Abstract
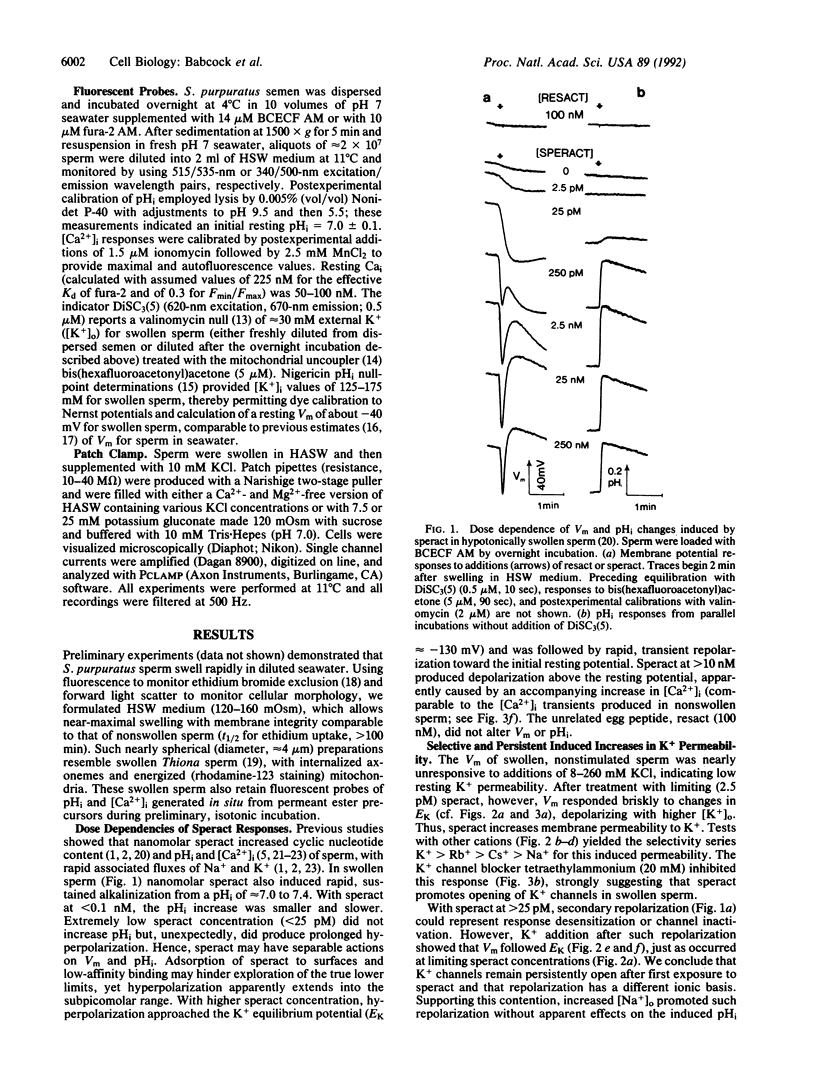
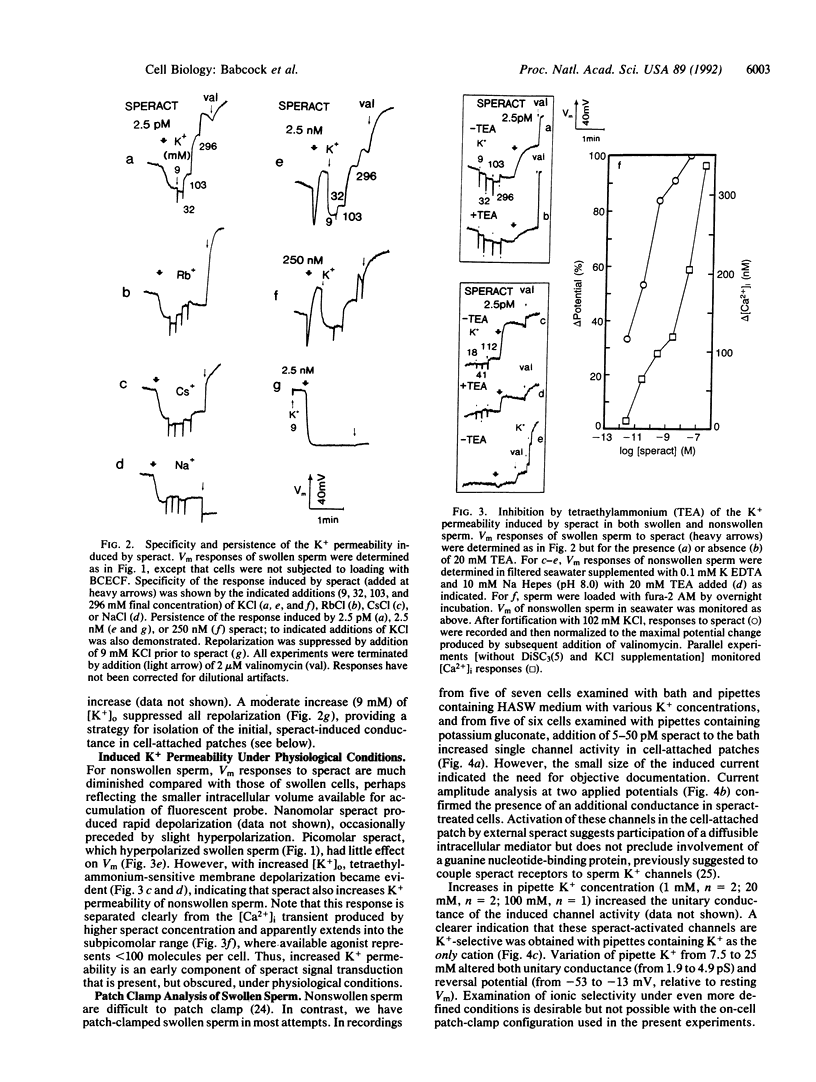
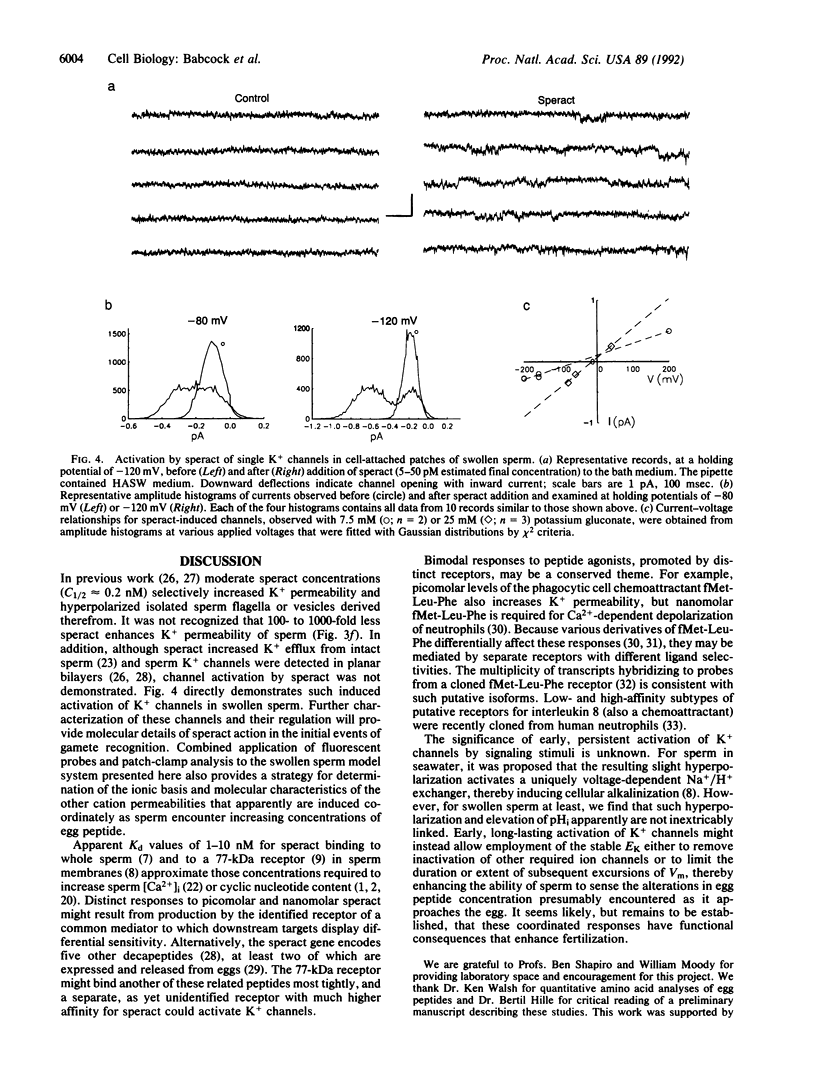
Transduction by sperm of the instructive signal provided by the egg peptide speract involves rapid, complex changes in internal ion and cyclic nucleotide content. Here, investigations of hypotonically swollen sperm provide insight into the underlying processes and identify K+ channel activation as an initial ionic event in gamete recognition. A sustained hyperpolarization of swollen sperm is promoted by less than 2.5 pM speract and is followed (with greater than 100 pM speract) by transient repolarization and (with greater than 10 nM speract) by depolarization that is dependent on external Ca2+. Monophasic increases in pHi are produced only by greater than 25 pM speract, indicating that hyperpolarization may not directly promote alkalinization. Increased K(+)-selective (K+ greater than Rb+ greater than Cs+ greater than Na+) membrane permeability is found after all speract greater than 2.5 pM, suggesting that hyperpolarization results from persistent activation of K+ channels and that repolarization has a different ionic basis. Supporting this contention, the K+ channel blocker tetraethylammonium (20 mM) inhibits the increased K+ permeability that follows treatment of swollen sperm (and of sperm in seawater) with 2.5 pM speract. Such induced activation of K+ channels is observed in patch-clamped swollen sperm examined in the cell-attached configuration, upon application of 5-50 pM speract to the bath medium. The efficacy of externally applied speract and its potency indicate that activation is indirect and probably involves an as yet unidentified diffusible mediator whose production is promoted by speract at concentrations 0.01-0.001 times those predicted from reported estimates of the Kd for the known speract receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J Biol Chem. 1983 May 25;258(10):6380–6389. [PubMed] [Google Scholar]
- Babcock D. F., Pfeiffer D. R. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. J Biol Chem. 1987 Nov 5;262(31):15041–15047. [PubMed] [Google Scholar]
- Bentley J. K., Khatra A. S., Garbers D. L. Receptor-mediated activation of detergent-solubilized guanylate cyclase. Biol Reprod. 1988 Oct;39(3):639–647. doi: 10.1095/biolreprod39.3.639. [DOI] [PubMed] [Google Scholar]
- Boulay F., Tardif M., Brouchon L., Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990 Dec 18;29(50):11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Garbers D. L. Identification and partial characterization of the receptor for speract. J Biol Chem. 1984 Nov 25;259(22):13712–13716. [PubMed] [Google Scholar]
- Dangott L. J., Jordan J. E., Bellet R. A., Garbers D. L. Cloning of the mRNA for the protein that crosslinks to the egg peptide speract. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2128–2132. doi: 10.1073/pnas.86.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Watkins H. D., Hansbrough J. R., Smith A., Misono K. S. The amino acid sequence and chemical synthesis of speract and of speract analogues. J Biol Chem. 1982 Mar 25;257(6):2734–2737. [PubMed] [Google Scholar]
- García-Soto J., González-Martínez M., de De la Torre L., Darszon A. Internal pH can regulate Ca2+ uptake and the acrosome reaction in sea urchin sperm. Dev Biol. 1987 Mar;120(1):112–120. doi: 10.1016/0012-1606(87)90109-6. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Sánchez J. A., Darszon A. Single-channel activity in sea urchin sperm revealed by the patch-clamp technique. FEBS Lett. 1987 Aug 17;220(2):295–298. doi: 10.1016/0014-5793(87)80833-5. [DOI] [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Sodium-dependent activation of sea urchin spermatozoa by speract and monensin. J Biol Chem. 1981 Mar 10;256(5):2235–2241. [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981 Feb 10;256(3):1447–1452. [PubMed] [Google Scholar]
- Harvath L., Aksamit R. R. Oxidized N-formylmethionyl-leucyl-phenylalanine: effect on the activation of human monocyte and neutrophil chemotaxis and superoxide production. J Immunol. 1984 Sep;133(3):1471–1476. [PubMed] [Google Scholar]
- Heytler P. G. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–442. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- Hoffman J. F., Laris P. C. Determination of membrane potentials in human and Amphiuma red blood cells by means of fluorescent probe. J Physiol. 1974 Jun;239(3):519–552. doi: 10.1113/jphysiol.1974.sp010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari K. G., Proto P., Simons E. R. Neutrophil hyperpolarization in response to a chemotactic peptide. J Biol Chem. 1990 Jul 5;265(19):10959–10967. [PubMed] [Google Scholar]
- Lee H. C., Garbers D. L. Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J Biol Chem. 1986 Dec 5;261(34):16026–16032. [PubMed] [Google Scholar]
- Lee H. C. Internal GTP stimulates the speract receptor mediated voltage changes in sea urchin spermatozoa membrane vesicles. Dev Biol. 1988 Mar;126(1):91–97. doi: 10.1016/0012-1606(88)90242-4. [DOI] [PubMed] [Google Scholar]
- Lievano A., Sanchez J. A., Darszon A. Single-channel activity of bilayers derived from sea urchin sperm plasma membranes at the tip of a patch-clamp electrode. Dev Biol. 1985 Nov;112(1):253–257. doi: 10.1016/0012-1606(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Ramarao C. S., Burks D. J., Garbers D. L. A single mRNA encodes multiple copies of the egg peptide speract. Biochemistry. 1990 Apr 3;29(13):3383–3388. doi: 10.1021/bi00465a034. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Garbers D. L. A hydrogen ion flux mediates stimulation of respiratory activity by speract in sea urchin spermatozoa. J Biol Chem. 1983 May 25;258(10):6025–6029. [PubMed] [Google Scholar]
- Schackmann R. W., Chock P. B. Alteration of intracellular [Ca2+] in sea urchin sperm by the egg peptide speract. Evidence that increased intracellular Ca2+ is coupled to Na+ entry and increased intracellular pH. J Biol Chem. 1986 Jul 5;261(19):8719–8728. [PubMed] [Google Scholar]
- Schackmann R. W., Christen R., Shapiro B. M. Measurement of plasma membrane and mitochondrial potentials in sea urchin sperm. Changes upon activation and induction of the acrosome reaction. J Biol Chem. 1984 Nov 25;259(22):13914–13922. [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Shimomura H., Dangott L. J., Garbers D. L. Covalent coupling of a resact analogue to guanylate cyclase. J Biol Chem. 1986 Nov 25;261(33):15778–15782. [PubMed] [Google Scholar]
- Suzuki N., Garbers D. L. Stimulation of sperm respiration rates by speract and resact at alkaline extracellular pH. Biol Reprod. 1984 Jun;30(5):1167–1174. doi: 10.1095/biolreprod30.5.1167. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Kajiura H., Nomura K., Garbers D. L., Yoshino K., Kurita M., Tanaka H., Yamaguchi M. Some more speract derivatives associated with eggs of sea urchins, Pseudocentrotus depressus, Strongylocentrotus purpuratus, Hemicentrotus pulcherrimus and Anthocidaris crassispina. Comp Biochem Physiol B. 1988;89(4):687–693. doi: 10.1016/0305-0491(88)90309-4. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nomura K., Ohtake H., Isaka S. Purification and the primary structure of sperm-activity peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1238–1244. doi: 10.1016/0006-291x(81)90752-x. [DOI] [PubMed] [Google Scholar]
- Tilney L. G. The polymerization of actin. II. How nonfilamentous actin becomes nonrandomly distributed in sperm: evidence for the association of this actin with membranes. J Cell Biol. 1976 Apr;69(1):51–72. doi: 10.1083/jcb.69.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. E., Brokaw C. J., Garbers D. L., Vacquier V. D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985 Dec;101(6):2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]



