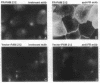
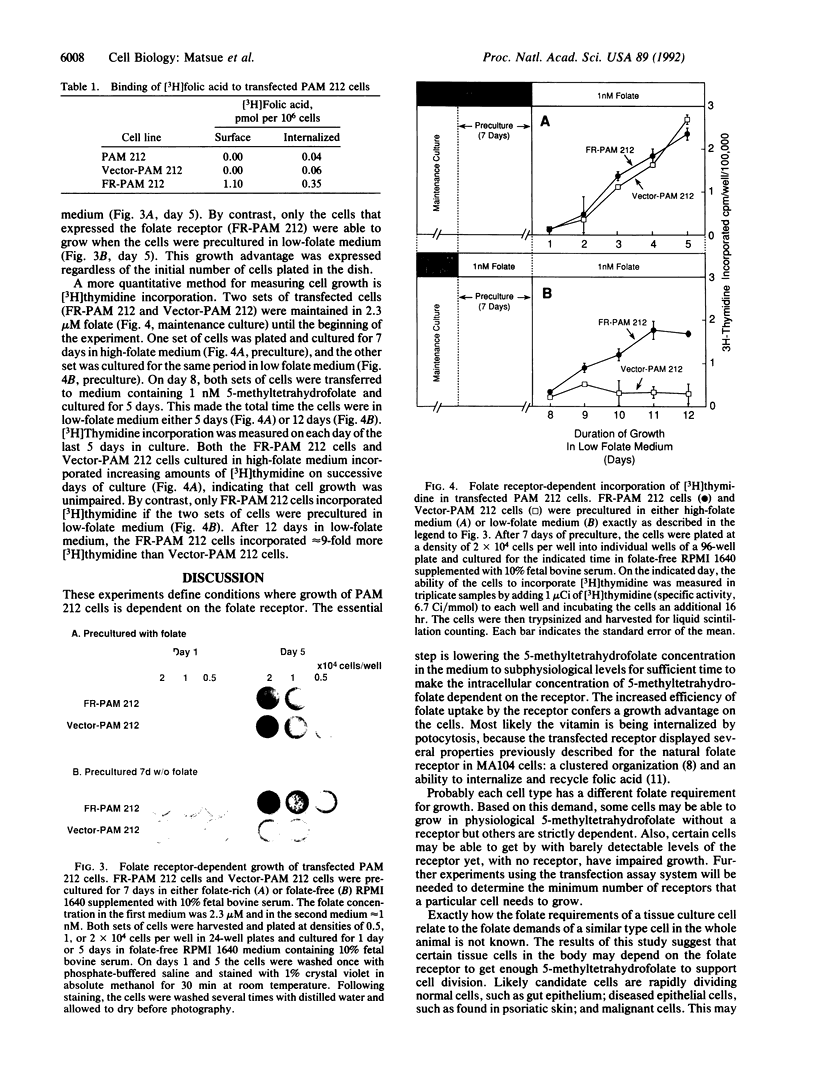
Abstract
The folate receptor mediates the uptake of 5-methyltetrahydrofolate in certain cultured cells by a process called potocytosis. When these cells are grown in physiological concentrations of folate, the receptor increases the efficiency of vitamin uptake by 30-fold. We now show that PAM 212 cells, a mouse keratinocyte cell line, are unable to grow in 1 nM 5-methyltetrahydrofolate unless they express a functional folate receptor. These results suggest that under certain conditions, tissue cells in the body may depend on the folate receptor to obtain enough 5-methyltetrahydrofolate for growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coney L. R., Tomassetti A., Carayannopoulos L., Frasca V., Kamen B. A., Colnaghi M. I., Zurawski V. R., Jr Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991 Nov 15;51(22):6125–6132. [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Johnson C. A., Wang M. T., Anderson R. G. Regulation of the cytoplasmic accumulation of 5-methyltetrahydrofolate in MA104 cells is independent of folate receptor regulation. J Clin Invest. 1989 Nov;84(5):1379–1386. doi: 10.1172/JCI114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Smith A. K., Anderson R. G. The folate receptor works in tandem with a probenecid-sensitive carrier in MA104 cells in vitro. J Clin Invest. 1991 Apr;87(4):1442–1449. doi: 10.1172/JCI115150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Wang M. T., Streckfuss A. J., Peryea X., Anderson R. G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988 Sep 25;263(27):13602–13609. [PubMed] [Google Scholar]
- Lacey S. W., Sanders J. M., Rothberg K. G., Anderson R. G., Kamen B. A. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989 Aug;84(2):715–720. doi: 10.1172/JCI114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- YAMADA E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955 Sep 25;1(5):445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]