Abstract
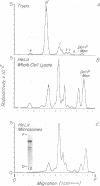
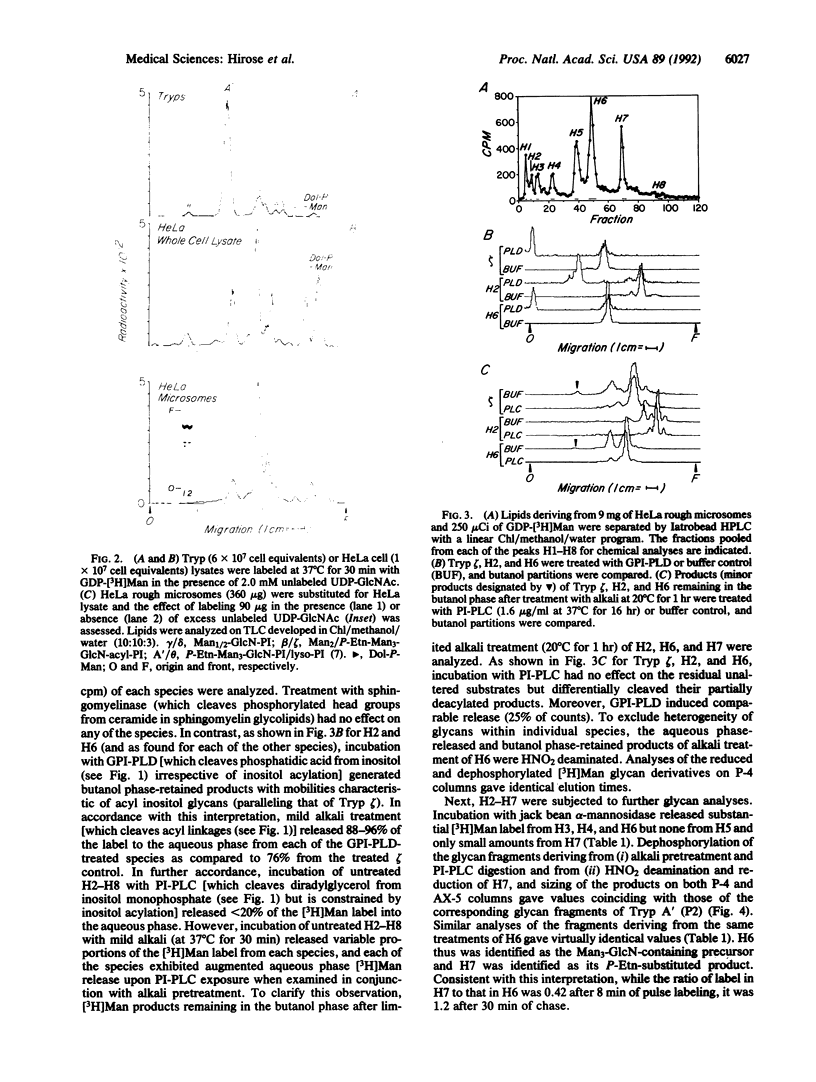
To identify mannosyl (Man)-containing intermediates of the human glycoinositol phospholipid (GPI) anchor pathway and examine their expression in paroxysmal nocturnal hemoglobinuria (PNH), mannolipid products deriving from in vitro guanosine diphosphate [3H]Man labeling of HeLa cell microsomes were characterized. The defined GPI species were correlated with products deriving from in vivo [3H]Man labeling of normal and (GPI-anchor defective) affected leukocytes. In vitro analyses in HeLa cells showed dolichol-phosphoryl (Dol-P)-[3H]Man and a spectrum of [3H]Man lipids exhibiting TLC mobilities approximating those of Trypanosoma brucei (Tryp) GPI precursors. Iatrobead HPLC separations and partial characterizations of the major isolated [3H]Man species (designated H1-H8) showed that all but H1 (Dol-P-Man) were sensitive to HNO2 deamination and serum GPI-specific phospholipase D digestion but were resistant to phosphatidylinositol-specific phospholipase C digestion unless previously deacylated with mild alkali. [3H]Man label in H3, H4, and H6 but not in H5 or H7 was efficiently released into the aqueous phase by jack bean alpha-mannosidase digestion. BioGel P-4 and AX-5 sizing of the dephosphorylated core glycan fragments of H6 and H7 gave values that coincided precisely with the corresponding glycan fragments from the fully assembled Tryp anchor donor A' (P2). Affected leukocytes from four patients with PNH supported formation of GlcNAc- and GlcN-PI but all failed to express H6 and H7 as well as H8 and two showed complete absence of earlier Man-containing intermediates. These findings argue that human intracellular GPI mannolipids are built on acylated inositol phospholipids, that H6 and H7 contain differentially phosphoethanolamine-substituted Man3-GlcN-inositol cores, and that PNH cells are defective in conversion of GlcN-PI into these more mature mannolipid structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carothers D. J., Hazra S. V., Andreson S. W., Medof M. E. Synthesis of aberrant decay-accelerating factor proteins by affected paroxysmal nocturnal hemoglobinuria leukocytes. J Clin Invest. 1990 Jan;85(1):47–54. doi: 10.1172/JCI114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ravi L., Hazra S. V., Medof M. E. Assembly and deacetylation of N-acetylglucosaminyl-plasmanylinositol in normal and affected paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3762–3766. doi: 10.1073/pnas.88.9.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemansky P., Gupta D. K., Meyale S., Tucker G., Tartakoff A. M. Atypical mannolipids characterize Thy-1-negative lymphoma mutants. Mol Cell Biol. 1991 Aug;11(8):3879–3885. doi: 10.1128/mcb.11.8.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson W. J., Doering T. L., Hart G. W., Englund P. T. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell. 1989 Mar 10;56(5):793–800. doi: 10.1016/0092-8674(89)90684-3. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Menon A. K., Schwarz R. T., Mayor S., Cross G. A. Cell-free synthesis of glycosyl-phosphatidylinositol precursors for the glycolipid membrane anchor of Trypanosoma brucei variant surface glycoproteins. Structural characterization of putative biosynthetic intermediates. J Biol Chem. 1990 Jun 5;265(16):9033–9042. [PubMed] [Google Scholar]
- Ogata S., Misumi Y., Miki K., Ikehara Y. Structural analysis of the asparagine-linked oligosaccharides of rat haptoglobin metabolically labeled in a hepatocyte culture system. Eur J Biochem. 1986 Dec 1;161(2):315–320. doi: 10.1111/j.1432-1033.1986.tb10449.x. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Roberts W. L., Santikarn S., Reinhold V. N., Rosenberry T. L. Structural characterization of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase by fast atom bombardment mass spectrometry. J Biol Chem. 1988 Dec 15;263(35):18776–18784. [PubMed] [Google Scholar]
- Thomas J. R., Dwek R. A., Rademacher T. W. Structure, biosynthesis, and function of glycosylphosphatidylinositols. Biochemistry. 1990 Jun 12;29(23):5413–5422. doi: 10.1021/bi00475a001. [DOI] [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]