Abstract
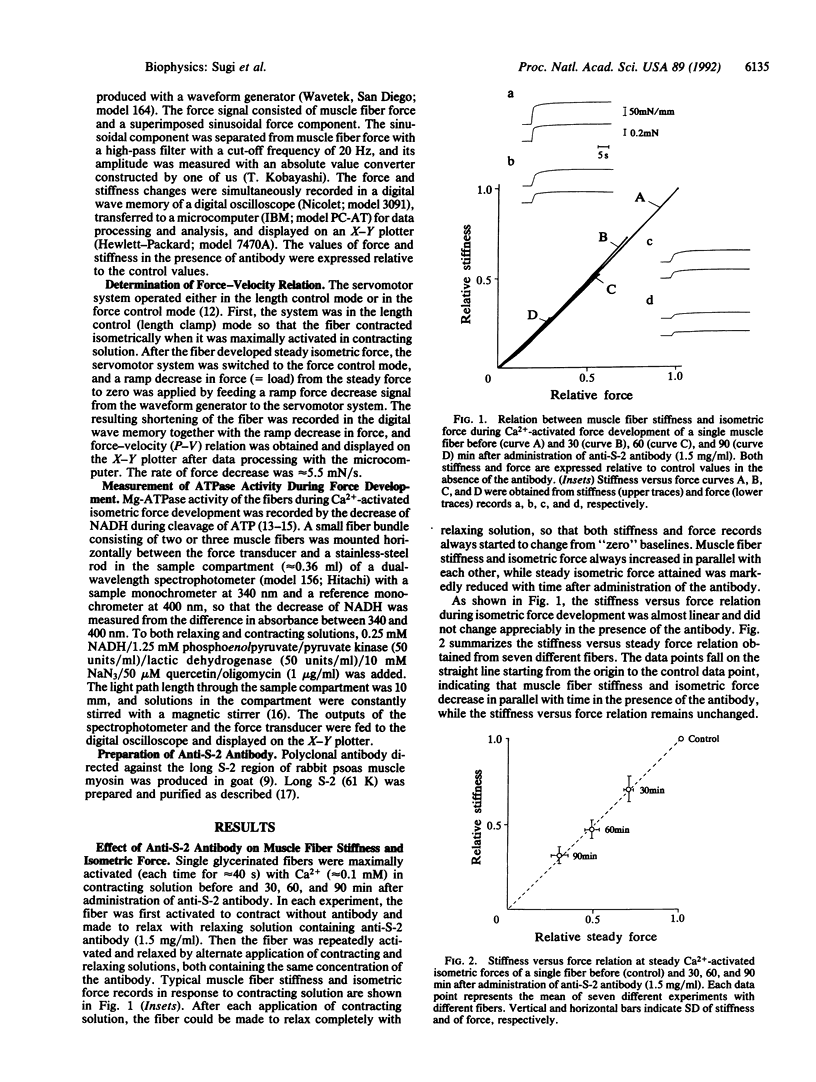
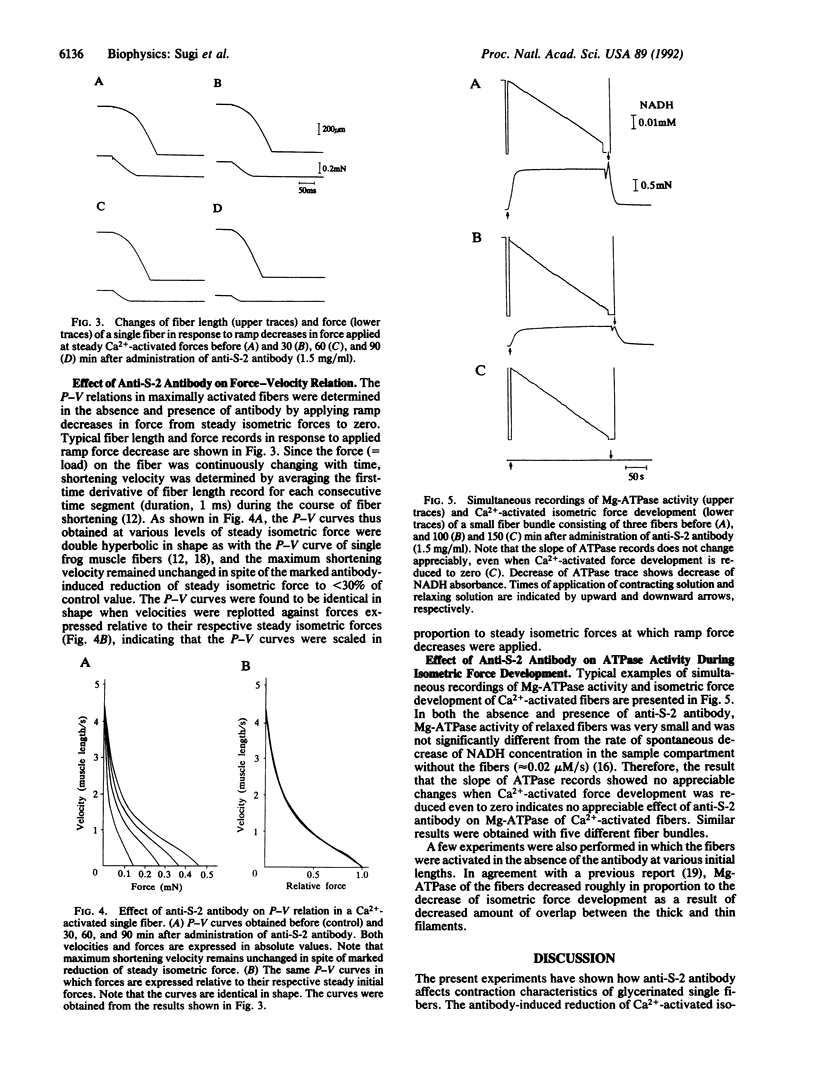
To investigate the role of the myosin hinge region in muscle contraction, we examined the contraction characteristics and Mg-ATPase activity of glycerinated muscle fibers prepared from rabbit psoas in the presence and absence of polyclonal antibody directed against the subfragment 2 (S-2) region of myosin. The antibody-induced reduction of Ca(2+)-activated isometric force was always accompanied by a parallel decrease of muscle fiber stiffness, so that the stiffness versus force relation remained unchanged by the antibody treatment. Force-velocity relations of the fibers, obtained by applying ramp decreases in force at steady isometric forces, indicated that the antibody had no effect on maximum shortening velocity or on the shape of force-velocity curves. Simultaneous measurements of Mg-ATPase activity and Ca(2+)-activated force showed that Mg-ATPase activity of the fibers remained unchanged despite the antibody-induced reduction of isometric force even to zero. These results indicate that when anti-S-2 antibody attaches to the S-2 region of myosin molecules, their heads still hydrolyze ATP but no longer contribute to both force generation and muscle fiber stiffness.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate D., Reisler E. Crossbridge release and alpha-helix-coil transition in myosin and rod minifilaments. J Mol Biol. 1983 Sep 15;169(2):455–468. doi: 10.1016/s0022-2836(83)80061-8. [DOI] [PubMed] [Google Scholar]
- Chaen S., Shimada M., Sugi H. Evidence for cooperative interactions of myosin heads with thin filament in the force generation of vertebrate skeletal muscle fibers. J Biol Chem. 1986 Oct 15;261(29):13632–13636. [PubMed] [Google Scholar]
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Harrington W. F., Karr T., Busa W. B., Lovell S. J. Contraction of myofibrils in the presence of antibodies to myosin subfragment 2. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7453–7456. doi: 10.1073/pnas.87.19.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Sugaya R., Sugi H. Force-velocity relation of frog skeletal muscle fibres shortening under continuously changing load. J Physiol. 1990 Mar;422:185–202. doi: 10.1113/jphysiol.1990.sp017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Lovell S., Karr T., Harrington W. F. Suppression of contractile force in muscle fibers by antibody to myosin subfragment 2. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1849–1853. doi: 10.1073/pnas.85.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxdale H. D. A method for the continuous assay of picomole quantities of ADP released from glycerol-extracted skeletal muscle fibres on MgATP activation [proceedings]. J Physiol. 1976 Sep;260(2):4P–5P. [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Krueger J. W., Sellers J. R., Cuda G., Caulfield J. B., Norton P., Slayter H. S. Influence of the cardiac myosin hinge region on contractile activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4941–4945. doi: 10.1073/pnas.88.11.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Stewart A. W., Wilson G. J. Dissociation of force from myofibrillar MgATPase and stiffness at short sarcomere lengths in rat and toad skeletal muscle. J Physiol. 1989 Mar;410:351–366. doi: 10.1113/jphysiol.1989.sp017537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashi R., Putnam S. A fluorimetric method for continuously assaying ATPase: application to small specimens of glycerol-extracted muscle fibers. Anal Biochem. 1979 Jan 15;92(2):375–382. doi: 10.1016/0003-2697(79)90674-2. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Tanaka M., Sugi H. The effect of sarcomere length and stretching on the rate of ATP splitting in glycerinated rabbit psoas muscle fibers. J Biochem. 1979 Nov;86(5):1587–1593. doi: 10.1093/oxfordjournals.jbchem.a132676. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Karr T., Harrington W. F. Rapid helix--coil transitions in the S-2 region of myosin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1109–1113. doi: 10.1073/pnas.76.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. An enzyme-probe study of motile domains in the subfragment-2 region of myosin. J Mol Biol. 1984 Dec 15;180(3):667–701. doi: 10.1016/0022-2836(84)90032-9. [DOI] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Cross-linking within the thick filaments of muscle and its effect on contractile force. Biochemistry. 1987 Jun 16;26(12):3589–3596. doi: 10.1021/bi00386a051. [DOI] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Local melting in the subfragment-2 region of myosin in activated muscle and its correlation with contractile force. J Mol Biol. 1986 Jul 5;190(1):69–82. doi: 10.1016/0022-2836(86)90076-8. [DOI] [PubMed] [Google Scholar]



