Abstract
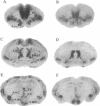
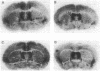
The neuropeptide oxytocin has been implicated in the mediation of several forms of affiliative behavior including parental care, grooming, and sex behavior. Here we demonstrate that species from the genus Microtus (voles) selected for differences in social affiliation show contrasting patterns of oxytocin receptor expression in brain. By in vitro receptor autoradiography with an iodinated oxytocin analogue, specific binding to brain oxytocin receptors was observed in both the monogamous prairie vole (Microtus ochrogaster) and the polygamous montane vole (Microtus montanus). In the prairie vole, oxytocin receptor density was highest in the prelimbic cortex, bed nucleus of the stria terminalis, nucleus accumbens, midline nuclei of the thalamus, and the lateral aspects of the amygdala. These brain areas showed little binding in the montane vole, in which oxytocin receptors were localized to the lateral septum, ventromedial nucleus of the hypothalamus, and cortical nucleus of the amygdala. Similar differences in brain oxytocin receptor distribution were observed in two additional species, the monogamous pine vole (Microtus pinetorum) and the polygamous meadow vole (Microtus pennsylvanicus). Receptor distributions for two other neurotransmitter systems implicated in the mediation of social behavior, benzodiazepines, and mu opioids did not show comparable species differences. Furthermore, in the montane vole, which shows little affiliative behavior except during the postpartum period, brain oxytocin receptor distribution changed within 24 hr of parturition, concurrent with the onset of maternal behavior. We suggest that variable expression of the oxytocin receptor in brain may be an important mechanism in evolution of species-typical differences in social bonding and affiliative behavior.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arletti R., Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985 Jun;6(3):247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Caldwell J. D., Jirikowski G. F., Greer E. R., Stumpf W. E., Pedersen C. A. Ovarian steroids and sexual interaction alter oxytocinergic content and distribution in the basal forebrain. Brain Res. 1988 Apr 19;446(2):236–244. doi: 10.1016/0006-8993(88)90882-7. [DOI] [PubMed] [Google Scholar]
- Caldwell J. D., Prange A. J., Jr, Pedersen C. A. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986 Feb-Mar;7(2):175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Carter C. S., Witt D. M., Schneider J., Harris Z. L., Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster). Horm Behav. 1987 Mar;21(1):74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Elands J., Barberis C., Jard S., Tribollet E., Dreifuss J. J., Bankowski K., Manning M., Sawyer W. H. 125I-labelled d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988 Mar 1;147(2):197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Cador M., Robbins T. W. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30(1):63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Morrell J. I., Pfaff D. W. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984 Sep;18(3):267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Morrell J. I., Pfaff D. W. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985 Jun;40(6):526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Slotnick B. M. Disruption of maternal behavior in rats with lesions of the septal area. Physiol Behav. 1978 Aug;21(2):189–200. doi: 10.1016/0031-9384(78)90041-0. [DOI] [PubMed] [Google Scholar]
- Gray J. A., McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983 Summer;7(2):119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Hansen S., Ferreira A., Selart M. E. Behavioural similarities between mother rats and benzodiazepine-treated non-maternal animals. Psychopharmacology (Berl) 1985;86(3):344–347. doi: 10.1007/BF00432226. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Gelhard R., Shapiro L. E. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43(2-3):623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Harbaugh C. R. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989 May;45(5):1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Hill J. L., Mayor R. B. Rat pup ultrasonic isolation calls: possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986 May;24(5):1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Kinsley C. H., Mann P. E., Bridges R. S. Prenatal stress has long-term effects on brain opiate receptors. Brain Res. 1990 Mar 12;511(1):93–97. doi: 10.1016/0006-8993(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Insel T. R. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel T. R. Postpartum increases in brain oxytocin binding. Neuroendocrinology. 1986;44(4):515–518. doi: 10.1159/000124694. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Winslow J. T. Central administration of oxytocin modulates the infant rat's response to social isolation. Eur J Pharmacol. 1991 Oct 2;203(1):149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Kendrick K. M., Keverne E. B., Baldwin B. A. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987 Jun;46(1):56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kendrick K. M., Keverne E. B., Chapman C., Baldwin B. A. Microdialysis measurement of oxytocin, aspartate, gamma-aminobutyric acid and glutamate release from the olfactory bulb of the sheep during vaginocervical stimulation. Brain Res. 1988 Feb 23;442(1):171–174. doi: 10.1016/0006-8993(88)91447-3. [DOI] [PubMed] [Google Scholar]
- Keverne E. B., Martensz N. D., Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14(1-2):155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Shinoda A., Yamanouchi K., Arai Y. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm Behav. 1990 Sep;24(3):421–434. doi: 10.1016/0018-506x(90)90019-t. [DOI] [PubMed] [Google Scholar]
- LeDoux J. E., Cicchetti P., Xagoraris A., Romanski L. M. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990 Apr;10(4):1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J., Herman B., Conner R., Bishop P., Scott J. P. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978 Oct;13(5):607–618. [PubMed] [Google Scholar]
- Pedersen C. A., Caldwell J. D., Johnson M. F., Fort S. A., Prange A. J., Jr Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985 Apr;6(2):175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Prange A. J., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973 Sep 15;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Phillips A. G. Brain reward circuitry: a case for separate systems. Brain Res Bull. 1984 Feb;12(2):195–201. doi: 10.1016/0361-9230(84)90189-8. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Dewsbury D. A. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus). J Comp Psychol. 1990 Sep;104(3):268–274. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Insel T. R. Infant's response to social separation reflects adult differences in affiliative behavior: a comparative developmental study in prairie and montane voles. Dev Psychobiol. 1990 Jul;23(5):375–393. doi: 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Insel T. R. Ontogeny of oxytocin receptors in rat forebrain: a quantitative study. Synapse. 1989;4(3):259–266. doi: 10.1002/syn.890040312. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Barberis C., Jard S., Dubois-Dauphin M., Dreifuss J. J. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988 Feb 23;442(1):105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Niehoff D. L., Kuhar M. J., Palacios J. M. Quantitative receptor autoradiography using [3H]ultrofilm: application to multiple benzodiazepine receptors. J Neurosci Methods. 1982 Jul;6(1-2):59–73. doi: 10.1016/0165-0270(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Witt D. M., Carter C. S., Walton D. M. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol Biochem Behav. 1990 Sep;37(1):63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Witt D. M., Insel T. R. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991 Jun;128(6):3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]