1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S. C., Baeuerlein E. Location of the basal disk and a ringlike cytoplasmic structure, two additional structures of the flagellar apparatus of Wolinella succinogenes. J Bacteriol. 1992 Jan;174(1):263–268. doi: 10.1128/jb.174.1.263-268.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Flattening and shrinkage of bacteriophage T7 after preparation for electron microscopy by negative staining. J Ultrastruct Res. 1977 Mar;58(3):235–243. doi: 10.1016/s0022-5320(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Steuer E. R., Schroer T. A. The mechanism and regulation of fast axonal transport. Trends Neurosci. 1989 Nov;12(11):474–478. doi: 10.1016/0166-2236(89)90099-4. [DOI] [PubMed] [Google Scholar]
- Shortle D. Probing the determinants of protein folding and stability with amino acid substitutions. J Biol Chem. 1989 Apr 5;264(10):5315–5318. [PubMed] [Google Scholar]
- Sockett H., Yamaguchi S., Kihara M., Irikura V. M., Macnab R. M. Molecular analysis of the flagellar switch protein FliM of Salmonella typhimurium. J Bacteriol. 1992 Feb;174(3):793–806. doi: 10.1128/jb.174.3.793-806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky G. E., Francis N. R., Stallmeyer M. J., DeRosier D. J. Substructure of the flagellar basal body of Salmonella typhimurium. J Mol Biol. 1992 Jan 5;223(1):171–184. doi: 10.1016/0022-2836(92)90724-x. [DOI] [PubMed] [Google Scholar]
- Stolz B., Berg H. C. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J Bacteriol. 1991 Nov;173(21):7033–7037. doi: 10.1128/jb.173.21.7033-7037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan M. A. Electron microscopic observations of structures associated with the flagella of Spirillum volutans. J Bacteriol. 1985 Mar;161(3):1137–1145. doi: 10.1128/jb.161.3.1137-1145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. L., Macnab R. M. Co-overproduction and localization of the Escherichia coli motility proteins motA and motB. J Bacteriol. 1990 Jul;172(7):3932–3939. doi: 10.1128/jb.172.7.3932-3939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. L., Macnab R. M. Overproduction of the MotA protein of Escherichia coli and estimation of its wild-type level. J Bacteriol. 1988 Feb;170(2):588–597. doi: 10.1128/jb.170.2.588-597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Berg H. C. Acetyladenylate plays a role in controlling the direction of flagellar rotation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6711–6715. doi: 10.1073/pnas.85.18.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Aizawa S., Kihara M., Isomura M., Jones C. J., Macnab R. M. Genetic evidence for a switching and energy-transducing complex in the flagellar motor of Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1172–1179. doi: 10.1128/jb.168.3.1172-1179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]