Abstract
Most patients treated with curative intent for colorectal cancer (CRC) are included in a follow-up program involving periodic evaluations. The survival benefits of a follow-up program are well delineated, and previous meta-analyses have suggested an overall survival improvement of 5%-10% by intensive follow-up. However, in a recent randomized trial, there was no survival benefit when a minimal vs an intensive follow-up program was compared. Less is known about the potential side effects of follow-up. Well-known side effects of preventive programs are those of somatic complications caused by testing, negative psychological consequences of follow-up itself, and the downstream impact of false positive or false negative tests. Accordingly, the potential survival benefits of CRC follow-up must be weighed against these potential negatives. The present review compares the benefits and side effects of CRC follow-up, and we propose future areas for research.
Keywords: Colorectal cancer, Follow-up, Surveillance, False positive, Cancer survivorship
Core tip: Most western countries have a national follow-up program for colorectal cancer (CRC) survivors. The reported reduction in absolute mortality from intensive follow-up is 5%-10%, though recent data from the follow-up after colorectal surgery randomized trial call this effect into question. There exists limited evidence of improved quality of life (QoL) due to participation in a follow-up program, and the impact of false positive tests on QoL might be considerable. Several national experts advocate for low-cost, low-intensity CRC follow-up programs.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the western world, and surgery is the only curative treatment. Approximately one-third of those surgically resected will experience recurrent disease with an expected survival of less than two years[1]. Patients treated with curative intent are usually included in some form of preventive follow-up program involving periodic evaluations. Reviews comparing various follow-up programs have suggested that more intensive follow-up strategies tend to increase the five-year survival rate by 5%-10%[2,3].
Most national follow-up programs recommend intensive follow-up. However, there exist controversies on how to define an “intensive” follow-up program. This is mirrored in the fact that two identical national follow-up programs do not exist. In general, an intensive follow-up program consists of regular testing (usually every 3 mo the first two years) and consultations, whereas a low intensive follow up program is defined as no regular testing and consultations. In addition, most national follow-up programs make a distinction between rectal cancer and colon cancer surveillance, which is reflected in the difference of recommended radiological test modalities.
However, all preventive programs have the potential to harm patients[4-6]. The potential survival benefits of a follow-up program for CRC cancer patients have been well described, but much less is known about the potential negative effects accruing to patients and their families[2,3]. Patients surgically treated for CRC have to decide in partnership with the treating surgeon or family physician, whether they should participate in a CRC follow-up program. In making this personal decision, it is important to know not only the magnitude of potential benefits, but also the magnitude and likelihood of the potential adverse and unintended effects[5].
Firstly the survival benefits of intensive CRC follow-up must be delineated. In general, the benefits of preventive programs can be described as: (1) relative reduction of mortality rate; (2) absolute reduction of mortality; (3) the number of patients needed to prevent one adverse event; (4) evaluation of treatment effect; (5) reassurance by follow-up leading to improved quality of life (QoL); and (6) detection of other diseases[4]. In this paper we will further elaborate these terms.
Secondly, the side effects of CRC follow-up must be compared to the survival benefits. Well-known side effects of preventive programs are (1) over-diagnosis; (2) somatic complications caused by testing; (3) negative psychological consequences of follow-up; and (4) impact of a false positive (leading the patient to believe that he or she has recurrent disease) or false negative (leading to a potential diagnostic delay) tests.
Thirdly, the net benefits of follow-up must be considered in light of the associated economic costs. The United Kingdom’s National Institute for Health and Clinical Excellence (http://www.nice.org.uk) has proposed a societal willingness-to-pay of £40000 per life year gained, but this upper limit is controversial. In the case of CRC follow-up, it means that the long-term benefits of a follow-up program (i.e., the attempted curative resection of recurrent disease and resulting gains in survival) have to be balanced against society’s willingness to pay for such a service. To our knowledge, a systematic comparison of the benefits vs side effects of CRC follow-up has not been performed. Thus, the objective of this paper is to summarize the existing evidence regarding the benefits and side effects of CRC follow-up. An overview of the potential benefits and harms of CRC follow-up is provided in Table 1.
Table 1.
Benefits and side effects of colorectal cancer surveillance
| Benefits | Harms |
| Reassurance of surveillance | Impact of false positive tests |
| For the CRC survivor | Over diagnoses |
| For spouses and family | Complications related to the screening tests |
| Improved survival | Labeled as sick or at high risk |
| Control of treatment effects | False assurance of disease free status |
| Is the societal harm-to benefit ratio acceptable? |
CRC: Colorectal cancer.
RESEARCH
We performed a systematic PubMed search with the medical subject heading (MeSH) keywords “colorectal” in combination with the keywords “follow-up”, “surveillance”, “cancer recurrence”; “risk benefit assessment” and “false positive reactions”. Inclusion of papers was decided by discussion among authors. All reference lists of included publications were searched for relevant publications. Finally we identified relevant publications from the author’s personal databases. This resulted in 60 publications included in the review.
Benefits of colorectal follow-up
Benefit: Improved survival: The recurrence rate in CRC has been reported to be 30%-40% within 5 years (Figure 1)[1]. This means that all follow-up programs must focus on the early detection of recurrent cancers, aiming to offer curative metastases surgery to as many patients as possible.
Figure 1.
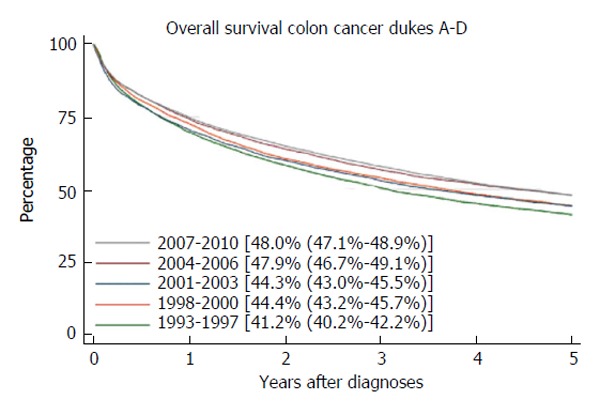
Overall survival of colon cancer dukes A-D. Eighty percent of the recurrences occur within the 3 first years after initial treatment, which is used as an argument to perform intensive surveillance the first 3 years. After 5 years, the survival curve is steady with few deaths caused by colon cancer. Courtesy of the Norwegian Cancer Registry (http://www.kreftregisteret.no/en/).
Two contemporary meta-analyses revealed that intensive and less intensive follow-up led to detection of a similar number of recurrences but that detection occurred between 5.91 mo (95%CI: 3.09-8.74) and 6.75 mo (95%CI: 2.44-11.06) earlier with intensive follow-up. Both analyses also found that curative reoperation for metastasis was significantly more likely in those subjects who were followed up intensively (Tjandra et al[2]: OR = 2.41, 95%CI: 1.63, 3.54. Jeffery et al[3]: OR = 2.81, 95%CI: 1.65-4.79). The survival benefits of intensive CRC follow-up has been reported to be a 5%-10% reduction in the total cohort mortality rate. The increased overall survival, earlier detection of recurrence, and higher reoperation rates observed provide only circumstantial evidence that intensive follow-up extends life by making cure of recurrent disease more likely. Neither meta-analyses found that cancer specific survival was improved by intensive follow-up.
However, there exists limited data regarding the relative reduction in mortality or number of patients who must be followed intensively in order to save one life from recurrent cancer death. Factors other than intensive follow-up have been postulated to contribute to the mortality reduction associated with CRC follow-up. Some combination of increased psychological well-being, improved health behavior, and improved treatment of coincidental disease may contribute to the mortality benefit. This issue represents an important direction for future studies[7].
Recently, the results from the follow-up after colorectal surgery (FACS) trial were reported[8,9]. The factorial randomized trial design, with independent allocation to the carcinoembryonic antigen (CEA) and computed tomography (CT) interventions, meant that patients received 1 of 4 types of follow-up: (1) CEA follow-up: measurement of blood CEA every 3 mo for 2 years, then every 6 mo for 3 years, with a single chest, abdomen, and pelvis CT scan at 12 to 18 mo if requested at study entry by hospital clinician; (2) CT follow-up: CT of the chest, abdomen, and pelvis every 6 mo for 2 years, then annually for 3 years; (3) CEA and CT follow-up: both blood CEA measurement and CT imaging as above; and (4) Minimum follow-up: no scheduled follow-up except a single CT scan of the chest, abdomen, and pelvis at 12 to 18 mo if requested at study entry by the hospital clinician.
Interestingly, there were no differences seen in overall or cancer-specific mortality between any of the intensive arms and the minimum follow-up group. Most patients with recurrence suffered from incurable disease. In fact, only 71 (5.9%) of 1202 patients followed were suitable for potentially curative treatment. Significantly more patients were treated with curative intent in the intensive follow up groups compared to minimalist follow-up, but there were no difference in the number of total deaths in the two groups. These data argue against very intensive follow-up schedules.
In conclusion, although two meta-analyses have reported a 5%-10% reduction in overall mortality among patients undergoing intensive follow-up, the existing evidence of any benefit in terms of cancer-specific survival is limited. The results from the FACS trial did not show any compelling evidence of a significant survival benefit of CRC follow-up. Hopefully, the final results of the ongoing COLOFOL trial will help answer the debate regarding which follow program enables the highest survival[10]. A summary of randomized controlled trails and their potential survival benefit is provided in Table 2.
Table 2.
Comparison of randomized trials assessing follow-up after colorectal cancer curative surgery n (%)
| Trial | Cancer stage included | Enrolled (n) | Recurrences | Time to cancer detection (mo) | Metastases surgeries (n) | Overall 5-yr survival | Disease free 5-yr survival | Survival after met surgery |
| Ohlsson 1995 | ||||||||
| Total | Dukes A, B, C | 107 | 35 (33) | |||||
| Intensive | 53 | 17 (32) | 20 (median) | 5 | 75 | 78 | 29% 5 yr | |
| Control | 54 | 18 (33) | 24 (median) | 3 | 67 | 71 | 22% 5 yr | |
| Makela 1995 | ||||||||
| Total | Dukes A, B, C | 106 | 43 (41) | 8 | 58 | Overall 3 pts mean 26 mo survival | ||
| Intensive | 52 | 22 (42) | 10 (mean) | 5 | 59 | |||
| Control | 54 | 21 (39) | 15 (mean) | 3 | 54 | |||
| Pietra 1998 | ||||||||
| Total | Dukes B, C | 207 | 82 (39) | Overall 8 pts mean 29 mo survival | ||||
| Intensive | 104 | 41 (39) | 10.3 (mean) | 21 | 73 | 68 | ||
| Control | 103 | 41 (40) | 20.2 (mean) | 6 | 58 | 53 | ||
| Rodriegez-Moranta 2006 | ||||||||
| Total | TNM II and III | 259 | 69 (26) | NA | ||||
| Intensive | 127 | 35 (27) | 39 (mean) | 18 | 75 | NA | ||
| Control | 132 | 34 (26) | 38 (mean) | 10 | 73 | |||
| Secco 2001 | ||||||||
| Risk adapted intensive | Low risk vs high risk | 108 | 74 (68) | Total | 31 | 48 | NA | NA |
| Risk adapted low intensive | 84 | 27 (32) | 13.5 (mean) | 82 | ||||
| Minimal follow-up: High risk | 84 | 58 (69) | 13 | 35 | ||||
| Minimal follow-up: Low risk | 61 | 25 (40) | 60 |
NA: Not available.
Benefit: Control of treatment effects: There exist several international controversies around treatment (drains vs no drains, laparoscopic technique vs open technique among others) and follow-up of patients with CRC[11,12]. There are for instance no similarly designed follow-up program at an international level[13-16]. It is therefore imperative for improved CRC treatment quality that the effects of radio-chemotherapy, surgical technique and postoperative follow-up are continuously evaluated, and a structured follow-up program might be a way to perform such a quality control[17,18].
Benefit: Reassurance of follow-up: There is no existing evidence that participation in a follow-up program leads to increased personal well-being. Some researchers have investigated the psychological effects of CRC follow-up[19-22]. None of the resulting studies have found improvement in the patient QoL with follow-up.
Harms of CRC follow-up
Harm: False positive tests: Table 3 summarizes the false positive rates of the most commonly used CRC follow-up tests. As an illustration, consider a patient followed according to the most recent United States follow-up recommendations from the National Comprehensive Cancer Network[16]. Based on the most optimistic estimates in Table 3 the annual probability of at least one false positive test for a patient with no actual recurrence would be 41% in each of years one and two, and 28% in each of years three, four, and five. Over the entire five-year period, the probability of at least one false positive would be 87%.
Table 3.
Probability of false positive test results (1-specificity) for commonly used colorectal cancer follow-up tests
| Test | False positive rate (1-specificity) | Ref. |
| Serum CEA | 10% | [54] |
| CT-hepatic metastases | 5%-28%1 | [55-58] |
| CT-other abdominal metastases | 2% | [58] |
| Contrast enhanced ultrasound-liver | 4%-33%2 | [56,57,59] |
| Ultrasound-liver | 50% | [59] |
| CT-lungs | 4% | [58] |
| Colonoscopy | 0% | [32] |
Based on specificity estimates from individual studies of 89%[55] (n = 24), 95%[58] (n = 115), 72%[56] (n = 87), and 91%[57] (n = 100);
Based on specificity estimates from individual studies of 96%[60] (n = 68), 96%[57] (n = 99), and 67%[59] (n = 56) subjects. The last was the only to employ intraoperative confirmation of hepatic metastases. The annual probability of at least one false positive test for a patient with no actual recurrence would be 41% in each of year one and two, and 28% in each of year three, four, and five. Over the entire five-year period, the probability of at least one false positive would be 87%. CT: Computed tomography; CEA: Carcinoembryonic antigen.
Given their high likelihood, it is important to consider the possible consequences of false positive follow-up tests. Primarily, these can come in the form of economic costs and psychological impact. None of the prospective studies or economic models focusing on CRC recurrence have reported the economic costs of false positive follow-up tests, but quantifying these costs could provide important perspective.
While no studies appear to have specifically addressed the psychological or quality-of-life impact of false positive follow-up tests in colorectal or other types of cancer, a small number of investigators have examined the quality-of-life impact of false positive cancer screening tests. In general, these studies have shown increased anxiety following false positive screening results for as long as 18[23] to 24[24] mo after the false positive result[23,25,26]. This data comes from populations who have not previously been diagnosed with and treated for cancer, so the results are difficult to extrapolate to CRC survivors.
Harm: Somatic complications caused by tests: Aside from any unlikely negative sequelae of CT radiation exposure, colonoscopy related colonic perforation and post-procedure bleeding represent the most likely serious complications arising from CRC follow-up. Endoscopic follow-up is endorsed in most comprehensive follow-up recommendations[16,27-31] primarily as a means to detect metachronous CRC’s (normally representing between 1.6% and 7.4% of CRC recurrences) or adenomas with advanced features[2,32,33]. The relatively invasive procedure has sensitivity of 95% and specificity of 100% for detecting high-risk polyps or tumours, however the major complication rate has been reported as 0.2%-1.2%[34-36].
To date, no trial has reported increased survival associated with colonoscopy follow-up after CRC resection. Because of the unproven benefit and non-trivial risk, some have argued against routine endoscopic follow-up after curative CRC resection[37-39]. Further study is needed to explore whether CT Colonography may eventually provide a better balance of risks and benefits[38].
Harm: QoL implications: There is limited evidence showing that enrolment in a follow-up program improves QoL among CRC survivors. In fact, available data from breast follow-up trails could be used to support the opposite viewpoint: such follow-up programs and tests might negatively influence QoL[40-42]. It is often claimed-and some evidence corroborates[22]-that follow-up tests can be reassuring for patients, and this may be true if all of the tests are completely normal every time. However, equivocal test results such as a slightly elevated CEA level, or questionable shadows on CT are quite common, and they commonly spur additional testing. This period between initial suggestive test result and subsequent conclusive work-up can be a stressful one for patients[21].
Some researchers have investigated the psychological effects of CRC follow-up[19-22]. None of the resulting studies have found improvement in the patient QoL with follow-up. In a recent published randomized trial comparing general practitioner vs surgeon-organized follow-up, there were no differences between the two groups in QoL measured by ERTOC-QLQ C30 and EQ-5D[21]. In fact, both groups had similar QoL levels as the general United Kingdom population at baseline (1 mo postoperatively). Results from a similar 2006 trial by Wattchow et al[19] told a similar story. There, study patients remained in the normal range for depression and anxiety with no difference between the two groups at either 12 or 24 mo[19,20]. In recent meta-analyses, it has been shown that anxiety rather than depression was a major problem among long-term cancer survivors. It is however unknown what impact an organized cancer follow-up program has on anxiety[43]. It has been shown that 46 percent of patients reported physiological distress while awaiting the results of a potential cancer diagnosis[44]. This and other trials suggest that tests recommended by a cancer screening or preventive program cause harm in terms of physiological distress[44-46].
The only survey showing a slight improvement in QoL among CRC survivors with intensive follow-up was published in 1997[47]. This survey included 350 Danish participants who reported a small but significant increase in QoL associated with more frequent follow-up, as measured by the Nottingham Health Profile.
In conclusion, there exists very limited evidence that CRC follow-up improves QoL among CRC survivors. Further research is needed, in particular, to address the impact of a false positive follow-up test on QoL among CRC survivors. From breast cancer follow-up trials, there is compelling evidence that postoperative follow-up does not improve QoL and that follow-up testing might cause physiological distress[48]. Factors that may impact QoL in a positive or negative way among colon cancer survivors enrolled in a follow-up program are shown in Figure 2.
Figure 2.
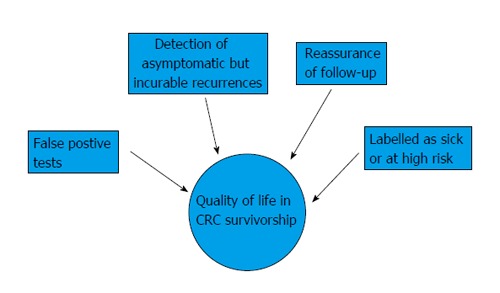
Factors influencing quality of life among colorectal cancer survivors enrolled in a follow-up program. CRC: Colorectal cancer.
DIRECTION OF FUTURE RESEARCH
According to the World Health Organisation, the success of preventive programs depends on three fundamental principles (www.who.int/cancer/detection/variouscancer/en/): The target disease should be a common form of cancer, with high associated morbidity or mortality; Effective treatment, capable of reducing morbidity and mortality, should be available; Test procedures should be acceptable, safe, and relatively inexpensive.
In CRC follow-up these principles are fulfilled: (1) CRC is the third most common cancer disease, and the risk of recurrence is as high as 30 to 40 percent; (2) if successful, metastasectomy can be curative (i.e., R0 resections); and (3) the tests in most programs are acceptable, relatively safe and relatively inexpensive. However, as discussed, there are several potential side effects of CRC follow-up; future research much be directed at further exploring these harms and weighing them against the expected survival benefit. Recently, a survey published in British Medical Journal found that the harms of screening and preventive programs were poorly reported[49]. Healthcare decision makers, surgeons, and patients therefore cannot make informed choices.
Personalized medicine is defined as a medical model that proposes the customization of healthcare, with medical decisions, practices and tests being tailored to the individual patient. To our knowledge there exist no individual risk stratification in the different national colorectal follow-up guidelines, and this is an area of future research.
Firstly we believe that genetic testing and biological determinants of tumor recurrence will gain increasingly importance[50,51]. The individualization of cancer care requires a deep understanding of tumor biology and the identification of tumor subsets that offer targets for tumor specific treatment. Of specific interest for CRC follow-up programs, are the promising results of the 12-gene recurrence score (RS), which is a quantitative assay integrating stromal response and cell cycle gene expression. It is shown that the 12-gene RS predicts recurrence in stage II colon cancer. This tool appears promising as a means to inform decision making around adjuvant chemotherapy following resection of stage II colon cancer. The use of the tool in planning post-treatment follow-up does not appear to have been explored, however[52].
Secondly, test intensity, test modality and the risk of false positive events has to be discussed in details with the patient. As shown in Table 3, the probability of at least one false positive event during a five-year follow-up program might be as high as 87%. High-test intensity programs should be offered to patients with a high probability of recurrent cancers, but this must be weighed against the patient’s preferences of experiencing a false positive test.
Finally, research must be aimed to identify the optimal combination of test, blood samples and clinical examinations that creates the highest possible overall follow-up sensitivity and specificity.
CONCLUSION
Any survival benefit (or lack of benefit) of the CRC follow-up must be considered along with the views of the patients to ensure that follow-up programs are accessible and acceptable, and that they address all patient needs and concerns. However, the problem of postoperative cancer follow-up is that a vast majority of patients must undergo a large number of tests without any benefit, or even with some harm, to identify a small number of patients with curable recurrence. Patients with asymptomatic but incurable disease (10%-20% of all recurrences) likely represent the group with the most potential to be harmed by follow-up[21,53].
In conclusion, little is known about the potential harms of CRC follow-up, especially when it comes to the impact of false positive tests. Tailored follow-up programs based on the individual’s risk of cancer recurrence and likely metastatic spread pattern must be developed. Further research is needed to settle these controversies, and new methods of decision-analytic modeling in combination with the emerging data from COLOFOL must be applied[9,10].
Footnotes
P- Reviewers: Chen CN, Steele SR S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
References
- 1.Larsen IK. Cancer Registry of Norway. Cancer in Norway 2011. 2013. pp. 1–90. Available from: http: //www.kreftregisteret.no/no/Generelt/Nyheter/Nokkeltall---kreft-2011/ [Google Scholar]
- 2.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2002;(1):CD002200. doi: 10.1002/14651858.CD002200. [DOI] [PubMed] [Google Scholar]
- 4.Marshall KG. Prevention. How much harm? How much benefit? 1. Influence of reporting methods on perception of benefits. CMAJ. 1996;154:1493–1499. [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall KG. Prevention. How much harm? How much benefit? 2. Ten potential pitfalls in determining the clinical significance of benefits. CMAJ. 1996;154:1837–1843. [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall KG. Prevention. How much harm? How much benefit? 3. Physical, psychological and social harm. CMAJ. 1996;155:169–176. [PMC free article] [PubMed] [Google Scholar]
- 7.Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Mechanisms of improved survival from intensive followup in colorectal cancer: a hypothesis. Br J Cancer. 2005;92:430–433. doi: 10.1038/sj.bjc.6602369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 9.Primrose JN. Follow-up after colorectal cancer surgery: Preliminary observational findings from the UK FACS trial. J Clin Oncol. 2011;29 Suppl:abstr 3521. [Google Scholar]
- 10.Wille-Jørgensen P, Laurberg S, Påhlman L, Carriquiry L, Lundqvist N, Smedh K, Svanfeldt M, Bengtson J. An interim analysis of recruitment to the COLOFOL trial. Colorectal Dis. 2009;11:756–758. doi: 10.1111/j.1463-1318.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 11.Augestad KM, Lindsetmo RO, Reynolds H, Stulberg J, Senagore A, Champagne B, Heriot AG, Leblanc F, Delaney CP. International trends in surgical treatment of rectal cancer. Am J Surg. 2011;201:353–357; discussion 357-358. doi: 10.1016/j.amjsurg.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Augestad KM, Lindsetmo RO, Stulberg J, Reynolds H, Senagore A, Champagne B, Heriot AG, Leblanc F, Delaney CP. International preoperative rectal cancer management: staging, neoadjuvant treatment, and impact of multidisciplinary teams. World J Surg. 2010;34:2689–2700. doi: 10.1007/s00268-010-0738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonen B. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft i tykktarm og endetarm. 2013. pp. 1–185. [Google Scholar]
- 14.Bülow S. Retningslinier for diagnostik og behandling af kolorektal cancer. DCCG. 2009;4:1–176. [Google Scholar]
- 15.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 16.Benson AB. Breast cancer. NCCN. 2013;2:1–117. Available from: http: //www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [Google Scholar]
- 17.Wille-Jørgensen P, Balleby L. Follow-up in colorectal cancer: questions to be answered. Colorectal Dis. 2011;13:959–960. doi: 10.1111/j.1463-1318.2011.02716.x. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair P, Singh A, Riaz AA, Amin A. An unsolved conundrum: the ideal follow-up strategy after curative surgery for colorectal cancer. Gastrointest Endosc. 2012;75:1072–1079. doi: 10.1016/j.gie.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, Platell C, Silagy C. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer. 2006;94:1116–1121. doi: 10.1038/sj.bjc.6603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gall CA, Weller D, Esterman A, Pilotto L, McGorm K, Hammett Z, Wattchow D. Patient satisfaction and health-related quality of life after treatment for colon cancer. Dis Colon Rectum. 2007;50:801–809. doi: 10.1007/s10350-006-0815-8. [DOI] [PubMed] [Google Scholar]
- 21.Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold T, Vonen B, Skrøvseth SO, Lindsetmo RO. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stiggelbout AM, de Haes JC, Vree R, van de Velde CJ, Bruijninckx CM, van Groningen K, Kievit J. Follow-up of colorectal cancer patients: quality of life and attitudes towards follow-up. Br J Cancer. 1997;75:914–920. doi: 10.1038/bjc.1997.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gram IT, Lund E, Slenker SE. Quality of life following a false positive mammogram. Br J Cancer. 1990;62:1018–1022. doi: 10.1038/bjc.1990.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen MR, Drescher CW, Zheng Y, Bowen DJ, Wilson S, Young A, McIntosh M, Mahony BS, Lowe KA, Urban N. Changes in cancer worry associated with participation in ovarian cancer screening. Psychooncology. 2007;16:814–820. doi: 10.1002/pon.1151. [DOI] [PubMed] [Google Scholar]
- 25.Hafslund B, Espehaug B, Nortvedt MW. Effects of false-positive results in a breast screening program on anxiety, depression and health-related quality of life. Cancer Nurs. 2012;35:E26–E34. doi: 10.1097/NCC.0b013e3182341ddb. [DOI] [PubMed] [Google Scholar]
- 26.Kauff ND, Hurley KE, Hensley ML, Robson ME, Lev G, Goldfrank D, Castiel M, Brown CL, Ostroff JS, Hann LE, et al. Ovarian carcinoma screening in women at intermediate risk: impact on quality of life and need for invasive follow-up. Cancer. 2005;104:314–320. doi: 10.1002/cncr.21148. [DOI] [PubMed] [Google Scholar]
- 27.Vonen B. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft i tynntarm og endetarm. 2010. pp. 1–162. Available from: http: //www.helsedirektoratet.no/kreft/publikasjoner/ [Google Scholar]
- 28.Bülow S. Retningslinier for diagnostik og behandling af kolorektal cancer. Danish Colorectal Cancer Group. 2009;4:1–176. [Google Scholar]
- 29.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v70–v77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- 30.Scholefield J. Guidelines for the Management of Colorectal Cancer. ACPGBI. 2007;3:1–117. [Google Scholar]
- 31.Tjandra JJ, Kilkenny JW, Buie WD, Hyman N, Simmang C, Anthony T, Orsay C, Church J, Otchy D, Cohen J, et al. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2005;48:411–423. doi: 10.1007/s10350-004-0937-9. [DOI] [PubMed] [Google Scholar]
- 32.Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. Int J Colorectal Dis. 1997;12:329–334. doi: 10.1007/s003840050118. [DOI] [PubMed] [Google Scholar]
- 33.Erenay FS, Alagoz O, Banerjee R, Cima RR. Estimating the unknown parameters of the natural history of metachronous colorectal cancer using discrete-event simulation. Med Decis Making. 2011;31:611–624. doi: 10.1177/0272989X10391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 35.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 36.Young PE, Womeldorph CM. Colonoscopy for colorectal cancer screening. J Cancer. 2013;4:217–226. doi: 10.7150/jca.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7–14. doi: 10.1016/s0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- 38.Søreide K. Endoscopic surveillance after curative surgery for sporadic colorectal cancer: patient-tailored, tumor-targeted or biology-driven? Scand J Gastroenterol. 2010;45:1255–1261. doi: 10.3109/00365521.2010.496492. [DOI] [PubMed] [Google Scholar]
- 39.Ramsey SD, Howlader N, Etzioni R, Brown ML, Warren JL, Newcomb P. Surveillance endoscopy does not improve survival for patients with local and regional stage colorectal cancer. Cancer. 2007;109:2222–2228. doi: 10.1002/cncr.22673. [DOI] [PubMed] [Google Scholar]
- 40.Gulliford T, Opomu M, Wilson E, Hanham I, Epstein R. Popularity of less frequent follow up for breast cancer in randomised study: initial findings from the hotline study. BMJ. 1997;314:174–177. doi: 10.1136/bmj.314.7075.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunfeld E, Yudkin P, Adewuyl-Dalton R, Vessey MP, Mant D. Follow up in breast cancer. Quality of life unaffected by general practice follow up. BMJ. 1995;311:54. doi: 10.1136/bmj.311.6996.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberati A. The GIVIO trial on the impact of follow-up care on survival and quality of life in breast cancer patients. Interdisciplinary Group for Cancer Care Evaluation. Ann Oncol. 1995;6 Suppl 2:41–46. doi: 10.1093/annonc/6.suppl_2.s41. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721–732. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 44.van den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, van Klaveren RJ, de Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154–161. doi: 10.1183/09031936.00123410. [DOI] [PubMed] [Google Scholar]
- 45.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med. 2007;146:502–510. doi: 10.7326/0003-4819-146-7-200704030-00006. [DOI] [PubMed] [Google Scholar]
- 47.Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–669. [PubMed] [Google Scholar]
- 48.Grunfeld E. Looking beyond survival: how are we looking at survivorship? J Clin Oncol. 2006;24:5166–5169. doi: 10.1200/JCO.2006.06.5953. [DOI] [PubMed] [Google Scholar]
- 49.Heleno B, Thomsen MF, Rodrigues DS, Jørgensen KJ, Brodersen J. Quantification of harms in cancer screening trials: literature review. BMJ. 2013;347:f5334. doi: 10.1136/bmj.f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594–603. doi: 10.1016/S1470-2045(10)70209-6. [DOI] [PubMed] [Google Scholar]
- 51.Vogelzang NJ, Benowitz SI, Adams S, Aghajanian C, Chang SM, Dreyer ZE, Janne PA, Ko AH, Masters GA, Odenike O, et al. Clinical cancer advances 2011: Annual Report on Progress Against Cancer from the American Society of Clinical Oncology. J Clin Oncol. 2012;30:88–109. doi: 10.1200/JCO.2011.40.1919. [DOI] [PubMed] [Google Scholar]
- 52.Venook AP, Niedzwiecki D, Lopatin M, Ye X, Lee M, Friedman PN, Frankel W, Clark-Langone K, Millward C, Shak S, et al. Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol. 2013;31:1775–1781. doi: 10.1200/JCO.2012.45.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Körner H, Söreide K, Stokkeland PJ, Söreide JA. Systematic follow-up after curative surgery for colorectal cancer in Norway: a population-based audit of effectiveness, costs, and compliance. J Gastrointest Surg. 2005;9:320–328. doi: 10.1016/j.gassur.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 54.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Bluemke DA, Paulson EK, Choti MA, DeSena S, Clavien PA. Detection of hepatic lesions in candidates for surgery: comparison of ferumoxides-enhanced MR imaging and dual-phase helical CT. AJR Am J Roentgenol. 2000;175:1653–1658. doi: 10.2214/ajr.175.6.1751653. [DOI] [PubMed] [Google Scholar]
- 56.Staib L, Schirrmeister H, Reske SN, Beger HG. Is (18)F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg. 2000;180:1–5. doi: 10.1016/s0002-9610(00)00406-2. [DOI] [PubMed] [Google Scholar]
- 57.Glover C, Douse P, Kane P, Karani J, Meire H, Mohammadtaghi S, Allen-Mersh TG. Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum. 2002;45:476–484. doi: 10.1007/s10350-004-6224-y. [DOI] [PubMed] [Google Scholar]
- 58.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, Myers RW, Greiss HB, Hofer GA. Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134:503–511; discussion 511-513. doi: 10.1001/archsurg.134.5.503. [DOI] [PubMed] [Google Scholar]
- 59.Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515–1526. doi: 10.1016/j.ultrasmedbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Staib L, Link KH, Beger HGXN. Follow-up in colorectal cancer: cost-effectiveness analysis of established and novel concepts. Langenbeck Arch Surg. 2000;385:412–20. doi: 10.1007/s004230000144. [DOI] [PubMed] [Google Scholar]


