Abstract
Lipids are diverse families of biomolecules that perform essential structural and signaling roles in platelets. Their formation and metabolism is tightly controlled by enzymes and signal transduction pathways, and their dysregulation leads to significant defects in platelet function and disease. Platelet activation is associated with significant changes to membrane lipids, and formation of diverse bioactive lipids that play essential roles in hemostasis. In recent years, new generation mass spectrometry analysis of lipids (termed “lipidomics”) has begun to alter our understanding of how these molecules participate in key cellular processes. While, the application of lipidomics to platelet biology is still in its infancy, seminal earlier studies have shaped our knowledge of how lipids regulate key aspects of platelet biology, including aggregation, shape change, coagulation and degranulation, as well as how lipids generated by platelets influence other cells, such as leukocytes and the vascular wall, and thus how they regulate hemostasis, vascular integrity and inflammation, as well as contribute to pathologies including arterial/deep vein thrombosis and atherosclerosis. This review will provide a brief historical perspective on the characterization of lipids in platelets, then an overview of the new generation lipidomic approaches, their recent application to platelet biology, and future perspectives for research in this area. The major platelet-regulatory lipid families, their formation, metabolism, and their role in health and disease, will be summarized.
Keywords: Platelets, Lipidomics, Mass spectrometry
1. Introduction
1.1 Lipids in platelets
Lipids are low molecular weight, typically hydrophobic and amphipathic molecules found in all cell types. They are either generated endogenously, or incorporated into cells from dietary sources. Their formation, trafficking and metabolism is tightly controlled by cellular proteins, that include members of large families of phospholipases, lipid synthetases, ligases, oxidases/reductases and transporters. Lipids fulfill three primary roles, structural, energy storage, and signaling. There are several distinct families of lipids in platelets, characterized by (i) common functional groups and structural motifs, such as phospholipids (PL), sphingolipids (SP), steroids (ST), and prenol lipids and (ii) minor structural differences including positional isomers, fatty acid (FA) chain length, and hydrocarbon saturation that render a complex mixture of molecular species. In common with all mammalian cells, the major structural lipids in platelets are PL, which arrange themselves in membranes with hydrophobic FAs orientated to the core and polar headgroups facing the aqueous phase (Figure 1). PL membranes include both the plasma membrane and also the numerous intracellular organelle membranes in platelets. During activation they provide substrates that are converted enzymatically to bioactive species including 1,2-diacyglycerol (DAG), FAs, eicosanoids/prostaglandins (PG), phosphatidylinositides (PI), lysophospholipids (LysoPL) and lysophosphatidic acid (LPA). They are also indirectly oxidized to form PL-esterified eicosanoids and PGs by lipoxygenases (LOX) and cyclooxygenases (COX). Major remodeling of lipids occurs during platelet activation and is associated with significant structural alterations to platelet membranes, including shape change, spreading, microvesicle formation and degranulation, as well as generation of bioactive pro-thrombotic species. Platelet membranes contain sphingomyelins (SM) and free cholesterol that is enriched in specialized signaling areas termed lipid rafts. Platelets also contain appreciable amounts of neutral lipids including di- and triglycerides (DAG, TAG) and cholesteryl esters (CE). Some additional lipids present in smaller amounts, but with important signaling roles include members of the SP and glycolipids/ceramide families.
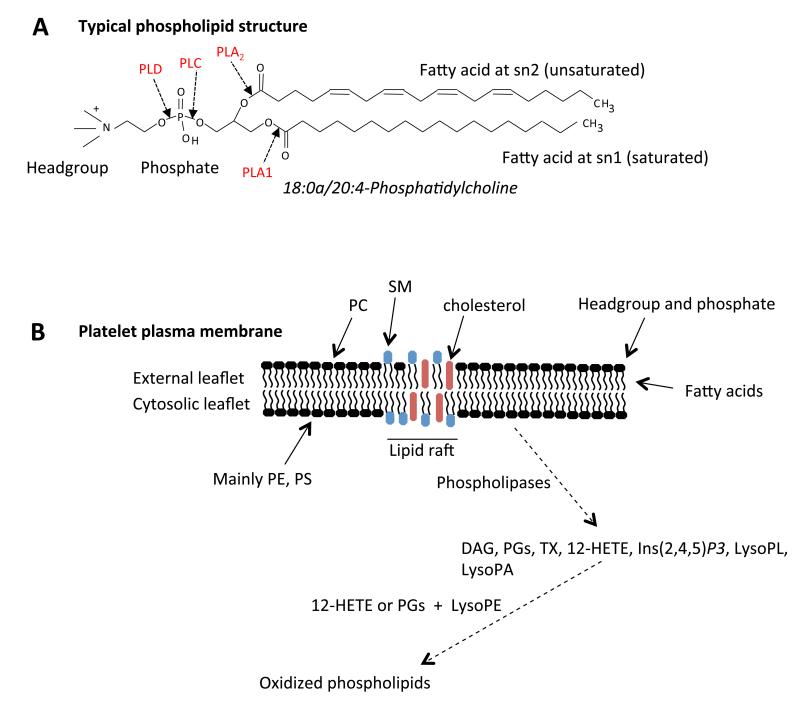
Figure 1. Phospholipids in the membrane.
Panel A. Typical structure of a phospholipid: 1-stearoyl-2-arachidonyl-phosphatidylcholine. Panel B. Structure of the plasma membrane lipid compartment and its relationship to formation of lipid mediators. Note, for simplicity, proteins have not been shown in this figure. PC: phosphatidylcholine, SP: sphingomyelin, PE: phosphatidylethanolamine, PS: phosphatidylserine, DAG: diacylglyceride, PG: prostaglandin, HETE: hydroxyeicosatetraenoic acid, Ins(2,4,5)P3: inositol triphosphate, PL: phospholipid, PA: phosphatidic acid.
In this review, we will summarize what is currently known about each lipid class in platelets, how they are metabolized and their major functions. Their roles in platelet-dependent pathologies will also be described.
1.2 Historical perspective, early studies on platelet lipids
Prior to the advent of lipidomics, cellular lipids were studied using traditional techniques that included thin layer chromatography (TLC), gas chromatography/mass spectrometry (GC/MS), and HPLC coupled to radiochemical, ultraviolet or fluorescence detection. Research using these approaches still informs most of what we know about platelet lipids today.
One of the earliest comprehensive analyses of platelet PLs was carried out in 1962 by Marcus and colleagues 1-4. Following this, studies were carried out in the 1970’s-1980’s comparing platelet lipid composition in a diverse and somewhat unusual array of population groups and species 5-15. Also, with interest in the role of lipids causing vascular disease increasing around that time, the effects of dietary supplementation with lipids on platelet lipid fatty acid composition (omega-3, fish oil, corn oil, or varying dietary fat) was characterized 16-18.
Platelet eicosanoids and related species were first identified in the early 1970’s by Bengt Samuelsson and colleagues in the Karolinska Institute, Stockholm, in parallel with Sir John Vane, in London. In 1974, the major platelet products 12S-hydroxyeicosatetraenoic acid (HETE) and 12-hydroxyheptadecatrienoic acid (HHT) were demonstrated, then in 1975, thromboxane A2 (TXA2) was discovered as a platelet-derived lipid that causes irreversible aggregation 19, 20. This seminal work contributed to the awarding of a Nobel Prize to Samuelsson and Vane for discoveries concerning PGs, thromboxanes and related biologically active substances. In the early 1980’s, thrombin was demonstrated to cause significant alterations to membrane lipids, with PI showing losses of up to 45%. AA specifically decreased in PI and PC pools suggesting these to be likely sources of substrate for eicosanoid generation 21, 22. Release of AA from PI was proposed to involve the sequential action of phospholipase C generating DAG followed by diacylglycerol lipase acting on DAG, representing an alternative to phospholipase A2 (PLA2) 22. These studies, established the overall lipid composition of resting and activated platelets, in particular regarding the more abundant species such as PLs and FAs. In contrast, 1980’s-2000’s saw intense interest in characterizing the role of specific platelet signaling lipids in regulating platelet function and contributing to both physiological hemostasis and human disease. Examples that will be discussed herein, and that deserve special mention include: (i) The central role of TXA2 in regulating hemostasis and contributing to pathological clot formation, (ii) The role of DAG, PI and inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) in promoting calcium mobilization following receptor-dependent activation of platelets, and (iii) The role of anionic phospholipids in promoting coagulation.
1.3 Lipidomics: current state-of-the-art
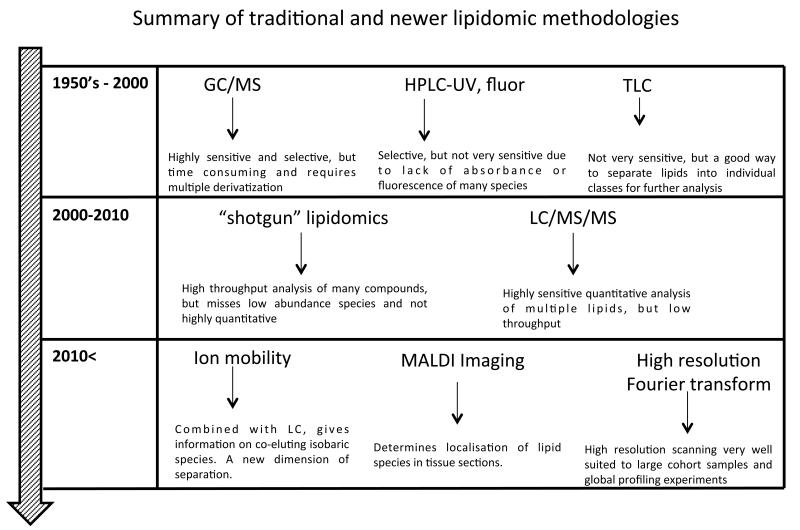
Since the mid 2000’s, the advent of ‘omics, largely driven by the development of sensitive benchtop mass liquid chromatography- mass spectrometry (LC/MS) instruments, for example, electrospray ionization (ESI) coupled to tandem (triple quadrupole or MS/MS) or time-of-flight (ToF) instruments, has revolutionized our ability to study small amounts of complex mixtures of diverse lipids in biological samples, hence the term lipidomics. These days, the term tends to be synonymous with the mass spectrometry of lipids. The major disadvantages of older approaches over LC/MS were (i) low sensitivity and selectivity (TLC, HPLC), (ii) the need for time-consuming derivatization methods (e.g. for GC/MS), and (iii) the requirement for radioisotopes with their inherent health issues, notably in regard to 32P-orthophosphate. Compared to this, LC/MS combined high sensitivity with the ability to directly detect, characterize and quantify individual molecular species without derivatization or purification. Older methods were generally unable to directly analyze specific lipids, e.g. 1-stearoyl-2-arachidonyl-PC would not have been measurable as a single species in a complex mixture. Traditional and newer lipidomic methodologies are summarized in Figure 2.
Figure 2. Summary of traditional and new lipidomic technologies used to discover and characterize cellular lipids.
GC/MS: gas chromatography/mass spectrometry, HPLCUV: high-pressure liquid chromatography/ultraviolet, LC/MS/MS: liquid chromatography/tandem mass spectrometry.
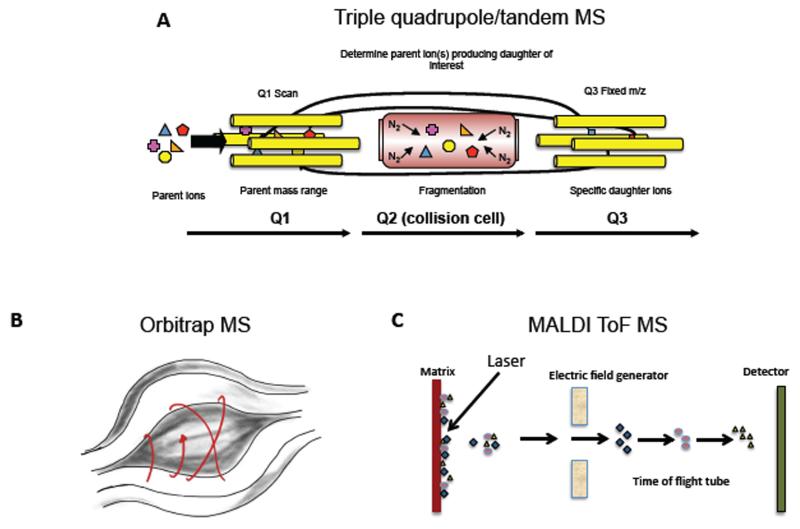
Lipidomics is broadly divided into two separate approaches, (i) liquid chromatography-tandem mass spectrometry (LC/MS/MS) which is targeted, highly sensitive and quantitative, and (ii) “shotgun” lipidomics, which is high throughput, but can only detect the most abundant species 23, 24. In LC/MS/MS, several molecular species are analyzed in the same sample following separation, by their characteristic parent m/z (mass to charge ratio, where mass is divided by the total net charge of the molecule) and daughter ions that form following collision-induced-fragmentation (where molecules are collisionally-activated using an inert gas then break apart into small daughter ions that can be separately analyzed). These methods typically use a tandem MS instrument (e.g. tandem in space as a triple quadrupole or tandem in time, as an ion trap). These comprise three separate chambers, the first and third of which are mass analyzers that house four parallel gold-plated rods (quadrupoles). The second is a collision cell where nitrogen or argon fragment the molecule for MS/MS analysis (Figure 3 A). Thus, LC/MS/MS methods are best suited to studies where researchers are interested in specific lipids and require accurate quantitation. However, separations can be long, for example up to 1 hr or more for PLs. On the other hand, shotgun lipidomics involves direct infusion of complex mixtures, without separation, into a mass spectrometer, followed by MS scanning of a defined mass window, often using high-resolution instruments (where the MS can distinguish molecules that are extremely close in mass, e.g. differing in mass by up to 1-5 ppm). The advantage is that many samples can be analyzed in a relatively short time, however, it is not generally quantitative, and low abundance (often highly biologically-relevant) species are missed. A newer quantitative approach combining LC separation with high resolution MS/MS on rapid scanning Fourier transform (FT) or Time-of-flight (ToF) instruments, termed multidimensional MS (MDMS) is now increasing in popularity in particular for profiling studies23. In Fourier transform mass spectrometry, the m/z is determined based on the cyclotron frequency of the ions in a fixed magnetic field. One example of this is the Orbitrap, a benchtop instrument currently popular for lipidomics that contains an outer barrel-like electrode and a coaxial inner spindle-like electrode that together generate an electrostatic field (Figure 3 B). In contrast, ToF instruments calculate mass based on the length of time it takes for molecules accelerated by an electric field to reach a detector at a known distance, with heavier particles having lower speeds (Figure 3 C).
Figure 3. Examples of MS instrument configurations.
Panel A. Triple quadrupole instrument. Ions are selected in Q1, fragmented in Q2, and daughter ions scanned out in A3. Panel B. Fourier transform Orbitrap MS. Ion trajectories in an Orbitrap mass spectrometer Panel C. Time-of-Flight MS. In the example shown, Matrix-assisted laser desorption ionization is used to generate ions that are selected in an electric field and m/z determined based on time taken to reach the detector.
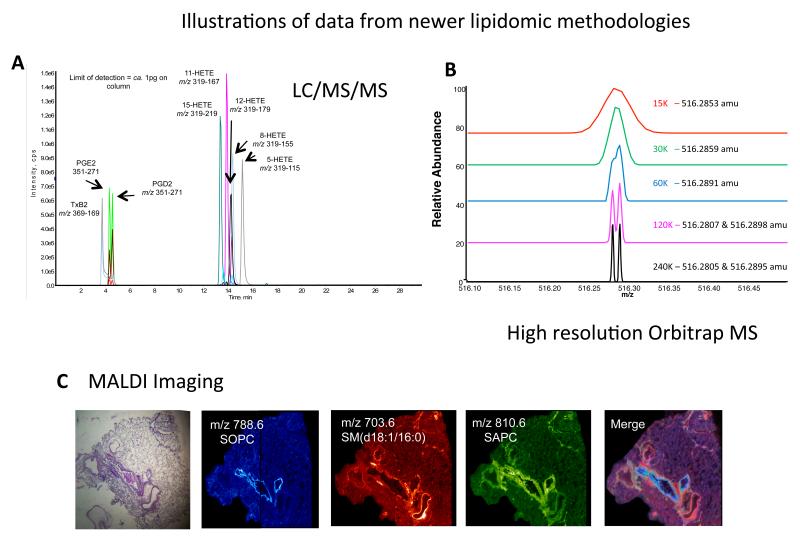
Up to now, platelet lipids have not been extensively studied using lipidomics. LC/MS/MS has been applied to quantitation of eicosanoids, such as thromboxane B2 (TXB2) and HETE, and to identification of SP molecular species 25, 26 (Figure 4 A). Shotgun methods have been applied to the characterization of platelet PLs, lysoPLs, CEs and ceramides in a small number of studies 27-29. Using traditional methods, individual lipid pools first had to be isolated using TLC, then hydrolyzed to release FAs for determination as free acid species, a considerably slower approach that requires more material 1-4. Recently, the composition of stored platelets and extracellular vesicles were characterized, showing that vesicles became enriched with LPA, cholesterol, and other lipids 28, 29.
Figure 4. Illustrations of data from three of the newer MS technologies.
Panel A. LC/MS/MS of eicosanoid standards (previously published in34). Panel B. Illustration of resolving power of Fourier transform MS on an Orbitrap Elite, at different resolution settings, as shown. Panel C left to right: H&E stain of a distal human lung slice (15 micron thick) showing both airways and blood vessels. MALDI mass spectrometric image of the positive ions (m/z 788.6 blue color) derived from 1-stearoyl-2-oleoyl-phosphatidylcholine (SOPC) a phospholipid found in the cells of the airways. MALDI mass spectrometric image of the positive ions (m/z 703.6, red) derived from the sphingomyelin molecular species SM(d18:1/16:0) an abundant phospholipid present in the pulmonary blood vessels. MALDI mass spectrometric image of the positive ions (m/z 810.6, green) derived from 1-oleoyl-2-arachidonoyl-phosphatidylcholine (SAPC) localized to both airways and blood vessels. Merged MALDI mass spectrometric images indicating co-localization of phospholipids in cells of the lung parenchyma.
In the last 2-3 years, MS technology has undergone significant increases in both sensitivity and scanning speed, with new benchtop instrument configurations coming available, and only now are that researchers are only now beginning to apply these to the study of cells and tissues. At this time, we are only learning what these new technologies are capable of, and what questions they might answer. One approach, ion mobility (Waters Synapt, AB Sciex SelexION), allows separate detection of lipids that have the same accurate mass (e.g. elemental composition) and retention time on HPLC, giving an extra layer of molecular differentiation that was not possible before. This is based on the differing “mobility” of the ions in a carrier gas. For example two triacylglycerides (TAG) each containing two stearic acids (SA) and one palmitic acid (PA), but with the PA at different positions on the glyceride backbone can be distinguished. A second approach, using Fourier transform instruments, such as the Orbitrap (ThermoFisher), allow rapid high-resolution scanning that differentiates between lipids based on mass differences down to 1-5 ppm (e.g. can distinguish co-eluting lipids with masses of m/z 516.280 and 516.289) (Figure 4 B). Coupled with HPLC, these instruments are particularly suited to structural characterization as well as global lipidomic screening approaches. Another configuration of relevance to lipidomics is the combination of matrix-assisted laser desorption/ionization (MALDI) with high resolution MS (e.g. the MALDI Synapt G2, Waters), which is currently being applied to lipid imaging in tissue samples to determine spatial localization of molecular species, e.g. in lung 30 (Figure 4 C) The resolution of lipid imaging, however, is not yet at the cellular or sub-cellular level. Potential applications and new frontiers for lipidomic MS will be described in the General Summary (Section 3).
1.4 Methodological issues, working with platelet lipids in vitro
Washed human platelets are isolated from whole blood using centrifugation. Care needs to be taken during blood letting, thus it is important to use a needle that allows the blood to flow freely to ensure that platelets are not exposed to shear. This can itself activate the cells to generate lipid mediators even before isolation. Several protocols are listed in detail in 31 and generally involve a low speed centrifugation of whole blood anticoagulated with acid-citrate-dextrose (ACD, lowers pH and chelates Ca2+) to generate platelet-rich-plasma followed by a faster centrifugation of the recovered plasma to pellet the platelets themselves. This is followed by a single wash step in Tyrode’s buffer with ACD (9:1). Anticoagulants such as prostacyclin and indomethacin are included during washing by many investigators, but should be avoided in studies of lipids, to avoid interference to lipid-sensitive signaling pathways. In this case, care needs to be taken, as the platelets may activate after the last spin when anticoagulant is removed. The plasma/cells need to be maintained at around 20°, and gently pipetted at all times. On final resuspension in Tyrode’s buffer they may appear slightly clumpy but after approximately 15-20 min will have dispersed to a single cell suspension, without requiring manipulation. It is advisable to avoid repeated pipetting, or shaking/inverting of the cells during isolation and handling. Once isolated, platelets should be kept at room temperature and used within 2-3 hrs.
Platelet lipids can be isolated using several different methods, including liquid:liquid extraction methods such as Bligh and Dyer using chloroform/methanol, or as in our lab, hexane/isopropanol based solvent mixtures that broadly extract most molecular species32, 33. The advantage of the latter is lipid extraction into the upper rather than lower organic phase. Specific lipids such as eicosanoids can also be more selectively isolated using solid phase extraction methods, such as C18 columns34. Once isolated, we typically resuspend in a small volume of methanol, or chloroform/methanol when highly concentrated, and store at −80 before analysis, under inert gas.
Unsaturated lipids can undergo facile oxidation ex vivo, so they need careful handling. Lipid extracts should be kept on ice when in use, but stored under inert gas at −80°C. While some investigators routinely include metal chelators and antioxidants when studying oxidized species, this isn’t generally necessary with platelet lipid extracts, if they are handled appropriately, and analyzed within a few weeks of generation. As confirmation, we only detect expected enantiomeric species of eicosanoids specific to platelet enzymes (e.g. PGE2, 12-HETE, but very little 8-iso-PGE2 or other HETE isomers) in our assays 26, 35. While antibody based ELISA kits for eicosanoids are commercially available, and may seem attractive where MS lipidomics is not locally available, these should be avoided for measurement of lipids in complex samples due to lack of antibody specificity. On the other hand, they may be acceptable for washed platelet eicosanoid measurements, since only a selected low number of eicosanoids and related species are formed, but careful validation and comparison should be performed with MS before use.
2. Specific lipid families in platelets
2.1 Aminophospholipids
Like all mammalian cells, the prominent PL in platelets are PC and PE accounting for approximately 40 % and 28 % of total phospholipids, respectively. SM and PS are also relatively abundant (approximately 18 %, 10 %) with smaller proportions of PI (3-5 %) 1, 36, 37. The FA composition of platelet PLs was described in Section 1.2 above, and varies for each PL class. Studies in the mid 1970’s, using chemical labeling or exogenous phospholipases established that platelet PLs are distributed asymmetrically between plasma membrane bilayers, with the aminophospholipids (aPL) PE and PS facing the cytosol, and PC and SM facing the outside. On stimulation, a substantial proportion of aPL is externalized generating a procoagulant or thrombogenic surface 38. In contrast, lipids required for intracellular signaling are generated from lipids that face the inside. These include Ins(1,4,5)P3 and DAG generated from phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), hydrolysis of AA to act as a substrate for TX generation and formation of phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P3, also known as PIP3). Thus, PLs act as a major reservoir for substrates for enzymes that generate key platelet signaling mediators. These will be described in more detail below.
In 1977, Zwaal et al noticed that unactivated platelets were inert to plasma coagulation, due to the absence of PS on their surface 39. Later, Bevers et al found that activation of platelets with thrombin/collagen caused exposure of aPL and correlated with procoagulant activity 40. Around this time, and for many years later, studies on mechanisms of phospholipid translocation, primarily in erythrocytes, revealed several regulatory mechanisms that maintain phospholipid asymmetry. These included a flippase (proposed as P4 ATPase) 41, 42, and a floppase which is promoted by ABCC1/multidrug resistance protein MRP1 43, 44. The identities and mechanisms of action of these proteins are still not fully known. Opposing enzymes, termed scramblases, are Ca2+-dependent and required for effective exposure of aPL on the platelet surface in order to promote coagulation. The role of the platelet membrane in acting as a critical mediator of coagulation, through promoting factor activity was first proposed in the 1980’s45. It is now known that at physiological pH, surface exposed PS provides the negatively charged platform that enables calcium ions to form bridges with gamma-carboxyglutamic acid-containing Gla domains on coagulation factors. In the case of the prothrombinase complex, FVa is believed to undergo a conformational change that forms a high-affinity binding site for FXa 46, 47. This brings thrombin generation to the site of platelet activation, enabling coagulation and aggregation to be focused together at the site of hemostatic need. The hunt for the platelet scramblase has been of clinical importance, since its absence accounts for the very rare bleeding disorder, Scott Syndrome, first described in 1979 by Weiss et al 48. In 2010, the involvement of a protein, TMEM16F in supporting scramblase activity in platelets was identified 49. Since then, the five Scott Syndrome patients so far identified have been reported to have different mutations in the TMEM16F gene, including in intron 6 (G-to-A), disrupting the donor splice site consensus sequence, and in exon 11 as a single-nucleotide insertion which predicts a frame shift and premature termination of translation at codon 41150. At this time, whether TMEM16F is the scramblase itself, or an accessory protein is the subject of debate.
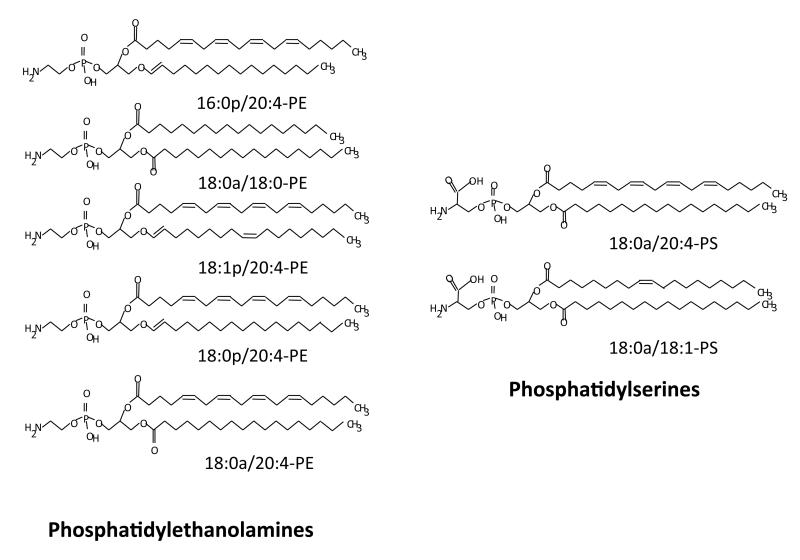
There appear to be at least two independent mechanisms for achieving aPL externalization. Recent studies have established that TMEM16F is required for agonist triggered scramblase, but not that mediated during platelet ageing/apoptosis 51, 52. Also, only agonist triggered trafficking of aPLs requires influx of extracellular Ca2+ 53. Up to now, aPL externalization has generally measured using the flow cytometry probe, Annexin VFITC. However, this gives no information on either the molecular species of aPL nor amounts. Using a newly developed lipidomic assay, where amine headgroups of external facing aPL are derivatized using a cell impermeable-reagent, we recently showed that platelets (agonist activated, apoptotic or energy depleted) externalize approximately 3-5% of the total cellular pool of five PE and two PS distinct molecular species (Figure 5). Those externalized were the most abundant platelet aPL molecular species, with the same for either apoptosis, agonist activation or energy-depletion, indicating that the process is not selective for aPL with particular FA chains 51. We also showed that these aPL were not efficiently externalized in Scott Syndrome platelets, and that for PE, the species externalized by platelets were the most effective in in vitro thrombogenic assays, compared to those with longer or shorter fatty acid chains 51. Thus, FA side chains are molecular determinants that can contribute to the coagulation-regulating activity of PL. The detailed biophysical mechanisms of this are not known, but may relate to accessibility of aPL phosphate groups with Ca2+ and Gla domains. Next, it will be important to compare how other membrane lipids, e.g. CEs, TAGs, and sphingolipids with platelet FAs influence coagulation factor activities. For this, knowing which FAs predominate in platelet plasma membrane lipids of these classes would also be important. Lipidomics will be of central importance in helping answering these questions through defining the composition of the platelet plasma membrane during both health and disease, and different activation states. This could be achieved through either targeted or global lipidomic strategies.
Figure 5. Molecular species of PE and PS that are externalized by activated, ageing or apoptotic human platelets.
2.2. Phosphatidylinositides
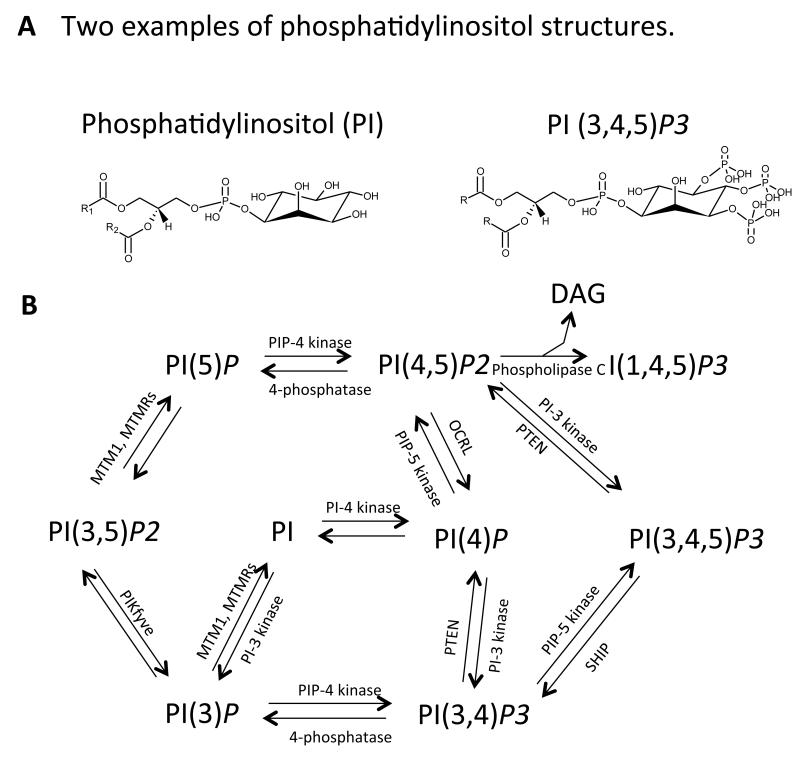
PIs comprise several interrelated species that contain an inositol headgroup. Two are shown in Figure 6, along with a diagram describing their inter-relationship. These lipids play important roles in cell signaling in health and disease, through reversible phosphorylation of the inositol headgroup that is tightly controlled by enzymes. The phosphoinositide PI(4,5)P2 is metabolized in response to receptor activation through two distinct pathways, namely (i) irreversible cleavage of the phosphodiester bond and (ii) reversible phosphorylation of the inositol headgroup. The latter generates the major product of the PI 3-kinase pathway, PI(3,4,5)P3 (or PIP3), and the former, the two second messengers, DAG and Ins(1,4,5)P3, which activate protein kinase C and mobilize intracellular Ca2+, respectively.
Figure 6. Phosphoinositides and their metabolic pathways.
Panel A. Structure of PI and PI(3,4,5)P3. Panel B. The known PIs, their kinases and phosphatases. DAG: diacyglyceride, SHIP: SH2 domain-containing inositol-5-phosphatase, MTMR, myotubularin-related phosphoinositides phosphatase, OCRL: oculocerebrorenal syndrome of Lowe, PIKfyve: phosphoinositides kinase with specificity for the five position containing a FYVE finger. R refers to fatty acid side chains.
(i) The phosphodiester cleavage of PI(4,5)P2 is mediated by the phospholipase C (PLC) family of phosphodiesterases, which is comprised of ten isoforms, several of which are regulated by the major G protein-coupled and tyrosine kinase-linked receptors in platelets. DAG activates several isoforms of PKC which, in combination with the release of intracellular Ca2+ by Ins(1,4,5)P3, leads to powerful platelet activation responses, such as shape change, aggregation and secretion. Platelets express variants of PLC-β (β1, β2, β3, β4 and PLC-γ2 in human) 54-57. PLC-β is stimulated by G protein-coupled receptors (including TX and PAR1/4), while PLC-γ is stimulated by single transmembrane glycoprotein receptors (e.g. GPIIb-IIIa, GPIba, GPVI) 58. Ins(1,4,5)P3 activates receptors that function as Ca2+ channels in the dense tubular system.
(ii) PI(4,5)P2 is also converted to the lipid second messenger, PI(3,4,5)P3, by the Class I family of PI 3-kinases. These catalyze the phosphorylation of PI, PI(4)P or PI(4,5)P2 at position of 3 of the inositol ring 59. PI3Ks are divided into Class I, II and III, with Class I further subdivided into α, β, δ and γ isoforms. Platelets contain all Class I isoforms of PI3K, although the level of the δ isoform is are lower than others 60. Stimulation of platelet G protein-coupled receptors results in phosphorylation of PI(4,5)P2 by the PI3Kγ isoform to generate PI(3,4,5)P3. Deficiency of α, β and γ isoforms of PI3K in mice results in a mild platelet aggregation defect and impaired thrombosis in vivo 61. In vitro, PI3Kγ-deficient platelets disaggregate faster following ADP activation and show mildly impaired ability to mobilize intracellular Ca2+ 62. Several studies show that this isoform controls a major part of platelet ADP responses 61, 63, 64. The protein targets for PI(3,4,5)P3 binding and activation downstream of PI3Kγ that promote ADP signaling include Akt isoforms. The PI3Kβ isoform has been proposed to play several roles in regulating platelet activation, including via promoting integrin-dependent Ca2+ flux and Gi dependent activation of Rap1b. In recent years, a small molecule inhibitor of PI3Kβ, TGX-221, has been developed as a potential antithrombotic therapy 60, 65, 66.
PI(3,4,5)P3 binds to a domain of approximately 120 amino acids, known as a pleckstrin homology (PH) domain, which takes its name from the major PKC substrate in platelets, pleckstrin (which is one of few proteins to have two PH domains). PH domains are found in a variety of signaling and cytoskeletal proteins including, in platelets, phospholipase C (PLC)γ2 and the tyrosine kinase Btk. PH domains are usually found with other domains such as SH2 and SH3 domains which bind to phosphotyrosine and proline rich regions, respectively. PH domains can also bind to other PIs. Additionally, PI(3,4,5)P3 is rapidly metabolized in platelets to PI(3,4)P2 by the Src homology 2 domain containing inositol 5-phosphatase1 (SHIP1), which itself is regulated by tyrosine phosphorylation. This enzyme removes the phosphate at D-5 of the inositol ring generating PI(3,4)P2 which can then be degraded by additional phosphatases. PI(3,4)P2 is generated in large amounts, accumulating slowly, but independently of aggregation and integrin activity 67, 68. It was originally suggested to regulate aggregation, integrin signaling and thrombus growth, but its targets and their overall significance in platelets remain unknown 69, 70. While SHIP1 is clearly established as a regulator of PI lipid levels in platelets, the roles of additional phosphatases also expressed in these cells (notably SHIP2 and PTEN) are currently unclear.
Thus, there is bewildering number of pathways of metabolism of PIs and regulation of PH domain-containing proteins in platelets such that we still have a relatively poor understanding of the role of the role of the ‘PI 3-kinase’ pathway at the molecular level. On the other hand, the functional significance of PI 3-kinase in platelets has been widely demonstrated using mutant mice and both broad spectrum (e.g. wortmannin and LY294002) and PI 3-kinase isoform-specific inhibitors.
Many PIs are generated rapidly and degraded at distinct cellular sites by specific PI-metabolizing enzymes that include lipid kinases, lipid phosphatases, and phospholipases, some of which have been described herein (Figure 4) 71, 72. Several of these enzymes are involved in platelet function during agonist-induced activation. For example, phosphatidylinositol-4-phosphate-5-kinase type I (PIP5KI) phosphorylates phosphatidylinositol-4-phosphate (PI(4)P) to generate PI(4,5)P2 on the plasma membrane 73. Platelets lacking PIP4KI-γ show impaired generation of PI(4,5)P2 and a significant defect in anchoring their cell membranes to the underlying cytoskeleton 74, 75. PI(4,5)P2 has also been proposed to bind talin, and to support its role in integrin activation 76-78. PI(3,4,5)P3 is generated very rapidly during platelet activation, and plays a key role in recruitment and activation of PLCγ2 and other PH domain containing enzymes 79.
Measurement and quantitation of PI molecular species using MS-based lipidomics approaches has been a major goal for several years, particularly in the area of cancer research. However, these lipids are extremely difficult to analyze using LC/MS due primarily to (i) difficulty in achieving efficient extraction, and (ii) in-source fragmentation that occurs on ionization of poly-phosphorylated forms, leading to loss of phosphate groups. A recent review highlighted available methods that allow detection and quantitation of some individual PI species, but to date, there is no single methodology that allows robust identification and quantitation of all 80. This has significantly hampered the study of these species in living cells, since they form transiently and at extremely low concentrations during cell signaling. For example, the specific molecular species of PI regulated in activated platelets including in terms of fatty acid chain length and saturation are still unclear. Development of sensitive and specific methods for quantifying PI molecular species is thus a major ongoing goal in lipidomics, that may become possible as newer methodologies become available, and is of importance in furthering our understanding of the roles of these important platelet lipids in platelet activation and platelet-driven pathologies.
2.3. Fatty acids, eicosanoids and related species
A major early response of platelets to activation is the switching on of several phospholipases including cytosolic phospholipase A2 (cPLA2α) isoforms, which cleave FAs from the sn2 bond of PLs. Early studies demonstrated that the prominent FAs released were AA, LA, PA and SA 81-83. However, the most important in terms of signaling is AA, the precursor for oxidative transformation to several eicosanoids via LOX and COX enzymes (Figure 7). Platelets express several PLA2 isoforms, including Ca2+ sensitive, and insensitive forms. Generally the Ca2+-sensitive cPLA2α is considered the source of AA for eicosanoid generation. In support, a recent study demonstrated that genetic deficiency of this isoform leads to significantly impaired platelet eicosanoid generation, along with platelet dysfunction in humans 84.
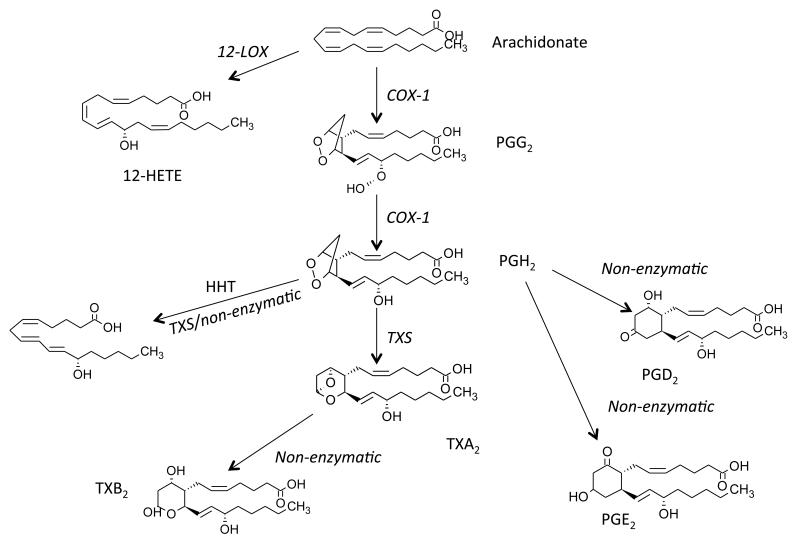
Figure 7. Arachidonate metabolism to form eicosanoids and prostaglandins by COX-1 in platelets.
AA, liberated by PLA2, is oxidized by COX-1 to form PGG2, then PGH2. PGH2 is further metabolized by thromboxane synthase (TXS) to TXA2, which rapidly decomposes to TXB2. PGH2 can also decompose to PGE2, PGD2 and HHT. Separately, AA can be oxidized by 12-LOX to 12-HETE.
Platelet generated eicosanoids are best exemplified by the potent proaggregatory TXA2, generated by coordinated action of COX-1 and thromboxane synthase. This lipid is extremely unstable and rapidly rearranges to TXB2, which is released by platelets at ng amounts per 2×108 cells (Figure 5). TXA2 binds the Gq-coupled receptor TPα with high affinity, triggering activation of PLCβ. A number of receptor agonists to TP have been developed, including U46619 and SQ 26,655 that mimic the action of TXA2. Due to its short plasma half-life, in vivo generation of platelet TX in both humans and mice is best measured through analysis of urinary metabolites, 11-dehydroTXB2 or 2,3-dinor TXB2 85-91. This has been achieved using either GC- or LC/MS, and is considered a reliable surrogate for measurement of in vivo platelet reactivity, that is significantly raised in both cardiovascular disease and smokers92-95.
On the other hand, 12-HETE, generated by 12-LOX is quantitatively more abundant, but does not display any potent bioactivity towards platelets in vitro. In contrast, the 12-LOX deficient mice display a mild hyper-responsiveness to ADP, however their collagen responses were normal96. 12-HHT is also abundant, accounting for up to 30% of the total AA flux through COX-1. This is formed through conversion of PGH2 either nonenzymatically or via thromboxane synthase, although its function is unknown 97-99. Platelets also generate smaller amounts of PGs, particularly PGE2 and PGD2, through non-enzymatic rearrangement of PGH2. They also generate isoprostanes via radical-based biochemistry, notably 8-epi-PGF2α, but levels are approximately 1,000 fold less than corresponding 12-HETE and TX 100, 101. PGE2 is either pro- (low dose) or anti-(high dose) aggregatory, through activation of either EP3 or IP receptors, respectively 102. In contrast, 8-epi-PGF2, a specific isoprostane isomer, is primarily anti-aggregatory through acting as a thromboxane receptor antagonist, although the physiological relevance of this is unclear103-105.
The importance of eicosanoid signaling in terms of platelet physiology and vascular disease is underscored by the widespread use of the COX inhibitor aspirin in prevention of cardiovascular events, as well as an antithrombotic agent in stroke, antiphospholipid syndrome and other pro-thrombotic conditions 106-108. This was established by seminal work from several laboratories notably those of Vane, Samuelsson, Roth & Majerus, Patrono and FitzGerald 20, 109-115, and was recently the subject of the 2013 Grand Prix Scientific (Lefoulon-Delalande Foundation, Institute of France) awarded to Garret FitzGerald and Carlo Patrono. More recently, a number of studies found that low dose (considered platelet-selective) aspirin can reduce the spread of existing cancers as well as the risk of developing others, in particular adenocarcinoma of the gut, some lung cancers and breast and prostate cancer. The mechanisms have not been elucidated, but have been suggested to involve an antiplatelet effect, particularly in terms of preventing spread of existing cancers through the bloodstream 116-121. The biological basis of this is uncertain, in part because platelets are implicated in multiple stages of cancer progression, but it may involve platelet derived signaling lipids that are generated either primarily or secondarily via COX-1 dependent signaling. Lipidomics could help address this question in the future. For example, through targeted or untargeted analysis of platelet lipids that are generated on activation in an aspirin-sensitive manner, candidate molecules that may play a role in cancer progression could be identified. Known lipids that could already be considered would include PGE2 which is known to help cancer cells both evade the immune system and resist drug treatment.
Additional eicosanoids, including leukotrienes (LT) and lipoxins (LX) can be generated through transcellular pathways involving platelet:leukocyte interactions. In 1989, Murphy and co-workers demonstrated that platelets efficiently converted neutrophil-derived LTA4 to LTC4, in mixed cell incubations122. Following this, neutrophil:platelet incubations were shown to generate lipoxin A4 from endogenous substrate, but this require activation with ionophore123. In 1990, receptor mediated interactions were also shown to promote this activity124, 125. However, whether transcellular biosynthesis itself is a significant source of these lipids in vivo is still unclear, since determining cellular origin of lipids in complex biological samples is extremely difficult.
Lipidomics has already been extremely powerful in facilitating studies of platelet eicosanoids, primarily through enabling rapid and accurate, high sensitivity quantitation of these species using LC/MS/MS approaches in both isolated cells and in human and murine disease. Newer lipidomics technologies could further our understanding of the biology of these species, through a number of ways including imaging their spatial and temporal generation in tissues (e.g. MALDI-MS), and discovery and structural characterization of new members of this important class of biomolecules, using high resolution scanning methods.
2.4. Oxidized phospholipids
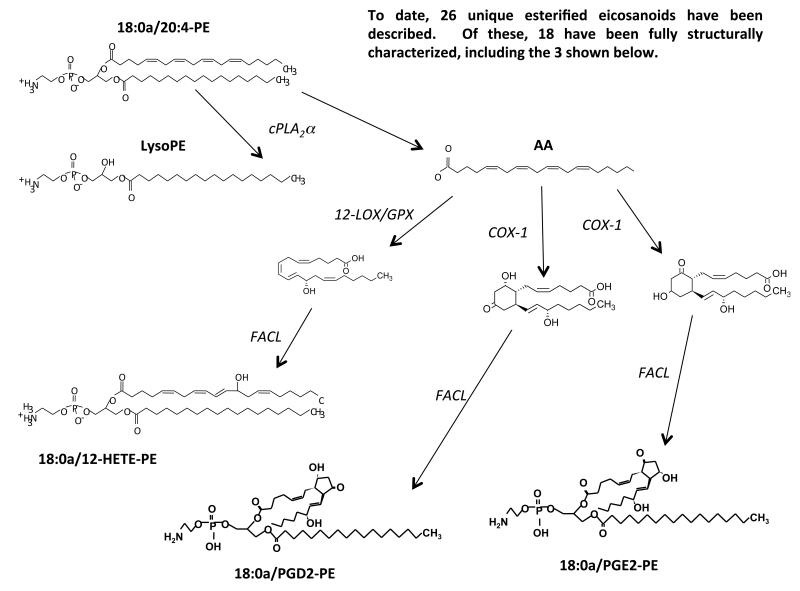
Oxidized PL have long been known as bi-products of non-enzymatic peroxidation, and several are found at relatively high concentrations in atheroma lesions. Recently, we used a targeted lipidomic approach to demonstrate that human platelets generate significant amounts of specific oxidized PL molecular species via enzymatic mechanisms, during acute activation by thrombin, collagen or Ca2+ ionophore 26, 126. This indicates that phospholipid oxidation is not simply an accidental consequence of inflammatory disease, but a regulated process of likely importance during physiological hemostasis. To date, three main families have been described, two from 12-LOX (10 total lipids) and four families of sixteen esterified PGs generated via COX-1 (total 26 lipids) (Figure 8).
Figure 8. Generation of oxidized PLs by enzymes.
Phospholipid substrates (PE is shown here) are hydrolyzed by PLA2 generating AA that is oxidized by COX-1 or 12-LOX to form eicosanoids, which are then re-esterified into PE by fatty acyl Co-A ligases (FACL).
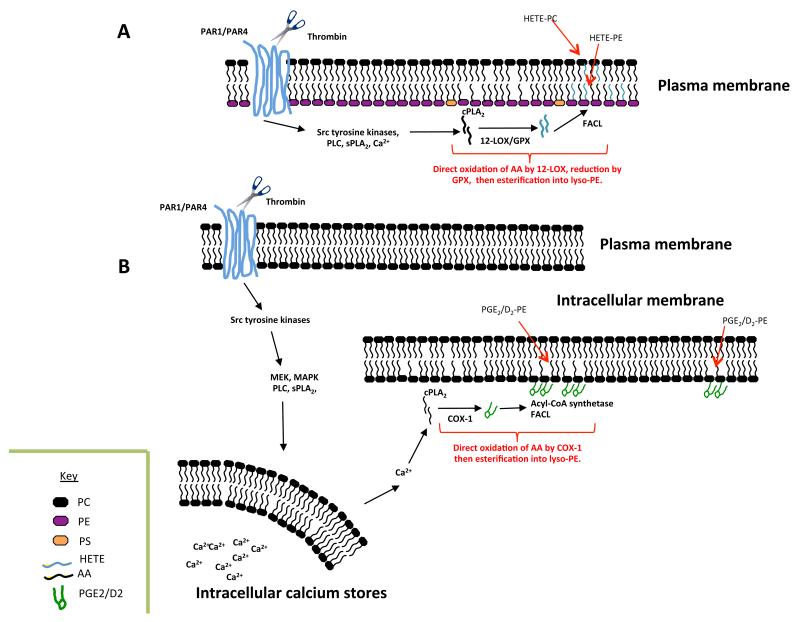
The approach used for their discovery, precursor-LC/MS/MS, is a tandem MS mode that enables molecules to be detected that contain a specific functional group. In this case, we used precursor LC/MS/MS to search specifically for esterified eicosanoids, since on collision-induced-fragmentation, they generate a robust carboxylate anion signal in negative ion mode. Initial studies demonstrated a family of six 12-HETE containing PLs, comprising four PEs and two PCs. The PEs include several plasmalogens and all represent oxidized forms of the most abundant PE and PC species in platelets. The positional and enantiomeric specificity of the HETE was confirmed using several chromatographic approaches, and the lipids are absent in platelets from 12-LOX deficient mice (Aldrovandi and O’Donnell, unpublished). Up to 30% of the total 12-HETE generated by platelet 12-LOX is incorporated into PL, representing a significant proportion of the endogenous pool 26. HETE-PL generation is highly coordinated using intracellular signaling pathways that include Src tyrosine kinases, Ca2+ mobilization and phospholipases, and they remain cell associated following their generation 26. Their formation requires PLA2-dependent hydrolysis of AA from the plasma membrane, followed by its oxidation by 12-LOX, then re-esterification using Co-A-dependent ligases 26. In vitro, liposomes containing physiological amounts of HETE-PLs significantly enhance tissue factor-dependent thrombin generation in plasma 26. This indicates that they may play a key role in hemostasis, and is consistent with the observation that in myeloproliferative disorders, patients with 12-LOX deficiency hemorrhage greater than those with normal levels of the enzyme 127. Pathways for their formation and cellular localization are summarized in Figure 9.
Figure 9. Enzymatic pathways that form oxidized phospholipids in platelets.
Panel A. Thrombin activation of protease activated receptors (PAR) triggers formation of HETE-PE and HETE-PCs, via several intracellular signaling cascades. Glutathione peroxidase (GPX) is required to reduce the initial hydroperoxide product to 12-HETE-PLs 26. Panel B. Formation of PGE2- and PGD2-PE in platelets following thrombin activation requires several intracellular signaling pathways, and most likely occurs in dense tubular membranes, where COX-1 has been localized35.
Platelet 12-LOX also generates four oxidized PLs containing 14-hydroxydocosahexanoic acid (HDOHE), in place of 12-HETE 126. Recently, we scanned for precursors of m/z 351, and uncovered 4 new families of PE-esterified PGs, including four molecular species each of PGE2-PE and PGD2-PE. Similar to the other esterified eicosanoids, these are generated acutely via coordinated pathways, and remain cell associated35. Their inhibition by aspirin or indomethacin in vitro, or in vivo by low dose aspirin indicates a requirement for platelet COX-135. PGs are considerably more hydrophobic than HETEs, and might not be expected to remain within the cell membrane itself. Thus, they may protrude out, anchored by the sn1 fatty acid, in a manner similar to that proposed in the Lipid Whisker Hypothesis, available to interact with receptors on adjacent cells and mediate paracrine signaling 128. Currently, their physiological functions are unknown.
In summary, human platelets generate a diverse array of oxidized PL acutely on activation. We have also found that LOX isoforms in other innate immune cells including neutrophils and monocytes/macrophages generate analogous lipids on activation, indicating that this is a common theme of likely importance in acute response to injury 33, 129-131. All these cell types undergo significant changes to the plasma membrane compartment on activation, including shape change, vesiculation, phagocytosis and microvilli generation. While we know much regarding how proteins control these events, far less is known regarding how lipid oxidation influences the dynamic behavior of the cell membrane. Chemical oxidation of model membranes has long been known to cause changes in headgroup distance, membrane thickness and water permeability, while high concentrations (mg/ml) of purified 15-LOX can cause pore formation in purified organelle membranes, through lipid peroxidation, and its overexpression in non-erythroid cells is associated with mitochondrial membrane collapse 132, 133. Thus, the significant oxidation that occurs on platelet activation might have similar consequences. Future work using biophysical methods will address these questions.
2.5. Lysophospholipids (LysoPL) and lysophosphatidic acid (LPA)
LysoPL and LPA are generated from phospholipids via the actions of phospholipases, either PLA2, PLD or through phosphorylation of DAG. They can also be generated nonenzymatically, although however there is no evidence currently that this happens in platelets134. The structure of LysoPC is shown (Figure 10). During platelet activation, powerful stimulation of cPLA2α occurs, generating AA for eicosanoid generation, and resulting in formation of lysoPLs. Using [14C]SA acid to prelabel PLs, generation of SA-lysoPLs of several classes, including PI, PC, PE and PS was reported in response to stimulation by collagen 135. LysoPE and –PC appeared within 30 sec, with lysoPI and –PS appearing, around 2-3 min. However, the overall increase from the baseline was relatively small (2-3 fold). Although only SA-containing species were studied, platelets might be expected to generate phosphatidic acid and plasmalogen species also for PC and PE, reflecting the predominant molecular species of PL in these cells 135. The temporal generation of specific lysoPL species in platelets is not currently known. These questions have not yet been addressed using lipidomics, but could easily be through either targeted quantitative or MDMS approaches.
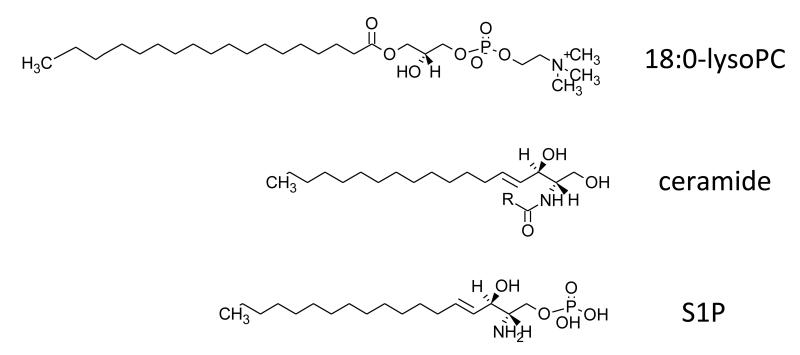
Figure 10. Structures for lysoPC, ceramide and S1P.
R refers to fatty acid group.
LPA is generated by thrombin-stimulated platelets, and can itself stimulate aggregation through G protein-coupled receptors 136. High concentrations exist in serum (5-10 μM) and were suggested to originate directly from platelets, although this would appear unlikely given their rate of production of lysoPL precursors 136, 137. More recently, a key role for autotaxin, a plasma lysophospholase D, in cleaving platelet derived lysoPL to generate high serum levels of LPA has been demonstrated using heterozygous knockout mice (the homozygous knockout is embryonically-lethal) 138, 139. LysoPL substrates themselves originate at least in part via the action of a platelet-derived PLA1 140. At this time, the exact physiological significance of platelet-derived LPA is still not clear, although it is thought to potentially contribute to development of the vasculature, and possibly in atherosclerosis and hypertension 141, 142.
2.6. Neutral lipids: Glycerides, cholesteryl esters and free cholesterol
Neutral lipids are uncharged species that include glycerides, CEs and free cholesterol. In platelets only very small amounts of these are present, with the most abundant being cholesterol at over 90% 3. Free cholesterol is essential for lipid raft function, described in more detail in Section 2.7. Glycerides include TAGs and DAGs, with TAGs representing approximately 2% of neutral lipids 3. Although present at only trace amounts in resting cells, DAG is formed during platelet activation by the action of PLC, as described earlier, and activates classical and novel isoforms of protein kinase C. Thus, DAG is an important second messenger involved in receptor-dependent activation of platelets. Currently, the detailed composition of platelet DAGs generated endogenously, in terms of FAs is not known, nor whether distinct DAG species are more effective as second messengers. These questions could potentially be answered in the future using new generation lipidomic approaches, e.g. through identifying and quantifying platelet-specific DAGs by LC/MS/MS or MDMS methods.
2.7. Sphingolipids and ceramides
SPs are a class of lipids that contain a sphingoid base backbone, the simplest of which is ceramide and consists of sphingosine and a FA (Figure 10). They are involved in both dynamic membrane functions (e.g. lipid raft dependent signaling), and acting as intracellular signaling messengers, although their role in platelets is uncertain 143. Sphingosine is phosphorylated by sphingosine kinases 1 and 2 to form sphingosine-1-phosphate (S1P) (Figure 10), which binds to specific G protein-coupled receptors to influence vascular development, carcinogenesis, chemotaxis and proliferation 25. S1P is generated in platelets via the action of sphingosine kinase, then stored and released on degranulation, contributing to the potent angiogenic activity of serum 144-147. Separately, a lipidomic study characterized the sphingolipid composition of murine platelets and demonstrated that resting cells do not contain S1P, in contrast to human25. Instead, they contained large amounts of dihydroS1P and C24 and C24:1 ceramides. Thrombin activation of mouse platelets in vitro leads to loss of dihydroS1P and ceramide, with major increases in sphingosine and dihydrosphingosine. In vivo, this was observed as an elevation in plasma dihydroS1P. In contrast to this, human plasma contains S1P and also dihydrosphingosine, but little ceramide 25. Separately, stored platelets were found to transfer phospholipids and sphingolipids to newly released extracellular vesicles resulting in increased ceramide and decreased S1P in the cells 28.
S1P has long been recognized as a regulator of hematopoietic cell trafficking, immune regulation, vascular development and brain inflammation, released from activated platelets. More recently, a role for this lipid in thrombopoiesis was demonstrated using genetically deficient mice 148. S1P signaling via the multifunctional S1P1 receptor is required for two specific events involved in platelet formation and release, the directional migration of proplatelet-containing cytoplasmic extensions into the circulatory compartment, and the shedding of proplatelets in a Rac-dependent manner 148, 149. Since it is known that S1P1 couples to Rac activation, this suggests that active signaling via S1P is required for key stages of thrombopoiesis. In support, S1P1 deficient mice display thrombocytopenia 148, 149.
Currently, relatively little is known about how lipid molecular dynamics during cell activation can regulate platelet function, however one area of intense investigation in recent years relates to the formation and action of cholesterol and SM-rich lipid rafts. These are specialized membrane microdomains that compartmentalize cellular processes relevant for signaling, membrane fluidity and protein trafficking. Lipid rafts were proposed in the 1970’s using biophysical approaches, but more formally recognized through the work of Simons and van Meer in the early 2000’s. Raft microdomains are estimated to account for up to 10% of the total surface area, with each containing approximately 3,500 SP molecules and 10-20 protein molecules 150. They are believed to enhance signaling functions by providing platforms that allow clustering of receptors, kinases and adaptor proteins involved in signaling pathways. The ability of functions of two additional proteins involved in adhesion, GP Ib-IX-V and GP VI/Fcγ to activate platelets is dependent on its translocation to lipid rafts 151-155. Furthermore, additional platelet proteins also reported to localize to rafts include the scavenger receptor CD36 and the tetraspanin CD63 156-158. Lipidomic methods have not yet been applied to the characterization of platelet lipid rafts, in terms of profiling individual molecular species. In other cells (KB oral, KBC pancreatic tumor cell lines), these domains are enriched in AA-containing plasmalogen PEs, although the molecular species of SM and PS were not determined 159. Whether platelet rafts differ from this is currently not known. Furthermore, MALDI imaging methodologies are not yet sufficiently high-resolution to enable sub cellular localization of lipids to be directly determined. If this were possible, then determination of raft lipids through approaches that don’t involve cellular disruption using detergents would significantly increase our knowledge of the composition of these specialized domains and generate new information on how lipids regulate key signaling events in platelets.
3. General Summary: Future perspectives and new horizons
New lipidomic methodologies, in particular those afforded by the latest high resolution rapid scanning instruments and imaging methodologies, are allowing us to open an exciting window into the world of lipid mediators in platelet and other cells, both in health and disease. The opportunities presented are considerable, but their potential is yet to be fully realized. Understanding the diversity and number of unique lipid species in cells is of potential importance for elucidating mechanisms of cell biology and disease, and also identifying biomarkers for stratified/personalized medicine. This is one area where new generation lipidomic MS has the potential for transforming our understanding of lipids in health and disease. While pre-lipidomic techniques reported overall composition of FAs in a particular lipid pool (e.g. PLs), individual molecular species could not be detected. Analysing all molecular species is important if particular species display biological actions not shared by others, or are key biomarkers of early disease. Although, this field of research is still in its infancy, it is exemplified by the observation that a particular molecular species of TAG identified using MS can be utilized for early diabetes prediction, and that a specific PC integrates hepatic lipogenesis and peripheral fatty acid use 160,161.
Combining this level of analytical ability with the study of large cohort sample sets, new generation MS technology has the potential to transform our understanding of disease mechanisms. As cohort collections become larger and more comprehensive, it becomes important that ‘omics’ technologies, including lipidomics are both analytically robust and used to investigate relevant questions. Considerable development work is still required in order to analyze large datasets from clinical studies using lipidomic MS screening, but the potential in terms of understanding disease mechanisms is significant. A recent study that highlights this approach used MS to demonstrate a series of PC metabolites in plasma that predict risk of cardiovascular disease. These were found to be generated in the gut through bacterial metabolism of dietary PC, absorbed into the bloodstream and exerted pro-inflammatory effects of relevance to cardiovascular disease 162.
A major challenge lies in development of computational and bioinformatic tools for analysis of the large datasets generated by high-resolution instruments. A number of packages for processing MS data have been developed both by instrumentation companies and research groups, but post-processing software is still lacking. Several databases provide spectral libraries for lipids including LipidMaps, HMDB and Metlin, however coverage of each alone is incomplete. Recently, Kind and colleagues described the creation of a database including in silico generated tandem MS spectra using cheminformatics, which is now freely available (LipidBlast: http://fiehnlab.ucdavis.edu/projects/LipidBlast/) 163. This begins to address the bioinformatics gap, but much remains to be done.
The application of lipidomics technologies can be either exploratory or hypothesis-driven. Many cohort and large dataset studies use lipidomics to compare sets of tissue or plasma samples, where large numbers of abundant lipids (e.g. PLs, CEs, etc) are profiled without a definitive biological or hypothesis-driven question. This can be accomplished using either targeted approaches (e.g. monitoring several lipids from well defined pathways, such as eicosanoids, quantitatively using a triple quad LC/MS/MS approach with deuterated internal standards for all species) but increasingly uses MDMS to profile several families of complex lipids simultaneously, but with less accurate quantitation (a single deuterated standard for one class of lipid is often employed). This is a powerful approach that may lead to discovery of biomarkers that could guide individualized treatment strategies or facilitate monitoring of drug responses (e.g. which patients should be prescribed aspirin, statins or other drugs of relevance to disorders of lipid metabolism) or open new avenues for understanding disease mechanisms, and could be considered analogous to genomic approaches, such as GWAS. On the other hand, targeted lipidomic approaches ask specific hypothesis-driven questions regarding the behavior of lipid mediators in health and disease. As examples, in platelets we have used targeted approaches to demonstrate that FA side chains regulate the procoagulant actions of PE and PS, and that platelets generate novel families of pro-coagulant oxidized PL through enzymatically-controlled pathways26, 51. Both approaches are equally relevant and powerful, but with the new era of personalized/stratified medicine and bioinformatics in medicine, it is likely that a new dawn of discovery for lipid mediators will be driven by exploratory approaches that will further our understanding of their direct roles in human disease. At this time, we can only speculate on the potential applications for lipidomics approaches in furthering our understanding of disease mechanisms and in the development of new therapeutic approaches, but these could involve: (i) predicting risk of disease, e.g. cardiovascular disease or cancer, (ii) making decisions on preventative or treatment strategies, and (iii) monitoring treatment efficacy and guiding ongoing clinical decisions, using plasma, urine or tissue global lipidome features or specific lipid levels.
We currently have no robust estimates of the total number of individual lipid molecular species in platelets, or in any other mammalian cell type. To address this, we are currently developing high resolution LC/MS/MS and in-house generated bioinformatic methodologies to define the total number of unique platelet lipids (knowns and unknowns) and how these change upon agonist stimulation (e.g. thrombin) and COX inhibition (e.g. aspirin) in genetically-unrelated donors (Mondhe and O’Donnell, unpublished data). This is a challenging endeavor, since the number of false signals is high and ensuring accurate identification of real lipids is laborious.
Lipidomic mass spectrometry can be used for screening, the identification of new lipids based on the presence of a characteristic functional group, or to study the movement of lipids from inside to the outside of the cell. In our laboratory, we applied a targeted lipidomic method, termed precursor scanning, to uncover several families of new platelet lipids comprising PLs with PGs, eicosanoids or docosanoids attached, that form within 2-5 min of platelet activation. This was based on the idea that during collision induced fragmentation, a characteristic eicosanoid or PG carboxylate anion would be generated. This is described in Section 2.4 26, 126. Separately, we developed a method that identifies molecular species of PE and PS that traffic to the cell surface upon platelet activation, apoptosis or ageing. This utilizes derivatization of external facing amino-containing PLs using a cell-impermeable reagent, and has allowed us to identify not only which lipids are externalized, but which platelet specific PE/PS are more procoagulant based on side chain FA composition (see Section 2.1) 51.
An important area where lipidomics can be utilized to provide new information is in the characterization of lipid adduction to non-lipid molecules during cell activation, signaling and proliferation. This generates novel species that are readily amenable to MS structural analysis. One example is the reaction of lipid electrophiles with key transcriptional regulators via Michael addition, that occurs during PG-dependent activation of the transcription factor Nrf2164. Additionally, lipids can adduct to proteins to form membrane anchors, e.g. via palmityolation, which is used to localize Src family kinases and the adapter LAT to lipid rafts. Lipidomics combined with proteomic MS could then be used to critically inform on position of lipidation within the protein, we well as the specific lipid itself.
While many challenges remain in ultimately defining the platelet ‘lipidome’, the new advances in technology are likely to make this a reality both in terms of characterization and quantitation of all lipid species. However, this on its own, is only the beginning of a new era. The challenge will be to establish the functional significance of this vast amount of information. Perhaps the biggest area of impact in relation to platelet regulation, will be around the therapeutic manipulation of products of phospholipases, in particular, phospholipases A2, C and D, and in the regulation of PI 3-kinases. It remains to be seen to what extent the variation in lipid backbone influences signaling, and how this varies between donors, health/disease and with diet/drug therapy. With an ageing population, and the critical role of platelets in thrombosis and bleeding, the question will emerge as to whether knowledge of the lipid composition, and functional activity of second messengers such as DAG, can be targeted therapeutically or in the diet. However, the challenge of this is perhaps illustrated by the demonstration of a critical role for PLD in pathological thrombus formation and ischemic stroke as shown using both mutant mice and a small pharmacological inhibitor 165, 166. Activation of PLD contributes to GPIb-mediated activation of platelet integrins, but the molecular basis of this effect, and the possible involvement of formation of DAG from phosphatidic acid, remains to be established.
Acknowledgements
This work was supported in part by grants from the British Heart Foundation (SPW, VOD) and Wellcome Trust (SPW, VOD), and grants from the National Institutes of Health (USA) U54 HL117798 The Personalized NSAID Therapeutics Consortium and R01 ES022172 (RCM). SPW is a British Heart Foundation Chair. We thank Drs Madhav Mondhe, David Slatter and Karin Zemski-Berry for generating panels for Figure 4.
Abbreviations
- aPL
aminophospholipid
- AA
arachidonic acid
- CE
cholesteryl ester
- COX
cyclooxygenase
- ESI
electrospray ionization
- FA
fatty acid
- GC
gas chromatography
- PL
glycerophospholipid
- HPLC
high pressure liquid chromatography
- HETE
hydroxyeicosatetraenoic acid
- HHT
hydroxyheptadecanoic acid
- Ins
inositol
- LA
linoleic acid
- LOX
lipoxygenase
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- LC/MS
liquid chromatography/mass spectrometry
- LPA
lysophosphatidic acid
- OA
oleic acid
- PA
palmitic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PLA2
phospholipase A2
- PG
prostaglandin
- SP
sphingolipids
- SM
sphingomyelin
- SA
stearic acid
- ST
steroid
- TLC
thin layer chromatography
- TX
thromboxane
- ToF
time-of-flight
- DAG
1,2-diacylglycerol
REFERENCES
- 1.Hamid MA, Kunicki TJ, Aster RH. Lipid composition of freshly prepared and stored platelet concentrates. Blood. 1980;55:124–130. [PubMed] [Google Scholar]
- 2.Marcus A, Ullman HL, Safier LB, Ballard HS. Platelet phosphatides. Their fatty acid and aldehyde composition and activity in different clotting systems. The Journal of Clinical Investigation. 1962;41:2198–2212. doi: 10.1172/JCI104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus A, Ullman HL, Safier LB. Lipid composition of subcellular particles of human blood platelets. Journal of Lipid Research. 1969;10:108–114. [PubMed] [Google Scholar]
- 4.Shattil SJ, Bennett JS, Colman RW, Cooper RA. Abnormalities of cholesterol-phospholipid composition in platelets and low-density lipoproteins of human hyperbetalipoproteinemia. The Journal of laboratory and clinical medicine. 1977;89:341–353. [PubMed] [Google Scholar]
- 5.Shastri KM, Carvalho AC, Lees RS. Platelet function and platelet lipid composition in the dyslipoproteinemias. J Lipid Res. 1980;21:467–472. [PubMed] [Google Scholar]
- 6.Strynadka K, McCoy EE. Phospholipid composition of down’s syndrome and normal platelets. Clinical biochemistry. 1978;11:35–37. doi: 10.1016/s0009-9120(78)90628-8. [DOI] [PubMed] [Google Scholar]
- 7.Innis SM, Dyer RA. Dietary canola oil alters hematological indices and blood lipids in neonatal piglets fed formula. The Journal of nutrition. 1999;129:1261–1268. doi: 10.1093/jn/129.7.1261. [DOI] [PubMed] [Google Scholar]
- 8.Renaud S, Dumont E, Godsey F, Suplisson A, Thevenon C. Platelet functions in relation to dietary fats in farmers from two regions of france. Thrombosis and haemostasis. 1979;40:518–531. [PubMed] [Google Scholar]
- 9.Santos MT, Valles J, Aznar J, Beltran M, Herraiz M. Effect of smoking on plasma and platelet fatty acid composition in middle-aged men. Atherosclerosis. 1984;50:53–62. doi: 10.1016/0021-9150(84)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Renaud S, Morazain R, Godsey F, Dumont E, Symington IS, Gillanders EM, O’Brien JR. Platelet functions in relation to diet and serum lipids in british farmers. British heart journal. 1981;46:562–570. doi: 10.1136/hrt.46.5.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood DA, Butler S, Riemersma RA, Thomson M, Oliver MF, Fulton M, Birtwhistle A, Elton R. Adipose tissue and platelet fatty acids and coronary heart disease in scottish men. Lancet. 1984;2:117–121. doi: 10.1016/s0140-6736(84)91044-4. [DOI] [PubMed] [Google Scholar]
- 12.Salo MK, Vartiainen E, Puska P, Nikkari T. Platelet aggregation in finnish men and its relation to fatty acids in platelets, plasma and adipose tissue. Thrombosis and haemostasis. 1985;54:563–569. [PubMed] [Google Scholar]
- 13.Aznar J, Santos MT, Valles J, Martinez-Sausor V. Effect of oral contraceptives on plasma and platelet lipid composition. Influence of the length duration of time of ingestion. Acta obstetricia et gynecologica Scandinavica. 1986;65:33–40. doi: 10.3109/00016348609158226. [DOI] [PubMed] [Google Scholar]
- 14.Matveev Iu A. essential fatty acid allowance in patients with malabsorption syndrome. Voprosy pitaniia. 1981:56–60. [PubMed] [Google Scholar]
- 15.Bottomley JM, Hanington E, Jones RJ, Chapman D. Platelet lipid composition in human migraine. Headache. 1982;22:256–260. doi: 10.1111/j.1526-4610.1982.hed2206256.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanders TA, Younger KM. The effect of dietary supplements of omega 3 polyunsaturated fatty acids on the fatty acid composition of platelets and plasma choline phosphoglycerides. The British journal of nutrition. 1981;45:613–616. doi: 10.1079/bjn19810139. [DOI] [PubMed] [Google Scholar]
- 17.Brox JH, Killie JE, Gunnes S, Nordoy A. The effect of cod liver oil and corn oil on platelets and vessel wall in man. Thrombosis and haemostasis. 1981;46:604–611. [PubMed] [Google Scholar]
- 18.Blomstrand R, Diczfalusy U, Sisfontes L, Svensson L. Influence of dietary partially hydrogenated vegetable and marine oils on membrane composition and function of liver microsomes and platelets in the rat. Lipids. 1985;20:283–295. doi: 10.1007/BF02534261. [DOI] [PubMed] [Google Scholar]
- 19.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skeaff CM, Holub BJ. Altered phospholipid composition of plasma membranes from thrombin-stimulated human platelets. Biochimica et biophysica acta. 1985;834:164–171. doi: 10.1016/0005-2760(85)90152-3. [DOI] [PubMed] [Google Scholar]
- 22.Prescott SM, Majerus PW. The fatty acid composition of phosphatidylinositol from thrombin-stimulated human platelets. J Biol Chem. 1981;256:579–582. [PubMed] [Google Scholar]
- 23.Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass spectrometry reviews. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balazy M. Eicosanomics: Targeted lipidomics of eicosanoids in biological systems. Prostaglandins Other Lipid Mediat. 2004;73:173–180. doi: 10.1016/j.prostaglandins.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Dahm F, Nocito A, Bielawska A, Lang KS, Georgiev P, Asmis LM, Bielawski J, Madon J, Hannun YA, Clavien PA. Distribution and dynamic changes of sphingolipids in blood in response to platelet activation. Journal of thrombosis and haemostasis : JTH. 2006;4:2704–2709. doi: 10.1111/j.1538-7836.2006.02241.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, Goodall AH, Hamali HA, Collins PW, O’Donnell VB. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285:6891–6903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leidl K, Liebisch G, Richter D, Schmitz G. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochimica et biophysica acta. 2008;1781:655–664. doi: 10.1016/j.bbalip.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Pienimaeki-Roemer A, Ruebsaamen K, Boettcher A, Orso E, Scherer M, Liebisch G, Kilalic D, Ahrens N, Schmitz G. Stored platelets alter glycerophospholipid and sphingolipid species, which are differentially transferred to newly released extracellular vesicles. Transfusion. 2013;53:612–626. doi: 10.1111/j.1537-2995.2012.03775.x. [DOI] [PubMed] [Google Scholar]
- 29.Ruebsaamen K, Liebisch G, Boettcher A, Schmitz G. Lipidomic analysis of platelet senescence. Transfusion. 2010;50:1665–1676. doi: 10.1111/j.1537-2995.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 30.Berry KA, Li B, Reynolds SD, Barkley RM, Gijon MA, Hankin JA, Henson PM, Murphy RC. Maldi imaging ms of phospholipids in the mouse lung. J Lipid Res. 2011;52:1551–1560. doi: 10.1194/jlr.M015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson S, Authi KS. Platelets, a practical approach. IRL Press; Oxford: 1996. [Google Scholar]
- 32.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 33.Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, Baker PR, Coles B, Coffey MJ, Kuhn H, O’Donnell VB. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem. 2007;282:20151–20163. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- 34.Maskrey BH, O’Donnell VB. Analysis of eicosanoids and related lipid mediators using mass spectrometry. Biochem Soc Trans. 2008;36:1055–1059. doi: 10.1042/BST0361055. [DOI] [PubMed] [Google Scholar]
- 35.Aldrovandi M, Hammond VJ, Podmore H, Hornshaw M, Clark SR, Marnett LJ, Slatter DA, Murphy RC, Collins PW, O’Donnell VB. Human platelets generate phospholipid-esterified prostaglandins via cyclooxygenase-1 that are inhibited by low dose aspirin supplementation. J Lipid Res. 2013 doi: 10.1194/jlr.M041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen P, Derksen A. Comparison of phospholipid and fatty acid composition of human erythrocytes and platelets. British Journal of Haematology. 1969;17:13. doi: 10.1111/j.1365-2141.1969.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 37.Owen JS, Hutton RA, Day RC, Bruckdorfer KR, McIntyre N. Platelet lipid composition and platelet aggregation in human liver disease. J Lipid Res. 1981;22:423–430. [PubMed] [Google Scholar]
- 38.Spangenberg P, Heller R, Wagner C, Till U. Localization of phosphatidylethanolamine in the plasma membrane of diamide-treated human blood platelets. Biomedica biochimica acta. 1985;44:1335–1341. [PubMed] [Google Scholar]
- 39.Zwall R, Comfurius P, van Deenen LLM. Membrane asymmetry and blood coagulation. Nature. 1977;268:3. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- 40.Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. European journal of biochemistry / FEBS. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 41.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of p-type atpases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 42.Paulusma CC, Elferink RP. P4 atpases--the physiological relevance of lipid flipping transporters. FEBS letters. 2010;584:2708–2716. doi: 10.1016/j.febslet.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 43.Dekkers DW, Comfurius P, Schroit AJ, Bevers EM, Zwaal RF. Transbilayer movement of nbd-labeled phospholipids in red blood cell membranes: Outward-directed transport by the multidrug resistance protein 1 (mrp1) Biochemistry. 1998;37:14833–14837. doi: 10.1021/bi981011w. [DOI] [PubMed] [Google Scholar]
- 44.Kamp D, Haest CW. Evidence for a role of the multidrug resistance protein (mrp) in the outward translocation of nbd-phospholipids in the erythrocyte membrane. Biochimica et biophysica acta. 1998;1372:91–101. doi: 10.1016/s0005-2736(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 45.Tracy PB, Eide LL, Mann KG. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J Biol Chem. 1985;260:2119–2124. [PubMed] [Google Scholar]
- 46.Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor va in the prothrombinase complex. J Biol Chem. 1980;255:274–283. [PubMed] [Google Scholar]
- 47.Bevers EM, Rosing J, Zwaal RF. Development of procoagulant binding sites on the platelet surface. Advances in experimental medicine and biology. 1985;192:359–371. doi: 10.1007/978-1-4615-9442-0_25. [DOI] [PubMed] [Google Scholar]
- 48.Weiss H, Vicic WJ, Lages BA, Rogers J. Isolated deficiency of platelet procoagulant activity. Am J Med. 1979;67:206–213. doi: 10.1016/0002-9343(79)90392-9. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by tmem16f. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 50.Castoldi E, Collins PW, Williamson PL, Bevers EM. Compound heterozygosity for 2 novel tmem16f mutations in a patient with scott syndrome. Blood. 2011;117:4399–4400. doi: 10.1182/blood-2011-01-332502. [DOI] [PubMed] [Google Scholar]
- 51.Clark SR, Thomas CP, Hammond VJ, Aldrovandi M, Wilkinson GW, Hart KW, Murphy RC, Collins PW, O’Donnell VB. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5875–5880. doi: 10.1073/pnas.1222419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Kruchten R, Mattheij NJ, Saunders C, Feijge MA, Swieringa F, Wolfs JL, Collins PW, Heemskerk JW, Bevers EM. Both tmem16f-dependent and tmem16f-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood. 2013;121:1850–1857. doi: 10.1182/blood-2012-09-454314. [DOI] [PubMed] [Google Scholar]
- 53.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O’Reilly LA, Henley KJ, Ono A, Hsiao S, Willcox A, Roberts AW, Huang DC, Salem HH, Kile BT, Jackson SP. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 54.Banno Y, Yu A, Nakashima T, Homma Y, Takenawa T, Nozawa Y. Purification and characterization of a cytosolic phosphoinositide-phospholipase c (gamma 2-type) from human platelets. Biochemical and biophysical research communications. 1990;167:396–401. doi: 10.1016/0006-291x(90)92035-x. [DOI] [PubMed] [Google Scholar]
- 55.Torti M, Lapetina EG. Role of rap1b and p21ras gtpase-activating protein in the regulation of phospholipase c-gamma 1 in human platelets. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7796–7800. doi: 10.1073/pnas.89.16.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 57.Rhee SG, Choi KD. Multiple forms of phospholipase c isozymes and their activation mechanisms. Advances in second messenger and phosphoprotein research. 1992;26:35–61. [PubMed] [Google Scholar]
- 58.Min SH, Abrams CS. Regulation of platelet plug formation by phosphoinositide metabolism. Blood. 2013;122:1358–1365. doi: 10.1182/blood-2013-05-427716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annual review of biochemistry. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 60.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. Pi 3-kinase p110beta: A new target for antithrombotic therapy. Nature medicine. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in pi3kgamma-deficient mice. FASEB J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 62.Lian L, Wang Y, Draznin J, Eslin D, Bennett JS, Poncz M, Wu D, Abrams CS. The relative role of plcbeta and pi3kgamma in platelet activation. Blood. 2005;106:110–117. doi: 10.1182/blood-2004-05-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lova P, Paganini S, Hirsch E, Barberis L, Wymann M, Sinigaglia F, Balduini C, Torti M. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the gi-dependent activation of platelet rap1b. J Biol Chem. 2003;278:131–138. doi: 10.1074/jbc.M204821200. [DOI] [PubMed] [Google Scholar]
- 64.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of rap1b by g(i) family members in platelets. J Biol Chem. 2002;277:23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 65.Bird JE, Smith PL, Bostwick JS, Shipkova P, Schumacher WA. Bleeding response induced by anti-thrombotic doses of a phosphoinositide 3-kinase (pi3k)-beta inhibitor in mice. Thrombosis research. 2011;127:560–564. doi: 10.1016/j.thromres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Laurent PA, Severin S, Gratacap MP, Payrastre B. Class i pi 3-kinases signaling in platelet activation and thrombosis: Pdk1/akt/gsk3 axis and impact of pten and ship1. Advances in biological regulation. 2013 doi: 10.1016/j.jbior.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Guinebault C, Payrastre B, Racaud-Sultan C, Mazarguil H, Breton M, Mauco G, Plantavid M, Chap H. Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85 alpha with actin filaments and focal adhesion kinase. The Journal of cell biology. 1995;129:831–842. doi: 10.1083/jcb.129.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Payrastre B, Missy K, Giuriato S, Bodin S, Plantavid M, Gratacap M. Phosphoinositides: Key players in cell signalling, in time and space. Cellular signalling. 2001;13:377–387. doi: 10.1016/s0898-6568(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 69.Maxwell MJ, Yuan Y, Anderson KE, Hibbs ML, Salem HH, Jackson SP. Ship1 and lyn kinase negatively regulate integrin alpha iib beta 3 signaling in platelets. J Biol Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 70.Severin S, Gratacap MP, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. The Journal of clinical investigation. 2007;117:944–952. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heck JN, Mellman DL, Ling K, Sun Y, Wagoner MP, Schill NJ, Anderson RA. A conspicuous connection: Structure defines function for the phosphatidylinositol-phosphate kinase family. Critical reviews in biochemistry and molecular biology. 2007;42:15–39. doi: 10.1080/10409230601162752. [DOI] [PubMed] [Google Scholar]
- 72.Krauss M, Haucke V. Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO reports. 2007;8:241–246. doi: 10.1038/sj.embor.7400919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, Petrich BG, Monkley SJ, Kanaho Y, Critchley DR, Sasaki T, Birnbaum MJ, Weisel JW, Hartwig J, Abrams CS. Loss of pip5kigamma, unlike other pip5ki isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. The Journal of clinical investigation. 2008 doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Zhao L, Suzuki A, Lian L, Min SH, Wang Z, Litvinov RI, Stalker TJ, Yago T, Klopocki AG, Schmidtke DW, Yin H, Choi JK, McEver RP, Weisel JW, Hartwig JH, Abrams CS. Platelets lacking pip5kigamma have normal integrin activation but impaired cytoskeletal-membrane integrity and adhesion. Blood. 2013;121:2743–2752. doi: 10.1182/blood-2012-07-445205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the ferm domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 77.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 78.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type i gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 79.Bodin S, Viala C, Ragab A, Payrastre B. A critical role of lipid rafts in the organization of a key fcgammariia-mediated signaling pathway in human platelets. Thrombosis and haemostasis. 2003;89:318–330. [PubMed] [Google Scholar]
- 80.Wakelam MJ, Clark J. Methods for analyzing phosphoinositides using mass spectrometry. Biochimica et biophysica acta. 2011;1811:758–762. doi: 10.1016/j.bbalip.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Derksen A, Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975;250:9342–9347. [PubMed] [Google Scholar]
- 82.Bills TK, Smith JB, Silver MJ. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. The Journal of clinical investigation. 1977;60:1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rittenhouse-Simmons S, Deykin D. The mobilization of arachidonic acid in platelets exposed to thrombin or ionophore a23187. Effects of adenosine triphosphate deprivation. The Journal of clinical investigation. 1977;60:495–498. doi: 10.1172/JCI108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adler DH, Cogan JD, Phillips JA, 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cpla(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. The Journal of clinical investigation. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belton OA, Duffy A, Toomey S, Fitzgerald DJ. Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein e knockout mouse model of atherosclerosis. Circulation. 2003;108:3017–3023. doi: 10.1161/01.CIR.0000104565.78013.AD. [DOI] [PubMed] [Google Scholar]
- 86.Egan KM, Wang M, Fries S, Lucitt MB, Zukas AM, Pure E, Lawson JA, FitzGerald GA. Cyclooxygenases, thromboxane, and atherosclerosis: Plaque destabilization by cyclooxygenase-2 inhibition combined with thromboxane receptor antagonism. Circulation. 2005;111:334–342. doi: 10.1161/01.CIR.0000153386.95356.78. [DOI] [PubMed] [Google Scholar]
- 87.Kearney D, Byrne A, Crean P, Cox D, Fitzgerald DJ. Optimal suppression of thromboxane a(2) formation by aspirin during percutaneous transluminal coronary angioplasty: No additional effect of a selective cyclooxygenase-2 inhibitor. Journal of the American College of Cardiology. 2004;43:526–531. doi: 10.1016/j.jacc.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 88.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd Formation of unique biologically active prostaglandins in vivo by a non-cyclooxygenase free radical catalyzed mechanism. Advances in prostaglandin, thromboxane, and leukotriene research. 1991;21A:125–128. [PubMed] [Google Scholar]
- 89.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane b2 by gas chromatography-mass spectrometry. Journal of chromatography. 1993;612:179–185. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 90.Morrow JD, Prakash C, Awad JA, Duckworth TA, Zackert WE, Blair IA, Oates JA, Roberts LJ., 2nd Quantification of the major urinary metabolite of prostaglandin d2 by a stable isotope dilution mass spectrometric assay. Analytical biochemistry. 1991;193:142–148. doi: 10.1016/0003-2697(91)90054-w. [DOI] [PubMed] [Google Scholar]
- 91.Morrow JD, Prakash C, Duckworth TA, Zackert WE, Blair IA, Oates JA, Roberts LJ., 2nd A stable isotope dilution mass spectrometric assay for the major urinary metabolite of pgd2. Advances in prostaglandin, thromboxane, and leukotriene research. 1991;21A:315–318. [PubMed] [Google Scholar]
- 92.Nowak J, Murray JJ, Oates JA, FitzGerald GA. Biochemical evidence of a chronic abnormality in platelet and vascular function in healthy individuals who smoke cigarettes. Circulation. 1987;76:6–14. doi: 10.1161/01.cir.76.1.6. [DOI] [PubMed] [Google Scholar]
- 93.Henriksson P, Wennmalm A, Edhag O, Vesterqvist O, Green K. In vivo production of prostacyclin and thromboxane in patients with acute myocardial infarction. British heart journal. 1986;55:543–548. doi: 10.1136/hrt.55.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knapp HR, Healy C, Lawson J, FitzGerald GA. Effects of low-dose aspirin on endogenous eicosanoid formation in normal and atherosclerotic men. Thrombosis research. 1988;50:377–386. doi: 10.1016/0049-3848(88)90267-8. [DOI] [PubMed] [Google Scholar]
- 95.Montine TJ, Sonnen JA, Milne G, Baker LD, Breitner JC. Elevated ratio of urinary metabolites of thromboxane and prostacyclin is associated with adverse cardiovascular events in adapt. PloS one. 2010;5:e9340. doi: 10.1371/journal.pone.0009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to adp in mice lacking platelet-type 12-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hecker M, Haurand M, Ullrich V, Diczfalusy U, Hammarstrom S. Products, kinetics, and substrate specificity of homogeneous thromboxane synthase from human platelets: Development of a novel enzyme assay. Archives of biochemistry and biophysics. 1987;254:124–135. doi: 10.1016/0003-9861(87)90088-9. [DOI] [PubMed] [Google Scholar]
- 98.Anderson MW, Crutchley DJ, Tainer BE, Eling TE. Kinetic studies on the conversion of prostaglandin endoperoxide pgh2 by thromboxane synthase. Prostaglandins. 1978;16:563–570. doi: 10.1016/0090-6980(78)90186-7. [DOI] [PubMed] [Google Scholar]
- 99.Sadowitz PD, Setty BN, Stuart M. The platelet cyclooxygenase metabolite 12-lhydroxy-5, 8, 10-hepta-decatrienoic acid (hht) may modulate primary hemostasis by stimulating prostacyclin production. Prostaglandins. 1987;34:749–763. doi: 10.1016/0090-6980(87)90297-8. [DOI] [PubMed] [Google Scholar]
- 100.Pratico D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of the isoprostane, 8-epi prostaglandin f2 alpha. J Biol Chem. 1995;270:9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- 101.Pratico D, Lawson JA, Fitzgerald GA. Cyclooxygenase dependent formation of 8-isoprostaglandin f2 alpha by human platelets. Advances in prostaglandin, thromboxane, and leukotriene research. 1995;23:229–231. [PubMed] [Google Scholar]
- 102.Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH. Activation of the murine ep3 receptor for pge2 inhibits camp production and promotes platelet aggregation. The Journal of clinical investigation. 2001;107:603–610. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morrow JD, Minton TA, Roberts LJ., 2nd The f2-isoprostane, 8-epi-prostaglandin f2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992;44:155–163. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 104.Leitinger N, Blazek I, Sinzinger H. The influence of isoprostanes on adp-induced platelet aggregation and cyclic amp-generation in human platelets. Thrombosis research. 1997;86:337–342. doi: 10.1016/s0049-3848(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 105.Smith JP, Haddad EV, Downey JD, Breyer RM, Boutaud O. Pge2 decreases reactivity of human platelets by activating ep2 and ep4. Thrombosis research. 2010;126:e23–29. doi: 10.1016/j.thromres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raju NC, Eikelboom JW. The aspirin controversy in primary prevention. Current opinion in cardiology. 2012;27:499–507. doi: 10.1097/HCO.0b013e328356ae95. [DOI] [PubMed] [Google Scholar]
- 107.Casado-Arroyo R, Bayrak F, Sarkozy A, Chierchia GB, de Asmundis C, Brugada P. Role of asa in the primary and secondary prevention of cardiovascular events. Best practice & research. Clinical gastroenterology. 2012;26:113–123. doi: 10.1016/j.bpg.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 108.Les I, Ruiz-Irastorza G, Khamashta MA. Intensity and duration of anticoagulation therapy in antiphospholipid syndrome. Seminars in thrombosis and hemostasis. 2012;38:339–347. doi: 10.1055/s-0032-1304720. [DOI] [PubMed] [Google Scholar]
- 109.Ferreira SH, Moncada S, Vane JR. Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. British journal of pharmacology. 1973;49:86–97. doi: 10.1111/j.1476-5381.1973.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, Brash AR. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. The Journal of clinical investigation. 1983;71:676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane a2 from prostaglandin endoperoxides. Nature. 1976;261:558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 112.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. The Journal of clinical investigation. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. The Journal of clinical investigation. 1975;56:624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Catella F, FitzGerald GA. Paired analysis of urinary thromboxane b2 metabolites in humans. Thrombosis research. 1987;47:647–656. doi: 10.1016/0049-3848(87)90103-4. [DOI] [PubMed] [Google Scholar]
- 115.Fitzgerald DJ, Fitzgerald GA. Historical lessons in translational medicine: Cyclooxygenase inhibition and p2y12 antagonism. Circulation research. 2013;112:174–194. doi: 10.1161/CIRCRESAHA.111.300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 117.Rothwell PM, Buchan AM. Mobile acute stroke units: Bringing the hospital to the patient. Lancet neurology. 2012;11:382–383. doi: 10.1016/S1474-4422(12)70069-8. [DOI] [PubMed] [Google Scholar]
- 118.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 119.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 120.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 121.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 122.Maclouf JA, Murphy RC. Transcellular metabolism of neutrophil-derived leukotriene a4 by human platelets. A potential cellular source of leukotriene c4. J Biol Chem. 1988;263:174–181. [PubMed] [Google Scholar]
- 123.Edenius C, Haeggstrom J, Lindgren JA. Transcellular conversion of endogenous arachidonic acid to lipoxins in mixed human platelet-granulocyte suspensions. Biochemical and biophysical research communications. 1988;157:801–807. doi: 10.1016/s0006-291x(88)80320-6. [DOI] [PubMed] [Google Scholar]
- 124.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. The Journal of experimental medicine. 1990;172:1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maclouf J, Murphy RC, Henson PM. Transcellular biosynthesis of sulfidopeptide leukotrienes during receptor-mediated stimulation of human neutrophil/platelet mixtures. Blood. 1990;76:1838–1844. [PubMed] [Google Scholar]
- 126.Morgan LT, Thomas CP, Kuhn H, O’Donnell VB. Thrombin-activated human platelets acutely generate oxidized docosahexaenoic-acid-containing phospholipids via 12-lipoxygenase. Biochem J. 2010;431:141–148. doi: 10.1042/BJ20100415. [DOI] [PubMed] [Google Scholar]
- 127.Schafer AI. Deficiency of platelet lipoxygenase activity in myeloproliferative disorders. The New England journal of medicine. 1982;306:381–386. doi: 10.1056/NEJM198202183060701. [DOI] [PubMed] [Google Scholar]
- 128.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 129.Clark SR, Guy CJ, Scurr MJ, Taylor PR, Kift-Morgan AP, Hammond VJ, Thomas CP, Coles B, Roberts GW, Eberl M, Jones SA, Topley N, Kotecha S, O’Donnell VB. Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection. Blood. 2011;117:2033–2043. doi: 10.1182/blood-2010-04-278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hammond VJ, Morgan AH, Lauder S, Thomas CP, Brown S, Freeman BA, Lloyd CM, Davies J, Bush A, Levonen AL, Kansanen E, Villacorta L, Chen YE, Porter N, Garcia-Diaz YM, Schopfer FJ, O’Donnell VB. Novel keto-phospholipids are generated by monocytes and macrophages, detected in cystic fibrosis, and activate peroxisome proliferator-activated receptor-gamma. J Biol Chem. 2012;287:41651–41666. doi: 10.1074/jbc.M112.405407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morgan AH, Dioszeghy V, Maskrey BH, Thomas CP, Clark SR, Mathie SA, Lloyd CM, Kuhn H, Topley N, Coles BC, Taylor PR, Jones SA, O’Donnell VB. Phosphatidylethanolamine-esterified eicosanoids in the mouse: Tissue localization and inflammation-dependent formation in th-2 disease. J Biol Chem. 2009;284:21185–21191. doi: 10.1074/jbc.M109.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]; A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 133.Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophysical Journal. 2007;93:4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi J, Zhang W, Gu X, Chen X, Hong L, Laird JM, Salomon RG. Lysophosphatidylcholine is generated by spontaneous deacylation of oxidized phospholipids. Chemical research in toxicology. 2011;24:111–118. doi: 10.1021/tx100305b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas LM, Holub BJ. Eicosanoid-dependent and -independent formation of individual [14c]stearoyl-labelled lysophospholipids in collagen-stimulated human platelets. Biochimica et biophysica acta. 1991;1081:92–98. doi: 10.1016/0005-2760(91)90255-g. [DOI] [PubMed] [Google Scholar]
- 136.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochimica et biophysica acta. 1989;1001:282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 138.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 139.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase d, is essential for blood vessel formation during development. Molecular and cellular biology. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bolen AL, Naren AP, Yarlagadda S, Beranova-Giorgianni S, Chen L, Norman D, Baker DL, Rowland MM, Best MD, Sano T, Tsukahara T, Liliom K, Igarashi Y, Tigyi G. The phospholipase a1 activity of lysophospholipase a-i links platelet activation to lpa production during blood coagulation. J Lipid Res. 2011;52:958–970. doi: 10.1194/jlr.M013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y, Meier KE. Lysophospholipase d and its role in lpa production. Cellular signalling. 2004;16:975–981. doi: 10.1016/j.cellsig.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. The Canadian journal of cardiology. 2003;19:1525–1536. [PubMed] [Google Scholar]
- 143.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO reports. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: A platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 145.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 146.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. Journal of biochemistry. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 147.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochimica et biophysica acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Bruhl ML, Tirniceriu A, Gaertner F, Proia RL, Graf T, Bolz SS, Montanez E, Prinz M, Muller A, von Baumgarten L, Billich A, Sixt M, Fassler R, von Andrian UH, Junt T, Massberg S. A novel role of sphingosine 1-phosphate receptor s1pr1 in mouse thrombopoiesis. The Journal of experimental medicine. 2012;209:2165–2181. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hla T, Galvani S, Rafii S, Nachman R. S1p and the birth of platelets. The Journal of experimental medicine. 2012;209:2137–2140. doi: 10.1084/jem.20122284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simons K, Toomre D. Lipid rafts and signal transduction. Nature reviews. Molecular cell biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 151.Shrimpton CN, Borthakur G, Larrucea S, Cruz MA, Dong JF, Lopez JA. Localization of the adhesion receptor glycoprotein ib-ix-v complex to lipid rafts is required for platelet adhesion and activation. The Journal of experimental medicine. 2002;196:1057–1066. doi: 10.1084/jem.20020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ezumi Y, Kodama K, Uchiyama T, Takayama H. Constitutive and functional association of the platelet collagen receptor glycoprotein vi-fc receptor gamma-chain complex with membrane rafts. Blood. 2002;99:3250–3255. doi: 10.1182/blood.v99.9.3250. [DOI] [PubMed] [Google Scholar]
- 153.Locke D, Chen H, Liu Y, Liu C, Kahn ML. Lipid rafts orchestrate signaling by the platelet receptor glycoprotein vi. The Journal of biological chemistry. 2002;277:18801–18809. doi: 10.1074/jbc.M111520200. [DOI] [PubMed] [Google Scholar]
- 154.Sullam PM, Hyun WC, Szollosi J, Dong J, Foss WM, Lopez JA. Physical proximity and functional interplay of the glycoprotein ib-ix-v complex and the fc receptor fcgammariia on the platelet plasma membrane. The Journal of biological chemistry. 1998;273:5331–5336. doi: 10.1074/jbc.273.9.5331. [DOI] [PubMed] [Google Scholar]
- 155.Wonerow P, Obergfell A, Wilde JI, Bobe R, Asazuma N, Brdicka T, Leo A, Schraven B, Horejsi V, Shattil SJ, Watson SP. Differential role of glycolipid-enriched membrane domains in glycoprotein vi- and integrin-mediated phospholipase cgamma2 regulation in platelets. The Biochemical journal. 2002;364:755–765. doi: 10.1042/BJ20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Claas C, Stipp CS, Hemler ME. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–7984. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- 157.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: Implications for human disease. The Journal of cell biology. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. Journal of thrombosis and haemostasis : JTH. 2005;3:1737–1744. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 159.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 160.Liu S, Brown JD, Stanya KJ, Homan E, Leidl M, Inouye K, Bhargava P, Gangl MR, Dai L, Hatano B, Hotamisligil GS, Saghatelian A, Plutzky J, Lee CH. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. The Journal of clinical investigation. 2011;121:1402–1411. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kind T, Liu KH, Lee do Y, Defelice B, Meissen JK, Fiehn O. Lipidblast in silico tandem mass spectrometry database for lipid identification. Nature methods. 2013;10:755–758. doi: 10.1038/nmeth.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: Role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elvers M, Stegner D, Hagedorn I, Kleinschnitz C, Braun A, Kuijpers ME, Boesl M, Chen Q, Heemskerk JW, Stoll G, Frohman MA, Nieswandt B. Impaired alpha(iib)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase d1. Science signaling. 2010;3:ra1. doi: 10.1126/scisignal.2000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stegner D, Thielmann I, Kraft P, Frohman MA, Stoll G, Nieswandt B. Pharmacological inhibition of phospholipase d protects mice from occlusive thrombus formation and ischemic stroke--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2212–2217. doi: 10.1161/ATVBAHA.113.302030. [DOI] [PubMed] [Google Scholar]