ABSTRACT
The apical surface of epithelial cells is often highly specialised to fulfil cell type-specific functions. Many epithelial cells expand their apical surface by forming microvilli, actin-based, finger-like membrane protrusions. The apical surface of Drosophila photoreceptor cells (PRCs) forms tightly packed microvilli, which are organised into the photosensitive rhabdomeres. As previously shown, the GPI-anchored adhesion protein Chaoptin is required for the stability of the microvilli, whereas the transmembrane protein Crumbs is essential for proper rhabdomere morphogenesis. Here we show that chaoptin synergises with crumbs to ensure optimal rhabdomere width. In addition, reduction of crumbs ameliorates morphogenetic defects observed in PRCs mutant for prominin and eyes shut, known antagonists of chaoptin. These results suggest that these four genes provide a balance of adhesion and anti-adhesion to maintain microvilli development and maintenance. Similar to crumbs mutant PRCs, PRCs devoid of prominin or eyes shut undergo light-dependent retinal degeneration. Given the observation that human orthologues of crumbs, prominin and eyes shut result in progressive retinal degeneration and blindness, the Drosophila eye is ideally suited to unravel the genetic and cellular mechanisms that ensure morphogenesis of PRCs and their maintenance under light-mediated stress.
Keywords: Microvilli, Retinal degeneration, Rhabdomere, Adhesion
INTRODUCTION
Photoreceptor cells (PRCs) are highly polarised cells originating from the neuroepithelium, which are characterised by an expanded apical plasma membrane, specialised to accommodate the large amount of the visual pigment rhodopsin. PRCs in the animal kingdom use two different strategies to expand their apical surface: vertebrate rods and cones, for example, expand the apical membrane by a microtubule-based modified primary cilium to form the outer segment, while many PRCs of invertebrate species form actin-based microvilli, which are organised into light harvesting compartments called rhabdomeres (Lamb, 2009; Fain et al., 2010). The microvilli of Drosophila rhabdomeres harbour the signalplex, a supramolecular protein complex organised by the scaffolding protein InaD, which directly binds to components of the light-dependent signalling cascade (Wang and Montell, 2007). Each rhabdomere of Drosophila is built from approximately 50,000 microvilli, which closely adhere to their neighbours. Each microvillus is about 1.5 µm in length and 50 nm wide. Actin filaments span the entire length of the microvilli (Arikawa et al., 1990).
The apical plasma membrane of vertebrate and Drosophila PRCs contains a second distinct domain, called inner segment and stalk membrane, respectively, which separates the photoreceptive outer segment/rhabdomere from the adherens junctions (AJs). Molecularly, this membrane domain is marked by the Crumbs (Crb) protein complex. The core components of this evolutionarily conserved complex are the transmembrane protein Crb, which is linked via its short cytoplasmic tail to the scaffolding proteins Stardust (Sdt)/MPP5/Pals1, DPATJ/PATJ and DLin-7/Lin-7/Veli (Bazellieres et al., 2009; Bulgakova and Knust, 2009). In Drosophila, loss of any core component leads to light dependent retinal degeneration (Johnson et al., 2002; Berger et al., 2007; Bachmann et al., 2008; Chartier et al., 2012; Soukup et al., 2013). Strikingly, mutations in CRB1, one of the three human Crb genes, lead to blindness (den Hollander et al., 1999). This suggests that Crb proteins control similar mechanisms required to prevent PRC degeneration in vertebrates and invertebrates.
Drosophila PRCs develop from a simple epithelium, the eye imaginal disc. During larval development, PRCs become gradually specified and are organised into groups of eight cells, which, after recruitment of additional support cells, form the ommatidia, the units of the compound eye. At ∼37% pupal development (pd), the apical surfaces of the PRCs undergo a shift of 90°, thus adopting a lateral position, with the apical poles of the eight PRCs of an ommatidium oriented towards each other and being closely associated. At around 50% pd, the stalk membrane can be identified as a distinct portion of the apical membrane, while the microvilli of the incipient rhabdomeres increase in number and length and start to separate from those of the other rhabdomeres. At the same time, the interrhabdomeral space (IRS) is formed. This process is accompanied by a tremendous increase in the size of the PRCs, including the rhabdomere, resulting in a retinal thickness of about 100 µm (Longley and Ready, 1995).
The genetic regulation of this complex morphogenetic process has been described to some extent. The specification of the apical membrane depends on Bazooka, the Drosophila orthologue of Par-3, and PTEN (Pinal et al., 2006). The stalk membrane becomes visible as distinct membrane from 50% pd onwards, when Crb, which is initially spread across the entire apical plasma membrane, becomes restricted to the stalk. In the absence of Crb, the stalk membrane is reduced in length and the rhabdomeres only span the distal third of the retina (Izaddoost et al., 2002; Johnson et al., 2002; Pellikka et al., 2002). The core of the microvilli is formed by actin filaments. Actin also participates in the organisation of the rhabdomeral terminal web (RTW), a tensile sheet at the base of the rhabdomere required for microvillar actin termini linkage via Moesin. The RTW is embedded in the apical, organelle-poor cytoplasm, called ectoplasm in Drosophila PRCs (Xia and Ready, 2011). Moesin, the single Drosophila member of the ERM (ezrin–radixin–moesin) protein family, links the actin cytoskeleton to the plasma membrane. RNAi-mediated knock down of Moesin results in loosely organised microvilli, which starts being visible at around 50% pd, and strongly disorganised microvilli later on due to disrupted F-actin organisation at the rhabdomere base (Karagiosis and Ready, 2004). Microvilli formation requires actin binding proteins, such as the Wiskott–Aldrich syndrome protein WASp (Zelhof and Hardy, 2004), the actin-depolymerising factor cofilin, encoded by Drosophila twinstar (tsr) (Pham et al., 2008) or motor proteins, such as Myosin V (Li et al., 2007). The RTW not only provides a mechanical support for the microvilli but also acts as trafficking route for Rab11-dependent vesicle delivery of rhabdomeral membrane components (Satoh et al., 2005; Li et al., 2007). At around 78% of pupal development, expression of Rhodopsin 1 (Rh1), encoded by ninaE, is required to stabilise the RTW of PRCs R1–R6 by localising the small GTPase DRac1 (Chang and Ready, 2000). In the absence of Rh1 during this period, the alignment of rhabdomeral microvilli is not maintained, resulting in their involution into the cytoplasm (Kumar and Ready, 1995; Kumar et al., 1997). However, recent results showed that DRac is dispensable for this process and suggested that it may act redundantly with Cdc42 (Pinal and Pichaud, 2011).
At early pupal stages, the apical compartments of all PRCs in each ommatidium are initially attached to each other, a process mediated by the GPI (glycosylphosphatidylinositol)-anchored glycoprotein protein Chaoptin (Chp) (Krantz and Zipursky, 1990; Hirai-Fujita et al., 2008). Separation of the apical membranes requires the function of the pentaspan membrane protein Prominin (Prom) and the secreted protein Eyes shut (Eys) [also known as Spacemaker (Spam)] (Husain et al., 2006; Zelhof et al., 2006; Gurudev et al., 2013). Prom and Eys cooperatively antagonise the function of Chp in order to form an open rhabdom, in which a single, continuous IRS separates the rhabdomeres from each other. Once separated, the microvilli expand in length. Chp is further required to ensure the tight adhesion between microvilli, thus allowing the formation of a compact rhabdomere. chp mutant PRCs of adult flies lack fully formed microvilli (Van Vactor et al., 1988).
So far, the genetic control of rhabdomere formation by chp, prom and eys on the one hand and stalk membrane development, mediated by the Crb complex, on the other hand, was studied separately. Here we show that chp acts synergistically with crb to form the rhabdomere and that crb is part of a genetic network, which comprises crb, chp, prom and eys. Furthermore, not only crb, but also prom and eys are required in adult PRCs to prevent light-dependent retinal degeneration, supporting an additional functional interaction at later stages. Strikingly, all three genes are conserved, and their loss-of-function has been associated with a clinically and genetically heterogeneous group of blindness in humans. Therefore, the fly eye provides an excellent model system to further study the role of these genes during development and disease progression.
RESULTS
Genetic interaction between crb and chp
crb loss of function mutations induce a pleiotropic phenotype, in which the rhabdomeres of photoreceptor cells (PRCs) are bulky, occasionally fused with each other (compare Fig. 1A to Fig. 1B), develop a shorter stalk membrane and fail to expand throughout the depth of the retina (Johnson et al., 2002; Pellikka et al., 2002). We identified chp in a screen performed to identify genes involved in PRC morphogenesis. For this, we screened for rhabdomere phenotypes in crb heterozygous flies, which were additionally heterozygous for a defined deletion on either the second (crb11A22/+; Def/+) or the third chromosome (crb11A22 +/+ Def) (to be published elsewhere). Mutations in chp are homozygous viable and have been shown to result in a severe reduction and disorganisation of the apical rhabdomeral microvilli in the adult eye (compare Fig. 1A to Fig. 1C) (Van Vactor et al., 1988). chp encodes several differentially spliced transcripts, which encode GPI-anchored adhesion molecules with 28 leucine-rich repeats (LRRs) in their extracellular domain (SMART prediction) (Reinke et al., 1988; Krantz and Zipursky, 1990). While both crb and chp mutations are fully recessive and do not show any mutant rhabdomere phenotype when heterozygous, PRCs trans-heterozygous for crb and chp develop rhabdomeres with significantly smaller width (compare Fig. 1D,E to Fig. 1F; for quantification see Fig. 1G). The length of the rhabdomeres is not affected (data not shown). The result was confirmed using various combinations of three different crb alleles (crb11A22, crb8F105 and crbGX24) and two different chp alleles, chp2 and chpMB05115, as well as two deficiencies removing chp (Fig. 1G; supplementary material Fig. S1). These results suggest that crb and chp act together to control the width of the rhabdomeres.
Fig. 1. crb and chp synergistically control the width of the rhabdomere.
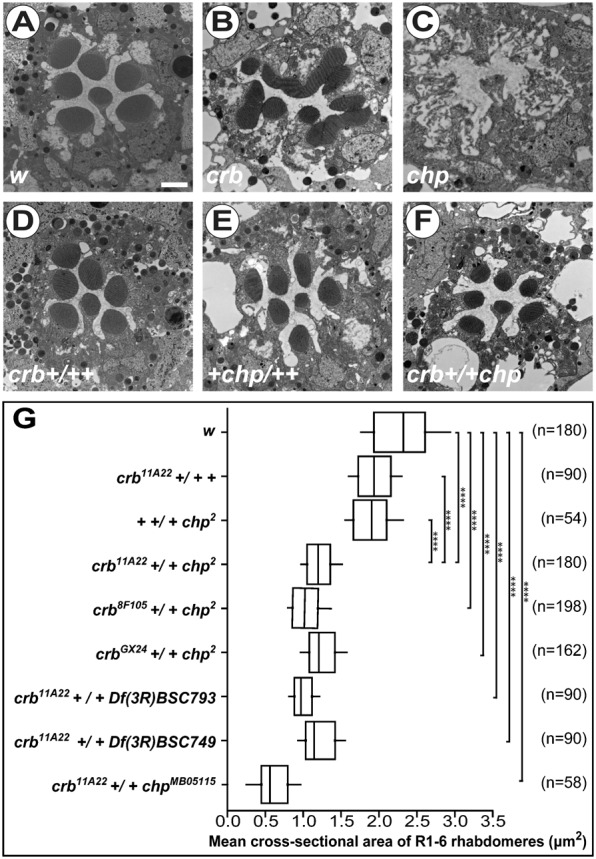
(A–F) Electron micrographs of cross-sections of control (w) (A), crb11A22 (B), chp2 (C), crb11A22 +/+ + (D); + +/+ chp2 (E) and crb11A22 +/+ chp2 (F) adult Drosophila ommatidia. Scale bar: 1 µm. (G) Box-plot representing the width of rhabdomeres of indicated genotypes. Whiskers indicate 10–90% confidence interval. The width is indicated in µm2 and estimated by measuring cross-sectional areas of rhabdomeres from outer PRCs (R1–R6) in 1–2-day-old adult female Drosophila eyes. n = number of ommatidia.
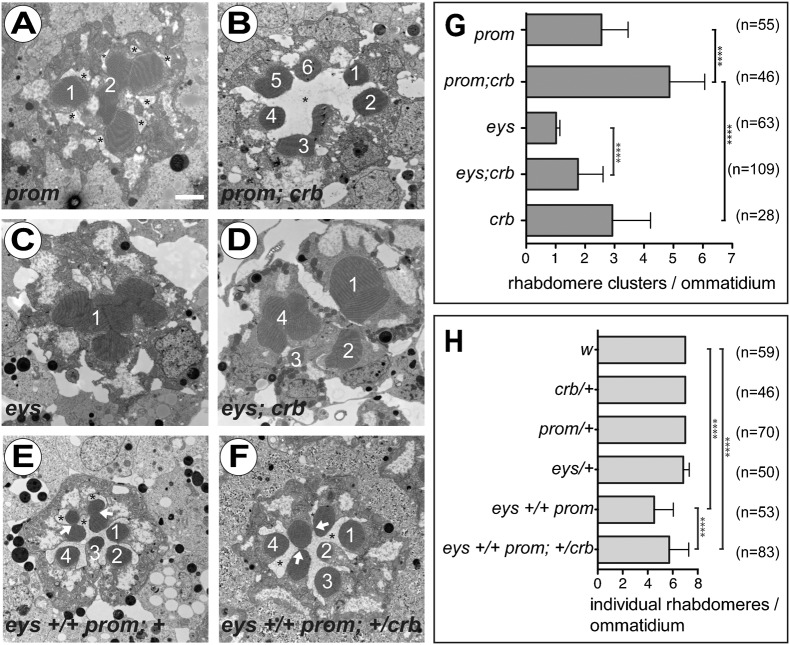
The apical membranes of all PRCs of each ommatidium are initially fused to each other, and only become separated during the second half of pupal development (Longley and Ready, 1995). Data suggest that this adhesion is mediated by homophilic interaction of Chp, while the separation requires the cooperative function of Prom and Eys. Ommatidia lacking prom or eys have fused rhabdomeres (Husain et al., 2006; Zelhof et al., 2006; reviewed by Gurudev et al., 2013). Given our observation on the synergistic function of crb and chp, we anticipated a genetic interaction of crb with prom and eys as well. prom mutant ommatidia fail to separate their rhabdomeres, resulting in one or two rhabdomere clusters rather than individual rhabdomeres and an irregular IRS (Fig. 2A). Strikingly, in the absence of crb the prom mutant phenotype is largely suppressed; most of the rhabdomeres are individualised and a single, continuous IRS is formed (Fig. 2B; quantified in Fig. 2G and supplementary material Fig. S2A). In eys mutants, all or nearly all rhabdomeres are fused to a single rhabdomeral cluster (Fig. 2C), and no IRS is formed. In the absence of both eys and crb the number of individual rhabdomeral clusters/individual rhabdomeres is increased, but unlike in prom;crb double mutant PRCs no IRS is formed in eys;crb double mutants (Fig. 2D; quantified in Fig. 2G and supplementary material Fig. S2A). Concomitant removal of one copy of prom and eys results in partial fusion of rhabdomeres (Fig. 2E, white arrows) (Zelhof et al., 2006), which is variable along the length of the retina. The fusion phenotype is rescued along the entire length of the rhabdomeres by removing just one copy of crb (Fig. 2F; quantified in Fig. 2H, supplementary material Fig. S2B and data not shown). Based on these results we suggest that loss of crb reduces the adhesive activity of Chp between individual rhabdomeres.
Fig. 2. Mutation in crb suppresses interrhabdomere adhesion in prom and eys mutants.
(A–F) Electron micrographs showing tangential sections of ommatidia of Drosophila with the following genotypes in w background: cn bw prom1 (A), cn bw prom1; crb11A22 (B), eys1 cn bw (C), eys1 cn bw; crb11A22 (D), + cn bw prom1/eys1 cn bw +; +/+ (E) and + cn bw prom1/eys1 cn bw +; crb11A22/+ (F). Numbers in A–F depict the numbers of individual rhabdomeres or rhabdomere clusters, not the identity of PRCs. Asterisk: IRS; white arrow: rhabdomere adhesion. Scale bar: 1 µm. (G,H) Quantification of interrhabdomeral adhesion of PRCs with different genotypes. Column chart (mean ± s.d.) represents the number of single rhabdomeres or rhabdomere clusters per ommatidium (G) and average individual rhabdomeres per ommatidium (H). n = number of ommatidia.
Crb and Chp affect localisation of each other in adult PRCs
The genetic interactions shown above suggest that Crb regulates Chp. Both in wild-type (Van Vactor et al., 1988; Zelhof et al., 2006; Kanie et al., 2009; Sanxaridis and Tsunoda, 2010; Rosenbaum et al., 2012; Yano et al., 2012) and in crb heterozygous PRCs, Chp is strongly enriched in the rhabdomere (Fig. 3A,A″ and data not shown). In crb mutant PRCs, identified by the absence of Crb protein from the stalk membrane, Chp is still highly enriched in rhabdomeres, but it is also detected in intracellular dot-like structures (Fig. 3B,B″,D, white arrows; quantification in Fig. 3C). This intracellular localisation was confirmed by immuno-EM analysis (Fig. 3E–E″, magenta arrow). Whether these intracellular sites represent compartments of the degradation pathway, such as multivesicular bodies (Sapp et al., 1991) as suggested by Fig. 3E″, needs further analysis. These data suggest that Crb ensures the proper localisation of Chp in adult PRCs.
Fig. 3. Localisation of Crb and Chp in wild-type and mutant PRCs.
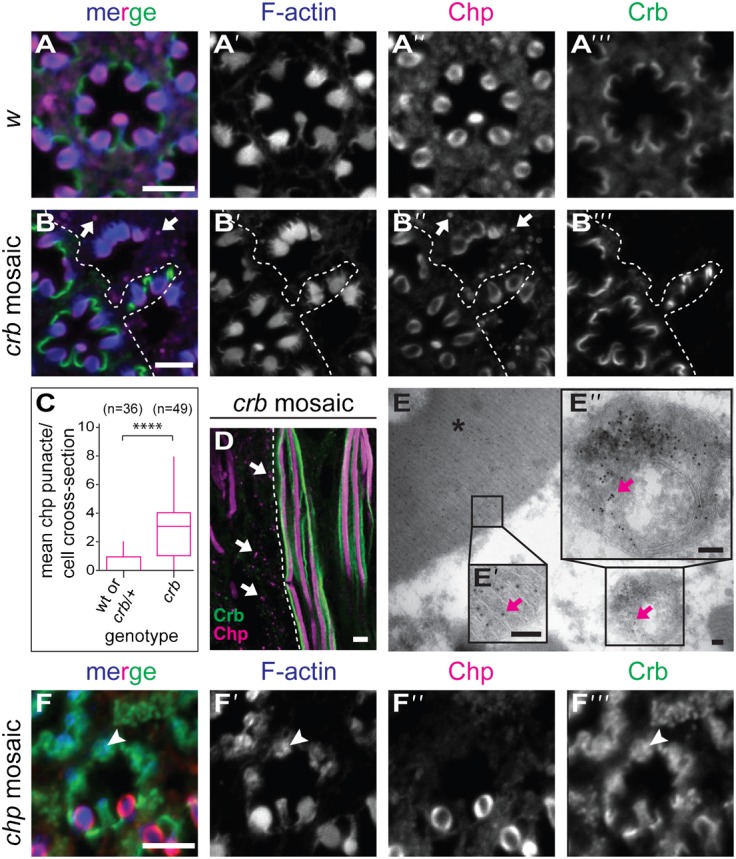
(A,B,D) Drosophila adult ommatidia. Genotypes are w (A), crb11A22 mosaic (B,D,E) and chp2 mosaic (F). Confocal images of immunostainings on tangential (A,B,F) and longitudinal (D) sections of PRCs stained for F-actin (blue), Chp (magenta) and Crb (green). White arrows in panels B,B″ and D point to intracellular Chp punctae in crb mutant cells. (C) Box-plot representing the number of cytosolic Chp positive punctae per cell per cross-section of wt, crb/+ and crb/crb mutant PRCs. Whiskers indicate 5–95% confidence interval. Statistical significance is analysed with Kruskal–Wallis test, followed by Dunn's multiple comparisons test. ****p<0.0001. (E–E″) Immunoelectron micrograph showing the localization of Chp (10 nm gold particle) (magenta arrows) in a cross-section of crb11A22 mutant PRC. Chp is localized in the rhabdomere (E,E′, asterisk) and in a multivesicular body (E″, arrow). (F) Confocal images of immunostainings on tangential sections of PRCs stained for F-actin (blue), Chp (magenta) and Crb (green). White arrowhead points to the apical membrane in chp mutant cells, which is no longer subdivided into rhabdomere and stalk. Scale bars: 5 µm (A,B,D,F), 100 nm (E).
To analyse the effect of chp on Crb, we made use of the allele chp2, which has been described as a functional null allele carrying a deletion that removes the 3′ 2785 nucleotides of the gene (Van Vactor et al., 1988). We confirmed that the deletion starts 3′ to exon 6 and removes the remaining coding region. The exact localisation of the second breakpoint could not be determined (supplementary material Fig. S3B). Other chp alleles were also analysed, including several published (Sanxaridis and Tsunoda, 2010) and newly induced alleles (see Materials and Methods), following determination of their molecular lesion (summarised in supplementary material Table S1 and Fig. S3). All null alleles gave rise to the same mutant phenotype (Fig. 3F; supplementary material Fig. S4). In wild-type PRCs, Crb is restricted to the stalk membrane (Fig. 3A,A′″). In all chp alleles tested and in chp-RNAi, Crb protein is still associated with the apical membrane of PRCs (Fig. 3F,F′″, arrowhead; supplementary material Fig. S5). However, Crb as well as actin cover the entire apical surface in PRCs of amorphic allele chp2 (Fig. 3F–F′″, white arrowhead), indicating that subdivision of the apical membrane into a defined stalk membrane and the rhabdomere is abolished. Nevertheless, the rudimentary rhabdomere spans the depth of the retina (supplementary material Fig. S5). From these results we conclude, that crb is required for optimal localisation of Chp at the apical membrane. chp is essential for microvilli stability, a prerequisite for the proper subdivision of the apical membrane into stalk and rhabdomere in adult PRCs.
The observed chp mutant phenotype starts at midpupal development
The adult phenotype of PRCs mutant for a loss-of-function chp allele is characterised by a severe reduction in the number and length of microvilli (Fig. 1C) and defective differentiation of the apical compartment (Fig. 3F; supplementary material Fig. S4). To determine the time point at which development of the microvilli starts to fail in the mutant, we compared PRCs of wild-type and chp mutant flies at different stages of pupal development. Shortly after puparium formation the apical surfaces of wild-type PRCs are tightly associated with each other (Longley and Ready, 1995) (Fig. 4A,E,E′). In chp mutants at a comparable stage, a small space can often be observed, suggesting that the apical membranes of opposing PRCs are less closely associated with each other (Fig. 4I,I′, blue asterisk). The difference between wild type and mutant becomes more pronounced at 54% pd. In wild type, PRCs at this stage are arranged in a stereotypic way, with their apical surface still in close association with that of their neighbours (Fig. 4B,F,F′). Thereby, the apical surface contacts either an incipient rhabdomere or a neighbouring stalk membrane, which is now clearly distinguishable from the incipient rhabdomere. The length of the microvilli is rather uniform (Fig. 4F′). In contrast, the microvilli of chp mutant PRCs are disorganised and more sparse than those in wild type (Fig. 4J,J′). Very often, they are pointing towards a central cavity, rather than being close to a neighbouring rhabdomere or stalk membrane. Wild-type PRCs at 79% pd exhibit clearly formed rhabdomeres, embedded in a now distinct IRS. They have tightly packed and properly aligned microvilli, which touch neighbouring PRCs only occasionally. The stalk membrane can be clearly distinguished (Fig. 4G,G′, highlighted in green in Fig. 4C). In chp mutant PRCs of this stage, the apical microvilli are less densely packed and rather stand out as individual microvilli. The stalk is visible as a smooth membrane (Fig. 4K,K′, indicted by magenta arrows). The rhabdomeres of adult wild-type flies show tightly packed microvilli and are well separated from each other by the IRS (Fig. 4D,H,H′). In contrast, microvilli of adult chp mutant PRCs deteriorate, with individual microvilli hard to distinguish (Fig. 4L,L′), hence termed “rudimentary rhabdomeres” (Van Vactor et al., 1988). Taken together, chp controls two distinct adhesion processes in developing PRCs: it ensures adhesion of the apical membranes of opposing PRCs at early stages, and elongation and tight packing of microvilli within the rhabdomeres at later stages.
Fig. 4. Development of the apical compartment in chp mutant PRCs.
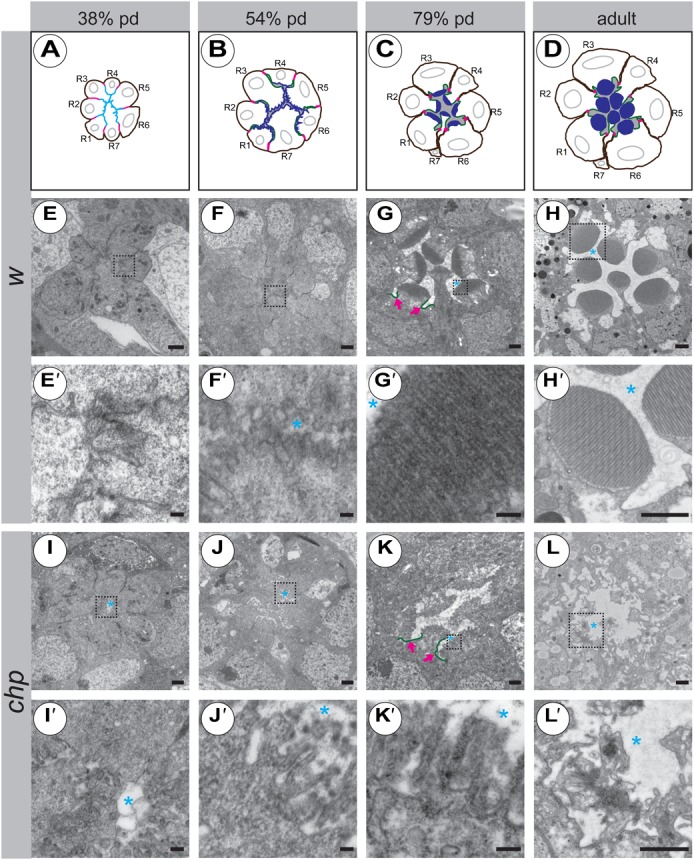
(A–D) Cartoons of different developmental stages in wild-type ommatidia (not taking into account the correct contacts made by individual rhabdomeres). Magenta: adherens junction. Cyan: common apical surface in panel A, as deduced by co-localisation of Crb and F-actin. Green: Crb, highlighting the stalk membrane. Blue: F-actin, highlighting the microvilli. Grey: interrhabdomerel space. R1–R7 = number of PRCs. pd = pupal development. (E–L′): Electron micrographs of tangential sections of wild-type (E–H′) and chp2 mutant (I–L′) ommatidia at 38% pd (E,E′,I,I′), 54% pd (F,F′,J,J′), 79% pd (G,G′,K,K′) and in the adult (H,H′,L,L′). The first defects in chp mutant ommatidia can already be detected at 38% pd, in that the microvilli are not as closely associated with each other as in wild type (compare panel E′ to panel I′). Scale bars: 1 µm (E–H,H′,I–L,L′) and 100 nm (E′–G′,I′–K′); blue asterisk, IRS; magenta arrows, stalk membrane (labelled in green).
prom and eys, but not chp mutant PRCs degenerate
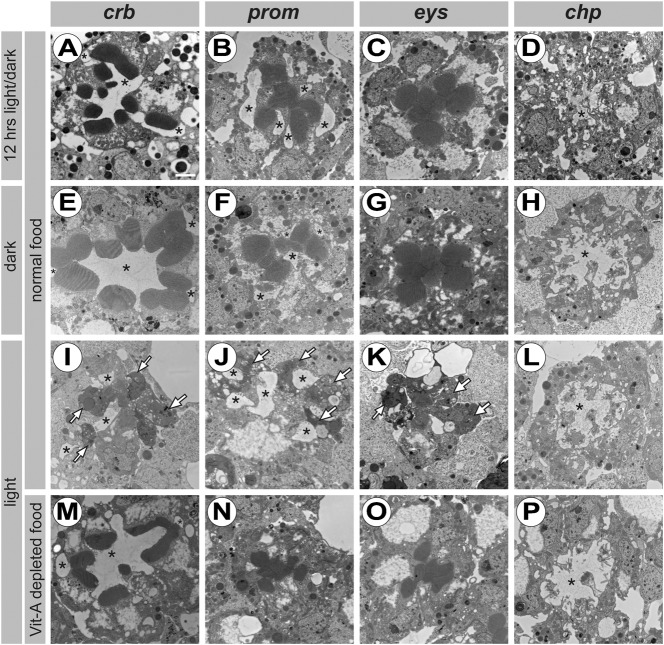
Given the close genetic interaction between crb, chp, prom and eys, and the observation that crb mutant PRCs undergo light-dependent degeneration (Johnson et al., 2002; Chartier et al., 2012), we analysed the effect of constant light exposure on the survival of PRCs mutant for chp, prom and eys. When kept in constant darkness or in a 12 hrs light/dark cycle, none of the mutant PRCs degenerated (Fig. 5A–H). However, when kept under constant illumination for 5 days, PRCs mutant for either prom or eys undergo light-dependent degeneration similar as crb mutant PRCs. The majority of PRCs show typical signs of degeneration, such as condensed cytoplasm (Fig. 5I–K, white arrows). Although the phenotype of chp mutants was previously interpreted as degeneration (Van Vactor et al., 1988; Rosenbaum et al., 2012), detailed inspection reveals that chp mutant PRCs do not degenerate when kept under any of these light conditions (Fig. 5D,H,L). Despite a nearly complete loss of microvilli, we could not detect any sign of apoptosis, such as cytoplasmic condensation, which is clearly visible in degenerating PRCs of the other genotypes (compare Fig. 5I–K to Fig. 1L).
Fig. 5. Effect of constant light exposure on the survival of crb, prom, eys and chp mutant PRCs.
Electron micrographs of ommatidial cross-sections of Drosophila raised on standard (A–L) or vitamin A-depleted Drosophila medium (M–P) and kept under various light conditions. Genotypes are: crb11A22 (A,E,I,M), cn1 bw1 prom1 (B,F,J,N), eys1 cn1 bw1 (C,G,K,O) and chp2 (D,H,L,P), all in a w genetic background. crb, prom and eys mutant PRCs do not show any signs of retinal degeneration when kept under a 12 hrs light/dark cycle (A–C) or in constant darkness (E–G). When kept in constant light, they display characteristics of degeneration (I–K), such as condensed cytoplasm (white arrows) and missing rhabdomeres. Degeneration under constant illumination is prevented when animals were raised in a vitamin A-depleted medium (M–O). Under all tested conditions, chp mutant PRCs do not show any major signs of retinal degeneration (D,H,L,P). Scale bar: 1 µm; asterisk: IRS.
We previously showed that degeneration of crb mutant PRCs can be prevented when larvae were raised in vitamin A-depleted food (Fig. 5M) (Johnson et al., 2002), which reduces the amount of Rh1 synthesis to about 3% of its normal amount in wild type. Strikingly, retinal degeneration of prom and eys mutant PRCs could also be prevented in the absence of dietary vitamin A (Fig. 5N,O). The phenotype of chp mutant PRCs was unaltered under light stress, with or without dietary vitamin A (Fig. 5D,H,L,P), which could be explained by a strongly reduced level of Rh1 in chp mutant PRCs (Rosenbaum et al., 2012). These results suggest that crb, prom and eys prevent light-dependent degeneration of PRCs by a similar cellular mechanism.
DISCUSSION
Two genetic systems have been described previously to control rhabdomere and stalk membrane development, chp, prom and eys on the one hand, and crb on the other hand. Here we show that the two systems form a common genetic network to assure the development of the highly elaborated apical surface of Drosophila PRCs. In addition, crb, prom and eys prevent light-dependent retinal degeneration, which can be prevented by dramatically reducing Rh1 levels. This suggests that these three genes are also functionally linked in adult PRC homeostasis.
The GPI-anchored protein Chp is required for three distinct processes during development of Drosophila PRCs. First, it ensures interrhabdomeral adhesion at early stages of pupal development. A failure to perform this function becomes obvious already at 38% pd in chp mutant PRCs, when the developing apical protrusions are less closely interdigitated with those of the neighbouring cells. Most insects, such as the honeybee Apis mellifera, the mosquito Anopheles gambiae or the flour beetle Tribolium castaneum, form a fused rhabdom without an IRS, in which all rhabdomeres of an ommatidium are closely attached to each other, even in the adult fly. In comparison to a closed rhabdome, the open rhabdome found in most flies, including Drosophila, confers increased sensitivity and a better signal-to-noise ratio (reviewed by Osorio, 2007). Interestingly, although the eys homologue exists in these species, it is not expressed in the visual system of pupae, whereas the chp and prom homologues are (Husain et al., 2006; Zelhof et al., 2006).
The second function of Chp is required later for the close alignment of neighbouring microvilli within a rhabdomere. Homophilic adhesion has been described for other systems to stabilise microvilli. In the cochlea of mammals, for example, protocadherin 15 (PCDH15) provides lateral links to connect the stereocilia, actin-based microvilli located on the apical surface of auditory hair cells, which are specialised to receive sensory input by sound. Mutations in PCDH15 are associated with Usher syndrome type 1F, a recessive disease characterised by retinopathy and hearing loss (Ahmed et al., 2001; Alagramam et al., 2001; El-Amraoui and Petit, 2005). Strikingly, the Drosophila homolog of PCDH15, Cad99C, is required for microvilli stability in the developing egg chamber (Schlichting et al., 2006).
Our data reveal that the phenotype of chp adult eyes is much stronger than that observed at 79% pd, suggesting that Chp is particularly important during the final stages of pupal development. This could be explained either by assuming that the increased length of microvilli require stronger adhesion for their stabilisation. Alternatively, other proteins, such as Rh1 or Php13, may contribute to stabilise microvilli at the end of pupal development. Rh1 plays an essential role in rhabdomere morphogenesis and is synthesised from 78% pd onwards (Kumar and Ready, 1995). In fact, Rh1 levels are strongly reduced in freshly eclosed chp2 adult flies (Rosenbaum et al., 2012). However, in contrast to mutations in ninaE, which encodes Rh1, microvilli do not protrude into the cytoplasm in chp mutant PRCs, suggesting that the reduced amount of Rh1 is sufficient to stabilise the RTW. PRCs lacking the transcription factor Php13 show a similar phenotype as chp mutant PRCs until 60% pd, which becomes more severe after 72% pd (Zelhof et al., 2003). Together with orthodenticle (otd)/ocelliless (oc), Pph13 regulates the expression of rhodopsin and chp, and probably other, not yet identified target genes required for microvillar morphogenesis (Mishra et al., 2010).
The third function of Chp is required, directly or indirectly, to subdivide the apical membrane into rhabdomere and stalk. Adult chp mutant PRCs have only few and strongly disorganised microvilli and no distinguishable stalk membrane, resulting in the spreading of the stalk membrane specific protein Crb throughout the apical surface.
Our data reveal an unexpected role of crb for the localisation of Chp in adult PRCs. The accumulation of Chp in multivesicular bodies in crb mutant PRCs suggests that crb is required for efficient localisation of Chp in the rhabdomeral membrane. We previously showed that in the absence of crb Rh1 accumulates in intracellular dots of unknown identity in adult PRCs (Pocha et al., 2011). Recently, an RNAi screen aimed to identify regulators of polarity proteins in PRCs was published. This screen identified several genes, the knock-down of which resulted in accumulation of the apical marker Chp in intracellular dots. The genes included regulators of protein/vesicle transport, such as Sec10, RabX4 and transportin, but also genes predicted to be involved in protein degradation (Yano et al., 2012). In addition, regulators of protein synthesis and modification are required to ensure proper delivery of Chp to and stabilisation at the apical surface (Rosenbaum et al., 2012). Whether a direct interaction between Crb and Chp occurs at any of these steps, or whether Crb is indirectly involved in Chp trafficking and stability is not known. It is tempting to speculate that the same relationship between Crb and Chp functions exists already at early stages of pupal development, when Prom and Eys antagonise Chp function (Zelhof et al., 2006). Loss of crb rescues the interrhabdomeral adhesion of prom and eys mutant PRCs, but whether it acts directly on any of these genes/proteins or in an indirect way has to be determined.
Beside their role in antagonising Chp function in the first half of pupal development, we present data showing that both Prom and Eys are additionally required for the survival of PRCs under light stress, a function also attributed to crb (Johnson et al., 2002). Several mechanisms are discussed, which prevent retinal degeneration in flies (Colley, 2012; Nie et al., 2012; Raghu et al., 2012). Accumulation of intracellular Rh1 has been suggested to trigger light dependent PRC degeneration in crb mutant PRCs (Pocha et al., 2011; Hollingsworth and Gross, 2012). It can be prevented by reduction of dietary vitamin A (Johnson et al., 2002), which has been shown to lower the amount of Rh1 to about 3% (Nichols and Pak, 1985). Raising prom and eys mutant animals in vitamin A depleted food also prevented light-dependent retinal degeneration, suggesting that prom and eys contribute to cell survival under light stress, directly or indirectly, by regulating Rh1. Interestingly, mutations in the human orthologues Prominin 1 (PROM1) and EYS are associated with autosomal-recessive retinitis pigmentosa and macular degeneration. Similar as in flies, mutations in mouse Prom1 result in defects in PRC morphogenesis, followed by degeneration (Maw et al., 2000; Zhang et al., 2007; Abd El-Aziz et al., 2008; Collin et al., 2008; Yang et al., 2008; Zacchigna et al., 2009). Considering that the expansion and organisation of the apical surface occurs by different mechanisms in vertebrates and Drosophila – formation of membrane discs vs microvilli – these proteins seem to control a very basic, evolutionarily conserved cell biological function, required for the maintenance of apical, light-sensing membrane integrity. This assumption is further strengthened by recent results showing that PROM1 can substitute the Drosophila protein in prom mutant fly PRCs and that expression of PROM1, which carries a mutation that has been associated with the development of blindness (hProm1R373C), results in morphologically defective rhabdomeres when expressed in fly PRCs (Nie et al., 2012).
In contrast to prom, eys and crb, PRCs mutant for chp do not degenerate, even when exposed to constant light. This is in contrast to previously published papers, which have interpreted the lack of rhabdomeral microvilli as indication of apoptosis (Van Vactor et al., 1988; Rosenbaum et al., 2012). However, in histological sections of chp mutant ommatidia we always detected seven cell bodies with rudimentary rhabdomeres, even after several days of light exposure. chp mutant PRCs have low levels of Rh1 (Rosenbaum et al., 2012), which is probably not sufficient to accumulate in toxic doses even under light stress and therefore no degeneration occurs.
Taken together, our work has unravelled genetic interactions between crb, chp, prom and eys, which build an important genetic network to ensure proper development of microvilli and formation of an open rhabdom in Drosophila ommatidia. Given the observation that all four proteins are localised apically, it is possible that they build an apical regulatory network to perform this function, but the molecular details of this interaction are not known. In addition, crb, prom and eys are required in adult eyes for PRC survival upon light stress, a function that is conserved during evolution. This makes the Drosophila eyes an ideal model to understand the corresponding processes in humans, which, when perturbed, often result in diseases such as retinitis pigmentosa or microvillus inclusion disease (Ameen and Salas, 2000; Bazellieres et al., 2009; Bulgakova and Knust, 2009; Gurudev et al., 2013).
MATERIALS AND METHODS
Drosophila stocks/experimental genotypes
Drosophila lines were maintained at 25°C on standard Drosophila food unless mentioned otherwise. The following Drosophila stocks were used: w1118, Oregon-R or w1118; cn1 bw1 as wild type. Loss-of-function alleles: crb11A22 and crb8F105 (Jürgens et al., 1984; Tepass and Knust, 1993; Wodarz et al., 1993), crbGX24 (Huang et al., 2009), chp2 (Van Vactor et al., 1988); chpZ3513, chpZ5240 and chpZ4345 (Zuker collection) (Koundakjian et al., 2004; Sanxaridis and Tsunoda, 2010), a kind gift from Tsunoda Lab; chpMB05115 and Df(3R)BSC793 and Df(3R)BSC749 (Bloomington); chpSS52 (this study), prom1 and eys1 (spam1) (Zelhof et al., 2006), a kind gift from C. Zuker, eyFLP; Rh1-GAL4; FRT82B w+ Bcl3R3/MKRS, eyFLP; Rh1-GAL4; FRT82B w+/MKRS, eyFLP; Rh1-GAL4; FRT82Bcrb11A22/TM6B (Richard et al., 2009). RNAi stocks V105053 and V39177 from Vienna Drosophila Research Centre (VDRC) (Dietzl et al., 2007). Drosophila manipulations were done in accordance with standard techniques.
Generating the hypomorphic allele chpSS52
chpMB05115 is a mutation induced by the insertion of a minos-element in the first intron (Flybase). Homozygous w; Mi{ET1}chpMB05115 flies (Bloomington stock no. 24321), carrying a Minos-transposon inserted in the chp locus (FlyBase) (supplementary material Fig. S3) were crossed with w1118; snaSco/SM6a, P{w[+mC] = hsILMiT}2.4 flies, which carry a heat-shock inducible Minos-transposase (Bloomington stock no. 24613). The following steps were performed as described previously (Metaxakis et al., 2005). Homozygous stocks obtained after mobilisation of the Minos element were screened for defects in the rhabdomere using the optical neutralisation assay (see below). One stock, chpSS52, showed mild rhabdomere defects severely affecting R7. The hypomorphic phenotype was confirmed by electron microscopic analysis of chpSS52/Df(3R)BSC793 adult ommatidia.
Optical neutralization assay
The technique was performed as described previously with modifications (Franceschini, 1972; Morante and Desplan, 2011). In brief, dissected Drosophila heads were mounted with immersion oil on a bridged glass slide to preserve the three-dimensional structure and covered with a glass slide. Bright field images were taken using an oil immersion objective lens 63×, Zeiss AxioImager.Z1.
Electron microscopy
Retinas from 1–2-day-old adult female flies were fixed, sectioned and photographed as described previously (Richard et al., 2006; Mishra and Knust, 2013) with minor modifications. Adult fly heads were bisected along the midline, and fixed for first 20 min in 25% glutaraldehyde in PB (0.1 M phosphate buffer [pH 7.2]), followed by fixation in 1% osmium tetroxide + 2% glutaraldehyde for 30 min at 4°C and followed by 2% osmium tetroxide for 30 min at 4°C. After dehydration with ethanol, eyes were infiltrated and embedded in Durcupan and semi- (2 µm) and ultra- (70 nm) thin sections were cut using a Leica Ultracut UCT. Semi-thin sections were stained with toluidine blue and imaged using Zeiss AxioImager.Z1. Ultra-thin sections were contrasted with 2% uranyl acetate in pure water for 10 min and lead citrate for 5 mins, and analyzed using a Morgagni electron microscope (FEI Company, 80 kV) and distal eye sections were imaged using Morada digital camera (SIS). Pupae were staged (Walther and Pichaud, 2006), fixed, sectioned, contrasted and imaged essentially as described previously (Longley and Ready, 1995), except that the retinal–brain complex from staged pupae was dissected and fixed on ice.
Quantification of rhabdomere size
All measurements were obtained from electron micrographs taken from cross-sections from the distal third of compound eyes of 1–2-day-old adult female Drosophila. The cross sectional area of R1–R6 rhabdomeres was measured from at least 3 different individual flies per genotype using Fiji software. Graphs were drawn and statistical analyses were performed using Prism software.
Immuno-electron microscopy
Heads from 1–2-day-old adult female flies were bisected and fixed in 4% formaldehyde in PB for 30 min at RT (room temperature) and 12 hrs at 4°C on a rotator. The samples were washed 3 times with PB and cryoprotected in 2.7 M sucrose in PB for 12 hrs at 4°C. Each eye was picked up with a pin and quickly frozen in liquid nitrogen. Ultrathin sections (70 nm) were cut using a Leica EM UC6, washed 2×10 min with PBG (PB with 0.5% BSA and 0.2% Gelatin), followed by incubation with MAb24B10 (1:20 in PBG) for 1 hr. Specimens were washed 6×5 min with PBG and incubated with secondary goat anti-mouse IgG coupled to 10 nm gold particle for 1 hr, followed by 6×5 min washes with PBG and 6×2 min washes with PB. After post-fixation with 1% glutaraldehyde for 5 min, specimens were washed 2×2 min with PB, 6×2 min with pure H20 and stained with 2% uranyl acetate. The grid was coated in 0.1% methylcellulose + 2% uranyl acetate. Grids with ultrathin sections were imaged using a Morgagni (FEI company, 80 kV) electron microscope and micrographs were taken with Morada camera and ITEH software (Olympus).
Light induced degeneration assay
For light induced degeneration assay, 1–2-day-old adult flies raised in 12-hr light/dark cycles at 25°C were exposed to constant light (1.700±20 lux) for 5 days at 25°C and 60% humidity. The carotenoid-free medium was prepared as previously described (Pocha et al., 2011).
Immunohistochemistry
1–2-day-old adult female Drosophilae were fixed, sectioned, stained and mounted as previously described (Muschalik and Knust, 2011). In brief, adult flies were fixed with Stefanini's fixative (8% PFA, 15% picric acid, and 75 mM Pipes; pH 7.4) for 60 min at RT, washed 3× with PBS (phosphate-buffered saline), pH 7.2. Heads were dissected from the body and cryopreserved by incubation in 10% sucrose in PBS, pH 7.2, for 30 min at RT and then in 25% sucrose in PBS, pH 7.2, overnight at 4°C. Heads were then embedded in Richard-Allan Scientific Neg-50 molds (Thermo Fisher Scientific), deep frozen on dry ice and stored at −80°C until used. 12-µm thick cryo-sections were cut on a cryostat microtome (HM560; Thermo Fisher Scientific). The cryo-sections were collected on coated glass slides (Marienfeld), surrounded with a layer of hydrophobic compound (ImmedgePEN, Vector), permeabilised for 1 hr in PBT [PBS with 0.1% Triton X-100, pH 7.2] and incubated in blocking buffer [PBS with 4% BSA (bovine serum albumin)] 2 hrs at room temperature, followed by incubation over night at 4°C with the primary antibody in blocking buffer. The primary antibody was removed and sections were washed for 3×20 min in blocking buffer before incubation with the secondary antibody and Alexa-Fluor-phalloidin for 2 hrs at RT. After washing (3×20 min) in blocking buffer, sections were mounted in Mowiol (Calbiochem)-containing 4% DABCO (Sigma). The following antibodies were used: rat anti-Crb2.8 antibody (1:1000) (Richard et al., 2006); mouse anti-Chp (24B10, 1:200, DSHB). Alexa-Fluor-647, Alexa-Fluor-555, Alexa-Fluor-488 (Invitrogen) were used as secondary antibodies at 1:400 dilution. Rhabdomeres were visualized by labelling F-actin with Alexa-Fluor-488-phalloidin at 1:40 (Invitrogen). Images were taken either on Zeiss LSM 510/710 confocal microscopes.
Production of anti-Chp N7A antibody
N7A is a polyclonal rabbit ant-Chp antibody, which was generated by immunizing rabbits with two synthetic peptides from the N-terminus [KLDLSGDRNDPTNLQT] of Chp-PA, PF and PD, and C-terminus [YNSSWSGRNEHGGMYH] of Chp-PA, PE, PF and PD (Speedy-28 day package, Eurogenetec Deutschland GmbH, Köln, Germany). The specificity of N7A antibody was confirmed by western blots (1:2000) using extracts from homogenized wild-type and chp mutant fly heads.
Image processing
Images were processed using Fiji and/or Adobe Photoshop-CS5. Image manipulation was fully compliant with the image guidelines for proper digital image handling outlined in Rossner and Yamada (Rossner and Yamada, 2004).
Supplementary Material
Acknowledgments
We thank Susan Tsunoda, Charles S. Zuker and Yang Hong for fly stocks, and Satoshi Goto for the N8A antibody. We thank Sylke Winkler and Katja Steinberg for sequence analysis of chp alleles, Sebastian Dunst and Xin Liang for help with statistical analysis of rhabdomere size, and Michaela Wilsch-Bräuninger for help with acquiring electron micrographs. We thank Shirin M. Pocha, Daiki Umetsu, Xin Liang, Sarita Hebbar, Michaela Wilsch-Bräuninger and David Flores for critical reading of the manuscript. We are indebted to the fly facility and the light and electron microscopy facilities of MPI-CBG.
Footnotes
Competing interests: The authors have no competing interests to declare.
Funding
This work was supported by the Max-Planck Society (MPG) and a grant from the EC [HEALTH-F2-2008-200234].
References
- Abd El-Aziz M. M., Barragan I., O'Driscoll C. A., Goodstadt L., Prigmore E., Borrego S., Mena M., Pieras J. I., El-Ashry M. F., Safieh L. A. et al. (2008). EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat. Genet. 40, 1285–1287 10.1038/ng.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. M., Riazuddin S., Bernstein S. L., Ahmed Z., Khan S., Griffith A. J., Morell R. J., Friedman T. B., Riazuddin S., Wilcox E. R. (2001). Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 69, 25–34 10.1086/321277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam K. N., Murcia C. L., Kwon H. Y., Pawlowski K. S., Wright C. G., Woychik R. P. (2001). The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 27, 99–102 10.1038/83837 [DOI] [PubMed] [Google Scholar]
- Ameen N. A., Salas P. J. (2000). Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1, 76–83 10.1034/j.1600-0854.2000.010111.x [DOI] [PubMed] [Google Scholar]
- Arikawa K., Hicks J. L., Williams D. S. (1990). Identification of actin filaments in the rhabdomeral microvilli of Drosophila photoreceptors. J. Cell Biol. 110, 1993–1998 10.1083/jcb.110.6.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Grawe F., Johnson K., Knust E. (2008). Drosophila Lin-7 is a component of the Crumbs complex in epithelia and photoreceptor cells and prevents light-induced retinal degeneration. Eur. J. Cell Biol. 87, 123–136 10.1016/j.ejcb.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Bazellieres E., Assemat E., Arsanto J. P., Le Bivic A., Massey-Harroche D. (2009). Crumbs proteins in epithelial morphogenesis. Front. Biosci. 14, 2149–2169 10.2741/3368 [DOI] [PubMed] [Google Scholar]
- Berger S., Bulgakova N. A., Grawe F., Johnson K., Knust E. (2007). Unraveling the genetic complexity of Drosophila stardust during photoreceptor morphogenesis and prevention of light-induced degeneration. Genetics 176, 2189–2200 10.1534/genetics.107.071449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova N. A., Knust E. (2009). The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 122, 2587–2596 10.1242/jcs.023648 [DOI] [PubMed] [Google Scholar]
- Chang H.-Y., Ready D. F. (2000). Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science 290, 1978–1980 10.1126/science.290.5498.1978 [DOI] [PubMed] [Google Scholar]
- Chartier F. J., Hardy E. J., Laprise P. (2012). Crumbs limits oxidase-dependent signaling to maintain epithelial integrity and prevent photoreceptor cell death. J. Cell Biol. 198, 991–998 10.1083/jcb.201203083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley N. J. (2012). Retinal degeneration in the fly. Adv. Exp. Med. Biol. 723, 407–414 10.1007/978-1-4614-0631-0_52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin R. W., Littink K. W., Klevering B. J., van den Born L. I., Koenekoop R. K., Zonneveld M. N., Blokland E. A., Strom T. M., Hoyng C. B., den Hollander A. I. et al. (2008). Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am. J. Hum. Genet. 83, 594–603 10.1016/j.ajhg.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. K., Christensen S. J., Deal J. A., Coburn R. A., Deal M. E., Gresens J. M., Kaufman T. C., Cook K. R. (2012). The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 13, R21 10.1186/gb-2012-13-3-r21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A. I., ten Brink J. B., de Kok Y. J., van Soest S., van den Born L. I., van Driel M. A., van de Pol D. J., Payne A. M., Bhattacharya S. S., Kellner U. et al. (1999). Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23, 217–221 10.1038/13848 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- El-Amraoui A., Petit C. (2005). Usher I syndrome: unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J. Cell Sci. 118, 4593–4603 10.1242/jcs.02636 [DOI] [PubMed] [Google Scholar]
- Fain G. L., Hardie R., Laughlin S. B. (2010). Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114–R124 10.1016/j.cub.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N. (1972). Pupil and pseudopupil in the compound eye of Drosophila. Information Processing in the Visual Systems of Arthropods Wehner R, ed75–82Berlin; Heidelberg; New York, NY: Springer Verlag. [Google Scholar]
- Gurudev N., Florek M., Corbeil D., Knust E. (2013). Prominent role of prominin in the retina. Adv. Exp. Med. Biol. 777, 55–71 10.1007/978-1-4614-5894-4_4 [DOI] [PubMed] [Google Scholar]
- Hirai-Fujita Y., Yamamoto-Hino M., Kanie O., Goto S. (2008). N-Glycosylation of the Drosophila neural protein Chaoptin is essential for its stability, cell surface transport and adhesive activity. FEBS Lett. 582, 2572–2576 10.1016/j.febslet.2008.06.028 [DOI] [PubMed] [Google Scholar]
- Hollingsworth T. J., Gross A. K. (2012). Defective trafficking of rhodopsin and its role in retinal degenerations. Int Rev Cell Mol Biol 293, 1–44 10.1016/B978-0-12-394304-0.00006-3 [DOI] [PubMed] [Google Scholar]
- Huang J., Zhou W., Dong W., Watson A. M., Hong Y. (2009). From the cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. USA 106, 8284–8289 10.1073/pnas.0900641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain N., Pellikka M., Hong H., Klimentova T., Choe K.-M., Clandinin T. R., Tepass U. (2006). The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev. Cell 11, 483–493 10.1016/j.devcel.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Izaddoost S., Nam S.-C., Bhat M. A., Bellen H. J., Choi K.-W. (2002). Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178–183 10.1038/nature720 [DOI] [PubMed] [Google Scholar]
- Johnson K., Grawe F., Grzeschik N., Knust E. (2002). Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr. Biol. 12, 1675–1680 10.1016/S0960-9822(02)01180-6 [DOI] [PubMed] [Google Scholar]
- Jürgens G., Wieschaus E., Nüsslein-Volhard C., Kluding H. (1984). Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Roux Arch. Dev. Biol. 193, 283–295 10.1007/BF00848157 [DOI] [PubMed] [Google Scholar]
- Kanie Y., Yamamoto-Hino M., Karino Y., Yokozawa H., Nishihara S., Ueda R., Goto S., Kanie O. (2009). Insight into the regulation of glycan synthesis in Drosophila chaoptin based on mass spectrometry. PLoS ONE 4, e5434 10.1371/journal.pone.0005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiosis S. A., Ready D. F. (2004). Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development 131, 725–732 10.1242/dev.00976 [DOI] [PubMed] [Google Scholar]
- Koundakjian E. J., Cowan D. M., Hardy R. W., Becker A. H. (2004). The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167, 203–206 10.1534/genetics.167.1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz D. E., Zipursky S. L. (1990). Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J. 9, 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. P., Ready D. F. (1995). Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development 121, 4359–4370. [DOI] [PubMed] [Google Scholar]
- Kumar J. P., Bowman J., O'Tousa J. E., Ready D. F. (1997). Rhodopsin replacement rescues photoreceptor structure during a critical developmental window. Dev. Biol. 188, 43–47 10.1006/dbio.1997.8636 [DOI] [PubMed] [Google Scholar]
- Lamb T. D. (2009). Evolution of vertebrate retinal photoreception. Philos. Trans. R. Soc. B 364, 2911–2924 10.1098/rstb.2009.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. X., Satoh A. K., Ready D. F. (2007). Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J. Cell Biol. 177, 659–669 10.1083/jcb.200610157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley R. L. J., Jr, Ready D. F. (1995). Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev. Biol. 171, 415–433 10.1006/dbio.1995.1292 [DOI] [PubMed] [Google Scholar]
- Maw M. A., Corbeil D., Koch J., Hellwig A., Wilson-Wheeler J. C., Bridges R. J., Kumaramanickavel G., John S., Nancarrow D., Röper K. et al. (2000). A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum. Mol. Genet. 9, 27–34 10.1093/hmg/9.1.27 [DOI] [PubMed] [Google Scholar]
- Metaxakis A., Oehler S., Klinakis A., Savakis C. (2005). Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics 171, 571–581 10.1534/genetics.105.041848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Knust E. (2013). Analysis of the Drosophila compound eye with light and electron microscopy. Methods Mol. Biol. 935, 161–182 10.1007/978-1-62703-080-9_11 [DOI] [PubMed] [Google Scholar]
- Mishra M., Oke A., Lebel C., McDonald E. C., Plummer Z., Cook T. A., Zelhof A. C. (2010). Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137, 2895–2904 10.1242/dev.051722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante J., Desplan C. (2011). Dissection and staining of Drosophila optic lobes at different stages of development. Cold Spring Harb. Protoc. 2011, pdb.prot5629 10.1101/pdb.prot5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschalik N., Knust E. (2011). Increased levels of the cytoplasmic domain of Crumbs repolarise developing Drosophila photoreceptors. J. Cell Sci. 124, 3715–3725 10.1242/jcs.091223 [DOI] [PubMed] [Google Scholar]
- Nichols R., Pak W. L. (1985). Characterization of Drosophila melanogaster rhodopsin. J. Biol. Chem. 260, 12670–12674. [PubMed] [Google Scholar]
- Nie J., Mahato S., Mustill W., Tipping C., Bhattacharya S. S., Zelhof A. C. (2012). Cross species analysis of Prominin reveals a conserved cellular role in invertebrate and vertebrate photoreceptor cells. Dev. Biol. 371, 312–320 10.1016/j.ydbio.2012.08.024 [DOI] [PubMed] [Google Scholar]
- Osorio D. (2007). Spam and the evolution of the fly's eye. Bioessays 29, 111–115 10.1002/bies.20533 [DOI] [PubMed] [Google Scholar]
- Pellikka M., Tanentzapf G., Pinto M., Smith C., McGlade C. J., Ready D. F., Tepass U. (2002). Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143–149 10.1038/nature721 [DOI] [PubMed] [Google Scholar]
- Pham H., Yu H., Laski F. A. (2008). Cofilin/ADF is required for retinal elongation and morphogenesis of the Drosophila rhabdomere. Dev. Biol. 318, 82–91 10.1016/j.ydbio.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal N., Pichaud F. (2011). Dynamin- and Rab5-dependent endocytosis is required to prevent Drosophila photoreceptor degeneration. J. Cell Sci. 124, 1564–1570 10.1242/jcs.082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal N., Goberdhan D. C., Collinson L., Fujita Y., Cox I. M., Wilson C., Pichaud F. (2006). Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16, 140–149 10.1016/j.cub.2005.11.068 [DOI] [PubMed] [Google Scholar]
- Pocha S. M., Shevchenko A., Knust E. (2011). Crumbs regulates rhodopsin transport by interacting with and stabilizing myosin V. J. Cell Biol. 195, 827–838 10.1083/jcb.201105144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P., Yadav S., Mallampati N. B. (2012). Lipid signaling in Drosophila photoreceptors. Biochim. Biophys. Acta 1821, 1154–1165 10.1016/j.bbalip.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Reinke R., Krantz D. E., Yen D., Zipursky S. L. (1988). Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell 52, 291–301 10.1016/0092-8674(88)90518-1 [DOI] [PubMed] [Google Scholar]
- Richard M., Grawe F., Knust E. (2006). DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev. Dyn. 235, 895–907 10.1002/dvdy.20595 [DOI] [PubMed] [Google Scholar]
- Richard M., Muschalik N., Grawe F., Ozüyaman S., Knust E. (2009). A role for the extracellular domain of Crumbs in morphogenesis of Drosophila photoreceptor cells. Eur. J. Cell Biol. 88, 765–777 10.1016/j.ejcb.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Rosenbaum E. E., Brehm K. S., Vasiljevic E., Gajeski A., Colley N. J. (2012). Drosophila GPI-mannosyltransferase 2 is required for GPI anchor attachment and surface expression of chaoptin. Vis. Neurosci. 29, 143–156 10.1017/S0952523812000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner M., Yamada K. M. (2004). What's in a picture? The temptation of image manipulation. J. Cell Biol. 166, 11–15 10.1083/jcb.200406019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanxaridis P. D., Tsunoda S. (2010). A forward genetic screen in Drosophila melanogaster to identify mutations affecting INAD localization in photoreceptor cells. Fly (Austin) 4, 95–103 10.4161/fly.4.2.11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp R. J., Christianson J., Stark W. S. (1991). Turnover of membrane and opsin in visual receptors of normal and mutant Drosophila. J. Neurocytol. 20, 597–608 10.1007/BF01215267 [DOI] [PubMed] [Google Scholar]
- Satoh A. K., O'Tousa J. E., Ozaki K., Ready D. F. (2005). Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132, 1487–1497 10.1242/dev.01704 [DOI] [PubMed] [Google Scholar]
- Schlichting K., Wilsch-Bräuninger M., Demontis F., Dahmann C. (2006). Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J. Cell Sci. 119, 1184–1195 10.1242/jcs.02831 [DOI] [PubMed] [Google Scholar]
- Soukup S. F., Pocha S. M., Yuan M., Knust E. (2013). DLin-7 is required in postsynaptic lamina neurons to prevent light-induced photoreceptor degeneration in Drosophila. Curr. Biol. 23, 1349–1354 10.1016/j.cub.2013.05.060 [DOI] [PubMed] [Google Scholar]
- Tepass U., Knust E. (1993). Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 159, 311–326 10.1006/dbio.1993.1243 [DOI] [PubMed] [Google Scholar]
- Van Vactor D., Jr, Krantz D. E., Reinke R., Zipursky S. L. (1988). Analysis of mutants in chaoptin, a photoreceptor cell-specific glycoprotein in Drosophila, reveals its role in cellular morphogenesis. Cell 52, 281–290 10.1016/0092-8674(88)90517-X [DOI] [PubMed] [Google Scholar]
- Venken K. J., Bellen H. J. (2007). Transgenesis upgrades for Drosophila melanogaster. Development 134, 3571–3584 10.1242/dev.005686 [DOI] [PubMed] [Google Scholar]
- Walther R. F., Pichaud F. (2006). Immunofluorescent staining and imaging of the pupal and adult Drosophila visual system. Nat. Protoc. 1, 2635–2642 10.1038/nprot.2006.379 [DOI] [PubMed] [Google Scholar]
- Wang T., Montell C. (2007). Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 454, 821–847 10.1007/s00424-007-0251-1 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Grawe F., Knust E. (1993). CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech. Dev. 44, 175–187 10.1016/0925-4773(93)90066-7 [DOI] [PubMed] [Google Scholar]
- Xia H., Ready D. F. (2011). Ectoplasm, ghost in the R cell machine? Dev. Neurobiol. 71, 1246–1257 10.1002/dneu.20898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chen Y., Lillo C., Chien J., Yu Z., Michaelides M., Klein M., Howes K. A., Li Y., Kaminoh Y. et al. (2008). Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Invest. 118, 2908–2916 10.1172/JCI35891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Yamamoto-Hino M., Awano W., Aoki-Kinoshita K. F., Tsuda-Sakurai K., Okano H., Goto S. (2012). Identification of proteasome components required for apical localization of Chaoptin using functional genomics. J. Neurogenet. 26, 53–63 10.3109/01677063.2012.661497 [DOI] [PubMed] [Google Scholar]
- Zacchigna S., Oh H., Wilsch-Bräuninger M., Missol-Kolka E., Jászai J., Jansen S., Tanimoto N., Tonagel F., Seeliger M., Huttner W. B. et al. (2009). Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J. Neurosci. 29, 2297–2308 10.1523/JNEUROSCI.2034-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof A. C., Hardy R. W. (2004). WASp is required for the correct temporal morphogenesis of rhabdomere microvilli. J. Cell Biol. 164, 417–426 10.1083/jcb.200307048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof A. C., Koundakjian E., Scully A. L., Hardy R. W., Pounds L. (2003). Mutation of the photoreceptor specific homeodomain gene Pph13 results in defects in phototransduction and rhabdomere morphogenesis. Development 130, 4383–4392 10.1242/dev.00651 [DOI] [PubMed] [Google Scholar]
- Zelhof A. C., Hardy R. W., Becker A., Zuker C. S. (2006). Transforming the architecture of compound eyes. Nature 443, 696–699 10.1038/nature05128 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zulfiqar F., Xiao X., Riazuddin S. A., Ahmad Z., Caruso R., MacDonald I., Sieving P., Riazuddin S., Hejtmancik J. F. (2007). Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum. Genet. 122, 293–299 10.1007/s00439-007-0395-2 [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Venkatesh T. R., Teplow D. B., Benzer S. (1984). Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell 36, 15–26 10.1016/0092-8674(84)90069-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.