Abstract
Objective:
Deep venous thrombosis (DVT) and pulmonary embolus (PE) are serious problems for patients admitted to the hospital with stroke, subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH) and transient ischemic attack (TIA). The purpose of this paper is to further understand the factors that place certain patients at increased risk of DVT/PE.
Methods:
At a 600 bed hospital, a retrospective analysis of data from 2613 patients admitted with a diagnosis of stroke, SAH, ICH or TIA in the time range 1/2008 through 3/2012 was carried out. The data was taken from the hospital’s Get with the Guidelines database and included 28 variables. These included initial NIH stroke scale, length of stay, heart failure, ambulatory by day 2 after admission, altered mental status,and renal failure among others. Multiple analyses were carried out to determine whether there were univariable or multivariable effects of any of the factors on the risk for DVT/PE.
Results:
The risk of DVT/PE was highest in patients with SAH and ICH and smallest with TIA. Multivariable analyses were performed and revealed only altered level of consciousness or heart failure as significant risks for DVT/PE. With the limited available data, administration of subcutaneous heparin or other chemoprophylaxis did not reduce the risk of DVT/PE.
Conclusion:
Although many of the variables used to describe the stroke patient are correlated, in multivariable analyses only heart failure and altered level of consciousness were important risk factors for DVT/PE. The risk of DVT/PE was 7 fold greater in patients in patients with both of these risk factors.
Keywords: Stroke, deep venous thrombosis, pulmonary embolus, risk factors.
INTRODUCTION
Deep venous thrombosis (DVT) and pulmonary embolus (PE) occur in up to 3-8.6% of patients hospitalized with stroke [1, 2]. This is a very serious issue in stroke care since 26% of patients with untreated PE will have a subsequent fatal embolic event [3]. Despite the frequency and serious nature of these complications, there is much controversy about diagnosis and treatment [3-6]. Although universal prophylaxis of patients with ischemic stroke using subcutaneous heparin or low molecular weight heparin has been recommended in patients where the risk of hemorrhage is low [6, 7], at least some guidelines have suggested that this treatment may not be effective [3, 8] or may lead to increased risk of hemorrahge [9]. The evidence and recommendations regarding the prophylactic treatment in patients with intracranial hemorrhage is even more confusing [5]. Part of the issue is the heterogeneity of patients not only in regard to the severity, size and location of the lesion but also medical issues. The purpose of this study is to further understand the factors that place a patient with either stroke or intracranial hemorrhage at risk for DVT/PE. This is an important step that may, in the future, allow patients to be classified into subgroups in which prophylactic therapy has the greatest value.
MATERIALS AND METHODOLOGY
As part of an IRB approved protocol, a retrospective analysis of data entered prospectively by the stroke program coordinators at the Department of Neuroscience was under-taken. The hospital is a 591 bed tertiary referral hospital. A total of 2613 patients admitted with stroke, transient ischemic attack (TIA) or intracranial hemorrhage from 1/2008 to 3/2012 were analyzed retrospectively. The data set included 28 variables including: diagnosis (TIA, stroke, subarachnoid hemorrhage, intracerebral hemorrhage), length of stay, age, race, height, weight and gender. It also included whether the patient had: afib/aflutter, heart failure, prior TIA, coronary artery disease, diabetes, hypertension, peripheral vascular disease, smoking, carotid stenosis, hyperlipidemia, prior stroke, or prosthetic heart valve. There was no additional information categorizing the severity of heart failure or coronary artery disease. The database also included the initial NIH stroke scale at admission and discharge, whether the patient had weakness, aphasia, altered level of consciousness, or was ambulatory by day 2. All of the NIH stroke scale values were obtained by providers certified in administering the scale. It also included whether patient received subcutaneous heparin, low molecular weight heparin, serial compression devices, or coumadin. Data regarding the particular type of chemoprophylaxis, dosing and timing was not available in that database. In addition, the database contained no information about the results of any possible hypercoagulable state or any systemic inflammatory response that might be evidenced in such laboratory values as the C-reactive protein.
All data was entered and double checked by nurse practitioners (KA and KM) specializing in stroke and included all patients admitted to the hospital whether they were in the intensive care unit or any service within the hospital.
There was no routine screening program for DVT/PE. In all cases the initial diagnosis was made on clinical grounds and confirmed with appropriate imaging.
Statistics
The initial statistical analysis was the computation of multiple univariate analyses of effects of each variable on the risk of DVT/PE. For factors that had more than 5 values or were continuous, Student’s t-test is used and the corresponding p value is tabulated. For variables that had less than 5 or fewer levels, a contingency table is constructed and analyzed using both the chi-square statistic and, for the 2x2 tables, Fisher’s exact test. In addition the relative risk of DVT/PE when the factor is positive is computed along with its 95% confidence interval using SPSS (IBM, Armonk NY). The DVT/PE rate are computed in each diagnosis group and compared using the chi-square test. All of the univariate analyses performed on the full patient group were repeated separately in each diagnosis group. An additional two level variable hemorrhage was created that took on the value of 1 in patients where there was an intracerebral hemorrhage and 0 for patients with subarachnoid hemorrhage. As multiple testing is performed, the Bonferroni correction was used to calculate the p value required for statistical significance. As there were 28 separate tests performed, this p value was chosen as 0.05/28=0.002.
Subsequently three multivariable tests are carried out. First, a forward stepwise logistic regression analysis with DVT/PE as the dependent variable. Independent variables were all variables with p<0.05 in the univariate analysis were the starting set entered. P=0.01 was used to enter variables and p=0.05 was used to remove variables from this analysis. A linear discriminant analysis using the same set of variables is also carried out. Variables were entered into the multivariable analyses only if the p value in the univariate analysis was less than 0.10. Finally a 2x2x2 crosstabulation table is created from the two independent variables that have significant effects in both of the multivariable analyses and DVT/PE. This is analyzed using the chi-square statistic.
RESULTS
A total of 33 patients had a diagnosis of DVT/PE and so the overall rate of DVT/PE was 1.3%. Of the 33 patients 25 had only DVT, 3 had only PE and 5 had both.
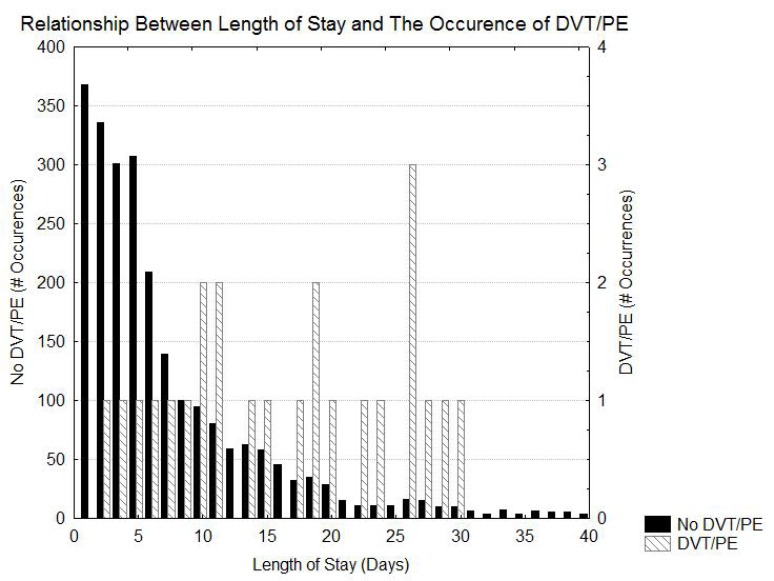
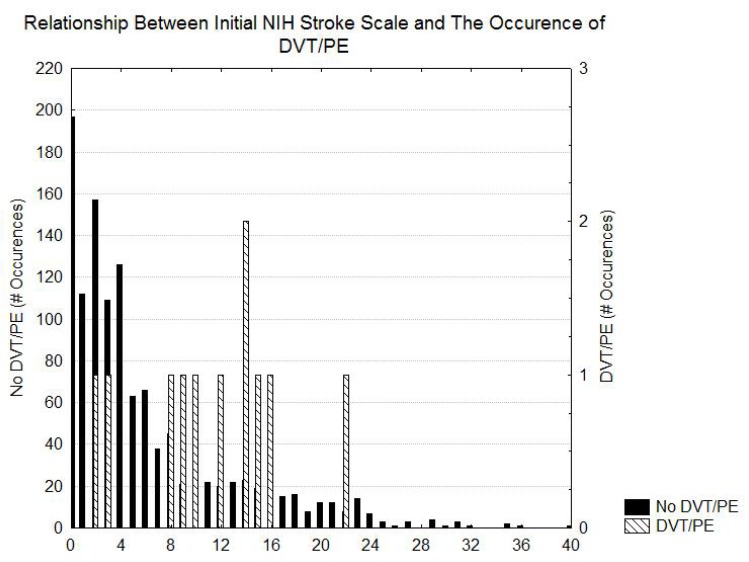
Table 1 shows the risk of DVT/PE as a function of the diagnosis. The greatest risk of DVT/PE is in patients that had SAH and ICH. Patients with ischemic stroke had intermediate risks of DVT/PE and the patients with TIA had the lowest risk of DVT/PE. Table 2 indicates that prolonged length of stay and possibly higher initial NIH stroke scale values, Heart failure, ambulatory by day2, weight, and altered level of consciousness were associated with an increased risk for DVT/PE. Figs. (1 and 2) demonstrate that, although the patients with DVT/PE generally have higher values of both length of stay and initial NIH stroke scale, many patients with longer length of stays and higher initial NIH stroke scales did not have DVT/PE.
Table 1.
Risk of DVT/PE for the different diagnoses. The relative risk of DVT/PE is normalized so that the risk for the stroke patients is 1.0.
| Diagnosis | #Patients Without DVT/PE | #Patients With DVT/PE | Total Number Patients | % Patients With DVT/PE | Relative risk DVT/PE (ischemic stroke=1) |
|---|---|---|---|---|---|
| Subarachnoid Hemorrhage | 183 | 6 | 189 | 3.1% | 2.69 |
| Ischemic stroke | 1317 | 16 | 1333 | 1.2% | 1 |
| Transient Ischemic Attack | 697 | 3 | 700 | 0.42% | 0.35 |
| Intracranial Hemorrhage | 264 | 8 | 272 | 2.9% | 2.5 |
χ2=14.8 df=3 p=0.002 (2 sided)
Table 2.
Factors Contributing to DVT/PE in the aggregated group of all patients. For the two level factors, the value in the second and third columns is the fraction of patients without and with DVT/PE who had that factor. NS indicates a p value of >0.1 in all statistical tests used. For the 2x2 tables the first p value is from the χ2 statistic and the second is from the 2-way Fisher’s Exact Test. For the other data the first p value is that for the t-test and the second for the Mann-Whitney U test (two-tailed).
| Factor | No DVT | DVT | Statistic | p* | Relative Risk for DVT/PE | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Length of Stay (days) | 8.6 (14) | 22.8 (20) | t(2434)=-5.2 | p<.0001 (<0.001) | ||
| Age (years) | 71.3 (16) | 70.7 (18) | t(2500)=0.21 | NS | ||
| Race | -- | -- | χ2=1.7 df=6 | NS | ||
| Gender | 56% female | 52% female | χ2=0.25 | NS | 1.08 female | 0.78-1.5 female |
| Height (inches) | 65.9 (6.2) | 66.6 (3) | t(2460)=-0.64 | NS | ||
| Weight (pounds) | 167 (45) | 182 (66) | t(2471)=-1.85 | p=0.06 (0.5) | ||
| BMI | 27 (6) | 28 (9) | t(2456)=-0.14 | NS | ||
| Afib/Flutter | 0.18 | 0.21 | χ2=0.28 | NS | 1.25 | 0.54-2.9 |
| Heart Failure | 0.06 | 0.15 | χ2=4.22 | p=0.04 (0.06) | 2.65 | 1.01-7.0 |
| Prior TIA | 0.13 | 0.1 | χ2=0.45 | NS | 2.2 | 0.2-2.2 |
| CAD/MI | 0.23 | 0.24 | χ2=0.01 | NS | 1.05 | 0.47-2.7 |
| Diabetes | 0.25 | 0.24 | χ2=0.02 | NS | 0.95 | 0.43-2.1 |
| Hypertension | 0.69 | 0.73 | χ2=0.19 | NS | 1.2 | 0.55-2.6 |
| Peripheral Vascular Disease | 0.02 | 0.0 | χ2=0.70 | NS | 0.987 | 0.98-0.991 |
| Smoking | 0.09 | 0.06 | χ2=0.37 | NS | 0.64 | 0.15-2.7 |
| Carotid Stenosis | 0.02 | 0.00 | χ2=0.75 | NS | 0.987 | 0.98-0.991 |
| Prior CVA | 0.15 | 0.21 | χ2=1.6 | NS | 1.7 | 0.7-4.0 |
| Prosthetic Heart Valve | 0.025 | 0.03 | χ2=0.04 | NS | 1.2 | 0.16-9.0 |
| Initial NIHSS | 5.8 (6.5) | 11.4 (6) | t(1145)=-2.84 | p=0.0046 (0.002) | ||
| Discharge NIHSS | 2.8 (5) | 9.8 (9.8) | t(853)=-2.8 | p=0.005 (0.07) | ||
| Weakness | 0.42 | 0.27 | χ2=3.01 | p=0.08 (0.11) | 0.5 | 0.2-1.1 |
| Altered LOC | 0.14 | 0.30 | χ2=7.13 | p=0.0076 (0.02) | 2.7 | 1.3-5.7 |
| Aphasia | 0.27 | 0.24 | χ2=0.12 | NS | 0.87 | 1.9 |
| Ambulatory by Day 2 | 0.254 | 0.1 | χ2=4.6 | p=0.03 (0.04) | 0.29 | 0.09-0.96 |
| Heparin or LMWH | 0.44 | 0.36 | χ2=0.74 | NS | 0.73 | 0.36-1.5 |
| Serial Compression Devices | 0.37 | 0.33 | χ2=0.65 | NS | 0.73 | 0.34-1.6 |
| Warfarin | 0.026 | 0.03 | χ2=0.02 | NS | 1.2 | 0.16-8.6 |
| Hyperlipidemia | 0.33 | 0.36 | χ2=0.2 | NS | 1.18 | 0.52-2.4 |
Fig. (1).
Histogram showing the distribution of length of stay in patients with and without DVT/PE.
Fig. (2).
Histogram showing the distribution of the initial NIH stroke scale in patients with and without DVT/PE.
Table 3 shows that there is a significant effect of increasing length of stay as a risk factor for DVT/PE in the patients with TIA and a statistical trend toward the same effect in patients with an intracranial hemorrhage. This effect is not seen in the patients with ischemic stroke. There is an effect of Altered level of consciousness and NIH stroke scale in the patients with TIA and less so in the patients with ICH and SAH. The presence of heart failure has a minor effect on the risk of DVT/PE in patients with ICH.
Table 3.
The effect of various factors that had a statistical effect (p<.1) in the aggregate group on the risk of DVT/PE in each diagnosis group.
| Factor | TIA | Stroke | ICH | SAH |
|---|---|---|---|---|
| Length of Stay | t(689)=-10.7 p<0.001 | t(1284)=-0.87 p=NS | t(261)=-2.5 p=0.012 | t(186)=-1.92 p=0.06 |
| Initial NIHSS | t(272)=-4.1 p<0.001 | t(762)=-1.74 p=0.08 | t(82)=-0.42 p=NS | Not Enough Data |
| Altered Level of Consciusness | χ2=18.6 p<0.001 (0.001) | NS | NS | NS |
| Heart Failure | χ2=4.7 p<0.03 (0.15) | χ2=6.4 p=0.01 (0.03) | NS | NS |
| Weight (lbs) | t(693)=0.43 NS | t(1313)=0.6 NS | t(267)=-1.3 NS | t(267)=-1.8 p=0.07 |
| Weakness | χ2=0.8 NS | χ2=0.27 NS | χ2=3.1 p<0.08 (0.11) | χ2=0.57 NS |
| Ambulatory by day 2 | χ2=0.28 NS | χ2=0.57 NS | χ2=0.55 NS | χ2=0.17 NS |
The problem with this data is that many of the “independent” factors may be related. For example, more patients with ICH will have longer lengths of stay and TIA patients may have shorter lengths of stay. In order to provide more insight into this, two multivariable analyses were undertaken. The variables entered into this analysis were all variables in Table 1 that were associated with DVT/PE with p values <0.05 which were length of stay, heart failure, initial NIHSS (discharge NIHSS had very few entries and hence was not entered), and ambulatory by day 2. In addition, a marker was entered to indicate if the patient had a TIA or intracranial hemorrhage. In the logistic regression analysis the factors that are significant are heart failure and altered level of consciousness. Heart failure was associated with an 11.7 fold increase in risk for DVT/PE (95% CI 28-49, p=0.001) and altered level of consciousness increased the risk of DVT/PE by a factor fo 6.2 (95% CI 1.5-26, p<0.02). The Hosmer-Lemeshow test (p=0.76) did not demonstrate problems with lack of fit. 99.3% of cases were correctly classified although none were classified into the DVT/PE category. This did not change with the addition of all variables. The linear discriminant analysis identified significant effects of only heart failure and altered level of consciousness (Wilks Lambda 0.97 χ2=33.8 df=2 p<0.001). However this model produced only an 80.2% correct classification of cases which increases only to 88% if all variables are included.
In order to get a better idea of the predictive of these two variables a 2x2x2 cross tabulation table of DVT/PE vs Heart Faiure vs altered level of consciousness (Table 4) shows that there is a 7 fold increase in the risk of DVT/PE in patients that had both heart failure and altered consciousness.
Table 4.
Cross tabulation table showing the risk of DVT/PE as a function of both heart failure and altered mental level of consciousness.
| Factor | #No DVT/PE | #DVT/PE | % Patients with DVT/PE |
|---|---|---|---|
| HF=0;Altered=0; | 1995 | 20 | 1.0 |
| HF=0;Altered=1; | 318 | 8 | 2.5 |
| HF=1;Altered=0; | 129 | 3 | 2.2 |
| HF=1;Altered=1; | 27 | 2 | 6.9 |
In none of the analyses did the administration of subcutaneous heparin or lovenox or coumadin have a significant effect on the risk of DVT/PE. However, it may be that chemprophylaxis for venous thromboembolism has a significant effect in the patient subgroup with the highest risk of DVT/PE. In order to answer this question, the risk of DVT/PE was studied in the group of patients with both heart failure and altered level of consciousness as a function of treatment. Although the risk of DVT was 11% in the group not given chemoprophylaxis and 0% in the group treated with chemoprophylaxis, this difference was not statistically significant because of the small number of patients. There was also no statistically significant effect of chemopro-phylaxis in the larger groups that had heart failure only or had altered level of consciousness only.
DISCUSSION
The major conclusion of this study is that the risk of DVT/PE in patients with stroke and intracranial hemorrhage can be influenced by different factors, many of which are interdependent. In univariate analyses, factors such as length of stay, diagnosis, weight, ambulatory status, and admission NIHSS, and heart failure all influenced the risk of DVT/PE. However, many of these factors are dependent on one another. For example, the length of stay for patients with intracranial hemorrhage is longer and the length of stay for patients with TIA is shorter than the length of stay for stroke. Thus, the multivariate analyses are critical in order to sort through all the factors to determine which are the primary risk factors. These multivariate analyses showed the most critical factors appear to be the presence of heart failure and the presence of altered mental status. This makes intuitive sense since heart failure might produce venous stasis as would the immobility that comes from altered level of consciousness. Many previous researchers have found that congestive heart failure is a significant risk for DVT [10-14].
Additional studies to confirm this hypothesis would be important given some of the statistical limitations that are inherent in this retrospective study. First, the NIH stroke scale is available for all of the stroke and TIA patients but less often the intracranial hemorrhage patients. Second, there are many differences in the treatment of patients with stroke, TIA and intracranial hemorrhage that are not codified including the full list of medications taken by the patient. In particular, we did not document which patients received platelets, other blood products or factor VII although no patients that were given factor VII at our hospital have developed DVT/PE. Third, is that despite the number of patients in the study, the number of patients with DVT/PE was small so that a larger data set could be helpful in elucidating risk factors. Fourth, other important factors that could modulate the risk of DVT/PE were not available in the database including the presence of a hypercoagulable state, and the dosing and timing of chemoprophylaxis for DVT.
It should be noted that we did not perform any routine screening with ultrasound for DVT in this study. This is likely the reason why our overall rates differ from those reported by some previous authors. Bemenek [1] reported a DVT rate of 8.7%, Paciaroni [5] 3-4%, Yi [15] 4.5% and the CLOTS trial [16] reported a rate of 6-9%. All of these used screening ultrasound. However many centers do not routinely use ultrasound in this patient group to find DVT and so in the large patient group analyzed by Skaf [17], the risk of DVT is 1.37% for stroke patients which is similar to that in our study.
Bembenek [18] in a study of less than 300 stroke patients found that C-reactive protein was the highest risk factor for DVT/PE and did not find statistically significant effects of heart failure or altered level of consciousness. Ogata [19], on the other hand found that D-dimer and high NIHSS were associated with a high risk of DVT/PE in patients with intracranial hemorrhage. Much of the differences between studies may be due to small patient populations and differences in the means of assessment of DVT/PE [4].
One interesting observation is that treatment with subcutaneous heparin or lovenox had no effect on the risk of DVT/PE statistically significant effect in either the univariate or multivariate analyses. This has been suggested by other authors [3] and may be due to the fact that many patients had a short length of stay and hence little time for prophylaxis to become effective. It may also be true that the factors that influence the decision to give prophylaxis covary with other factors that are not listed. A third explanation is that chemoprophylaxis of DVT/PE is effective only in the highest risk subgroups. Although our data showed such an effect, it was not statistically significant due to the small number of patients with DVT/PE in the study although other studies especially that of Piazza [12] have suggested the particular importance of chemoprophylaxis of DVT in patients with heart failure. Additional studies in the future may help answer this question.
CONCLUSION
DVT and/or PE are significant complications of stroke and intracranial hemorrhage. The task of elucidating risk factors is complex and complicated by the interdependence of many common risk factors. In this paper, the major risk factors for DVT/PE in the multivariable analysis were heart failure and altered level of consciousness. Although no effect of chemoprophylaxis was noted in the full group, it may be helpful in high risk groups. Additional studies using more patients may be helpful.
ACKNOWLEDGEMENTS
None decleared.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.Bembenek JP, Karlinski M, Kobayashi A , et al. Deep venous thrombosis in acute stroke patients. Clin Appl Thromb Hemost. 2012;18:258–64. doi: 10.1177/1076029611424575. [DOI] [PubMed] [Google Scholar]
- 2.Douds GL, Hellkamp AS, Olson DM , et al. Venous thromboem-bolism in the get with the guidelines-stroke acute ischemic stroke population: incidence and patterns of prophylaxis. J Stroke Cerebrovasc Dis. 2014;23(1):123–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Qaseem A, Chou R, Humphrey LL , et al. Venous thromboem-bolism prophylaxis in hospitalized patients: a clinical practice guideline from the american college of physicians. Ann Intern Med. 2011;55:625–32. doi: 10.7326/0003-4819-155-9-201111010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Eikelboom JW, Gould MK , et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e185S–94S. doi: 10.1378/chest.11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paciaroni M, Agnelli G, Venti M , et al. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: a meta-analysis of controlled studies. J Thromb Haemost. 2011;9:893–8. doi: 10.1111/j.1538-7836.2011.04241.x. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Amarenco P, Easton JD , et al. Antithrombotic and thrombolytic therapy for ischemic stroke 8th.American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;33:630S–69S. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- 7.Adams HPJr, del Zoppo G, Alberts MJ , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council Clinical Cardiology Council.Cardiovascular Radiology and Intervention Council and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 8.Sandercock PA, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;(4):CD000024. doi: 10.1002/14651858.CD000024.pub3. [DOI] [PubMed] [Google Scholar]
- 9.The International Stroke Trial (IST) a randomised trial of aspirin subcutaneous heparin both or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group Lancet. 1997;349:1569–81. [PubMed] [Google Scholar]
- 10.Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective. case-control study. J Clin Epidemiol. 2001;54:810–6. doi: 10.1016/s0895-4356(00)00373-5. [DOI] [PubMed] [Google Scholar]
- 11.Edelsberg J, Hagiwara M, Taneja C , et al. Risk of venous thromboembolism among hospitalized medically ill patients. Am J Health Syst Pharm. 2006;63:S16–22. doi: 10.2146/ajhp060389. [DOI] [PubMed] [Google Scholar]
- 12.Piazza G, Seddighzadeh A, Goldhaber SZ. Heart failure in patients with deep vein thrombosis. Am J Cardiol. 2008;101:1056–9. doi: 10.1016/j.amjcard.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 13.Galanaud JP, Sevestre-Pietri MA, Bosson JL , et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. 2009;102:493–500. doi: 10.1160/TH09-01-0053. [DOI] [PubMed] [Google Scholar]
- 14.Markovic-Denic L, Zivkovic K, Lesic A , et al. Risk factors and distribution of symptomatic venous thromboembolism in total hip and knee replacements: prospective study. Int Orthop. 2012;36:1299–305. doi: 10.1007/s00264-011-1466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi X, Lin J, Han Z , et al. The incidence of venous thromboembolism following stroke and its risk factors in eastern China. J Thromb Thrombolysis. 2012;34:269–75. doi: 10.1007/s11239-012-0720-z. [DOI] [PubMed] [Google Scholar]
- 16.First name Last name. CLOTS (Clots in Legs Or Stockings after Stroke) Trial Collaboration.Thigh-length versus below-knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153:553–62. doi: 10.7326/0003-4819-153-9-201011020-00280. [DOI] [PubMed] [Google Scholar]
- 17.Skaf E, Stein PD, Beemath A , et al. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005;96:1731–3. doi: 10.1016/j.amjcard.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 18.Bembenek J, Karlinski M, Kobayashi A , et al. Early stroke-related deep venous thrombosis: risk factors and influence on outcome. J Thromb Thrombolysi. 201;32:96–102. doi: 10.1007/s11239-010-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata T, Yasaka M, Wakugawa Y , et al. Deep venous thrombosis after acute intracerebral hemorrhage. J Neurol Sci. 2000;8 272:83–6. doi: 10.1016/j.jns.2008.04.032. [DOI] [PubMed] [Google Scholar]