Abstract
G protein-coupled receptors (GPCRs) play a central role in signal transmission, thereby controlling many facets of cellular function. Overwhelming evidence now implicates GPCRs, G proteins and their downstream signaling targets in cancer initiation and progression, where they can influence aberrant cell growth and survival, largely through activation of AKT/mTOR, MAPKs, and Hippo signaling pathways. GPCRs also play critical roles in the invasion and metastasis of cancer cells via activation of Rho GTPases and cytoskeletal changes, and angiogenesis to supply the tumor with nutrients and provide routes for metastasis. Lastly, GPCRs contribute to the establishment and maintenance of a permissive tumor microenvironment. Understanding GPCR involvement in cancer malignancy may help identify novel therapeutic opportunities for cancer prevention and treatment.
Introduction
Agonist binding to G protein coupled receptors (GPCRs) results in rapid conformational changes that lead to the activation of heterotrimeric G proteins, comprised of Gα, β and γ subunits, and the recruitment of proteins responsible for receptor internalization and desensitization, including arrestins and GPCR kinases (GRKs) [1,2]. A novel family of highly evolutionarily conserved α-arrestins has recently received attention due their implicated roles in GPCR trafficking and degradation [3]. Most GPCRs will activate one or multiple Gα proteins, which can be subdivided into 4 major families: Gαi, Gα12, Gαs, and Gαq, with each family activating distinct signaling pathways [4]. GPCRs can also trigger G protein-independent mechanisms, including signaling through β-arrestins and interactions with PDZ containing proteins and other GPCR-regulators/scaffolding proteins [5]. GPCRs act more as molecular rheostats rather than on-off switches, so the engagement of different G proteins and strength/duration of signaling may not only differ between GPCRs, but also for a given GPCR, depending on the ligand and cellular environment.
Early indications that GPCRs could function as oncogenes include characterization of the transforming capacity of the mas proto-oncogene and other GPCRs in the presence of excess ligand availability, the identification of activating oncogenic mutations in thyroid stimulating hormone receptor (TSHR), and the association of virally-encoded GPCRs with tumorigenesis [4]. Since then, many GPCRs were shown to be overexpressed in a variety of cancer types and linked to tumor-cell growth when activated by circulating or locally produced ligands. Yet, despite the association of GPCRs with cancer progression and the fact that GPCRs represent one of the most “druggable” classes of molecules, representing approximately 25% of all therapeutics on the market, there are relatively few cancer treatments targeting GPCRs [6]. By better understanding the molecular mechanisms underlying GPCR function in cancer, we can identify the best therapeutic targets for cancer prevention and treatment.
GPCRs signaling in normal and cancer cell proliferation and survival
Cell growth promotion has been traditionally associated with the activation of tyrosine kinase growth factor receptors (RTKs) [7]. The discovery and use of bacterial toxins inhibiting G protein αi subunits [8] established that multiple mitogens transduce proliferative signals through GPCRs, including thrombin and lysophosphatidic acid (LPA) [9-11]. Subsequent studies revealed that many mitogens act on GPCRs linked to the Gq and G12 G protein families, including many peptide hormones, bioactive lipid mediators, and neurotransmitters [4,12], supporting the involvement of GPCRs in cell proliferation in a variety of cell types [4,13,14]. The molecular mechanisms underlying cell growth promotion by GPCRs is still an active area on investigation, as it involves the coordinated activation of traditional second messenger generating systems with the regulation of protein-protein interaction based networks. Some of these signaling circuits may act in cell type specific manners to initiate or sustain cancer cell growth and the metastatic spread of primary tumor lesions.
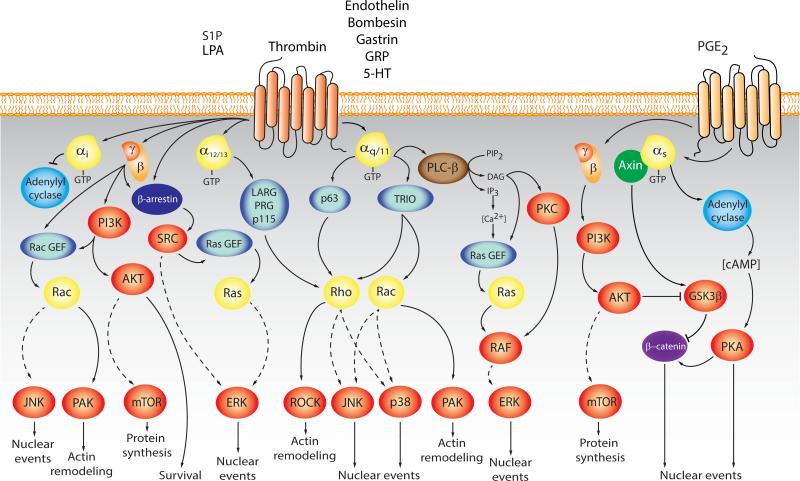
Second messenger generating systems
GPCR stimulation triggers the activation of heterotrimeric G proteins as GTP replaces GDP on the Gα subunit, promoting its dissociation from Gβγ subunits. Both α-GTP bound and Gβγ subunit complexes then stimulate multiple downstream signaling cascades [4, 2], including the rapid generation of multiple second messengers. For example, Gαs stimulates adenylyl cyclases, increasing the cytosolic levels of cAMP, while Gαi inhibits adenylyl cyclases and hence decreases cAMP levels [15]. Members of the Gαq family activate phospholipase-Cβ, which cleaves PIP2 into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3); the latter causes an increase in cytosolic calcium [16]. The targets of these diffusible second messengers include ion channels, calcium-sensitive enzymes, and kinases such as cAMP-dependent kinase (PKA), protein kinase C (PKC), cGMP-dependent kinase (PKG), and calcium-calmodulin regulated kinases (CAMKs), which are stimulated by cAMP, calcium/DG, cGMP, and calcium, respectively (Figure 1). Many of these kinases contribute to cancer progression and metastasis [17-22].
Figure 1. Activation of growth and survival pathways by GPCRs.
Stimulation of GPCRs results in the activation of multiple signaling pathways including second messenger generating systems, guanine nucleotide exchange factors (GEFs) for Ras and Rho GTPases, MAP kinases, PI3Ks, and their numerous downstream cytosolic and nuclear targets. This signaling network contributes to normal cell growth, survival, differentiation, and migration, but aberrant activation of GPCRs/G proteins and their downstream targets can result in tumor initiation, progression, and metastasis. In general, most mitogens acting on GPCRs stimulate Gαq/11, while others activate Gα12/13 and Gαi Gα subunits, which initiate intracellular signaling together with multiple pathways regulated by Gβγ subunits. In turn, these signaling routes converge in the nucleus to regulate the expression of growth promoting genes by the prolonged stimulation of transcription factors including c-FOS and c-JUN AP1 family members, YAP/TAZ, and c-MYC, among others. In parallel, activation of PI3K and AKT can induce cell proliferation by regulating cell cycle proteins, and promote cell survival through inactivation of pro-apoptotic proteins. AKT also activates an atypical kinase known as mTOR, which regulates protein synthesis, cell growth, and proliferation. In certain cancer cells, including colon cancer, activation of Gαs by COX-2 derived prostaglandins promotes cell proliferation by multiple mechanisms, including PKA-dependent regulation of multiple transcription factors and Gβs and Gβγ-initiated pathways controlling the accumulation of transcription factors such as β-catenin. See text for details.
Small GTPases and MAPK cascades
In addition to the regulation of second messengers, GPCRs can control cell migration, survival, and growth by activating multiple mitogen activated protein kinase (MAPK) cascades. These include ERK1/2, JNK1-3, p38α-δ MAPKs, and ERK5, which are a group of highly related serine/threonine kinases that link cell surface receptors to transcription factors [23]. In general, while Ras GTPases regulate ERK1/2, small GTPases of the Rho family, Rho, Rac, and Cdc42, control JNK and p38 MAPKs by a distinct kinase cascade [24] (Figure 1). These MAPKs play key roles in cell proliferation and metastasis, and their deregulation is a frequent event in human malignancies. Hence, how GPCRs regulate MAPKs, particularly through Ras and Rho GTPases, has been explored under multiple physiological and pathological conditions.
Specifically, many GPCRs coupled to Gi activate Rac and JNK through the direct interaction of Gβγ subunits with the P-REX1/2 family of Rac guanine nucleotide exchange factors (GEFs) [25,26]. Gαq activates Rho GTPases through p63-RhoGEF and Trio [27]. Receptors coupled to Gα12 and Gα13 activate Rho by stimulating a family of Rho GEFs, comprised of p115, PDZRhoGEF and LARG [28]. The JNK cascade is activated downstream of Rac and Cdc42 [24], which can mediate signaling from Gβγ dimers and Gα12, Gα13, Gαq and Gαi (reviewed in [4]). Activation of the ERK1/2 pathway by GPCRs is achieved in a highly cell-specific fashion (reviewed in [4]), promoted by Ras, tyrosine kinases, PI3Ks, PKCs, and/or arrestins. How GPCRs activate p38 and ERK5 is much less clear, but in general these MAPKs are activated primarily by Gαq, Gα12/13, and Gβγ dimers [4]. Activation of MAPK pathways stimulates the expression of growth promoting early-immediate responsive genes, including those encoding the AP-1 family of transcription factors. MAPKs are involved in the regulation of both gene expression and transactivating activity of AP-1 members by a complex and not fully understood mechanism (Figure 1).
Activation of the PI3K, AKT, and mTOR pathway
Activation of the PI3K-AKT-mTOR pathway plays a central role in cell metabolism, migration, growth and survival [29,30]. PI3K generates PIP3 inducing activation of AKT and mTOR [29,30]. PI3Kγ exhibits restricted tissue distribution and is activated by the direct interaction of its catalytic (p110γ) and regulatory subunit (p101) with Gβγ subunits [31]. PI3Kγ is involved in chemokine-induced migration of leukocytes, and plays significant roles in innate immunity [32]. In cells lacking PI3Kγ expression, GPCRs can utilize PI3Kβ to stimulate PIP3 synthesis [33,34]. One of the most studied PI3K-regulated events is the activation of the kinases AKT and mTOR, which phosphorylate multiple substrates involved in cell migration, survival, and metabolism [33,34] (Figure 2).
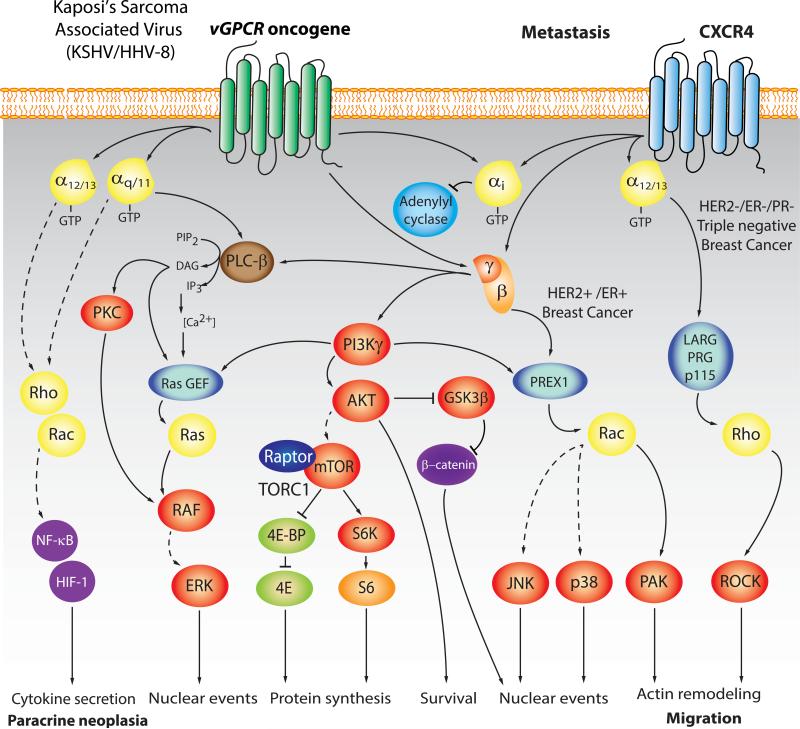
Figure 2. Signaling by virally-encoded oncogenic GPCRs, and metastasis-related signaling pathways elicited by chemokine receptors.
In general, chemokine receptors, such as CXCR4, drive cell migration primarily by acting on Gi and the stimulation of Gαi and Gβγ-initiated pathways controlling actin remodeling through PI3K and Rac GEFs, as well as the expression of pro-invasive gene programs. This is well exemplified by the role of CXCR4 in breast cancer metastasis. CXCR4 can also couple to G12/13 in basal-like breast cancer cells, where Gα13 protein expression is highly up-regulated, thereby driving metastasis in a Gα12/13-RhoA dependent manner. Similarly, LPA receptors and PAR-1 can activate Gα12/13-RhoA signaling in addition to Gαi. PI3K, Rac, and Rho signaling promotes the assembly of focal adhesions and actin polymerization important for inducing changes in cell shape and contraction, which are required for cell movement, thereby facilitating cancer cell migration, extravasation, and metastasis. Human oncogenic viruses, such as the Kaposi's sarcoma associated virus (KSHV/HHV8) express constitutively active GPCRs (vGPCRs) from their viral genome. Emerging evidence indicates that KSHV vGPCR initiates Kaposi's sarcoma, a highly angiogenic malignancy, by activating multiple intracellular signaling networks resulting in upregulation of expression and release of pro-angiogenic cytokines, such as VEGF, IL6, and IL8/CXCL8 and Gro-α/CXCL1, thus initiating paracrine neoplasia. Among these multiple pathways, the activation of AKT and mTOR through PI3Kγ represents a central pro-angiogenic and transforming mechanism deployed by KSHV-vGPCR, which has been successfully targeted in the clinic. The transforming effects of KSHV vGPCR also involves the activation of multiple MAPKs and their regulated transcription factors, including HIF-1α, AP-1, NF-kB, CREB, and NFAT transcription factors, all of which promote the expression and release of multiple KS-associated cytokines. Ultimately, KSHV vGPCR-expressing cells act in a paracrine fashion to stimulate the unrestricted growth of surrounding endothelial cells. See text for details.
Regulation of the Hippo signaling pathway
GPCRs involved in cell proliferation stimulate the activity of the transcriptional co-activator YAP [35], which is a critical component of the Hippo signaling pathway that controls organ size in mammals [36-39]. YAP (and related TAZ), is active in proliferating cells, but cell confluence triggers the activation of the growth-inhibitory Hippo kinase cascade. This causes the activation of two kinases known as LATS1/LATS2, which phosphorylate and thereby inhibit YAP [40]. GPCRs linked to Gα12/13 inhibit the activity of LATS, thus relieving YAP from LATS-dependent inhibition [35], while receptors activating Gαs promote LATS activation thus inhibiting YAP [35]. Recent work in our laboratory indicates that oncogenic mutations in the gene encoding Gαq activate YAP by a mechanosensing pathway initiated upon actin polymerization rather than by the inhibition of the Hippo pathway (unpublished results). YAP activation may represent a key pro-tumorigenic pathway activated by GPCRs, thereby representing a novel target for cancer treatment.
GPCR signal integration and crosstalk
While GPCRs can stimulate multiple diffusible second-messenger generating systems, their ability to promote normal and aberrant cell proliferation often relies on the persistent activation of PI3K/AkKT/mTOR, Ras and Rho GTPases, and MAPK cascades, thereby regulating the activity of nuclear transcription factors and co-activators, such as JUN, FOS and YAP [35,41]. Additionally, arrestin proteins contribute to G protein-dependent and G protein-independent events, initiating signaling and regulating receptor internalization and degradation/recycling kinetics [42,43].β-arrestins are now believed to scaffold a wide variety of signaling complexes [44,45]. Some β-arrestin-biased GPCR agonists initiate intracellular signaling independently of the activation of heterotrimeric G proteins [45]. By forming multimeric signaling complexes with ERK1/2 and JNK, β-arrestins can retain these MAPKs in the cytosol, thus restricting their nuclear translocation and leading to interaction with cytosolic substrates instead [45].
A more global view of the general systems by which GPCRs exert their numerous physiological and pathological roles is necessary to appreciate the overall implications to tumorigenesis. In particular, extensive cross-talk and co-regulation may occur between GPCR-and RTK-initiated signaling pathways and through receptor transactivation [46,47]. Therefore, the final biological outcome of GPCR activation results from the integration of the network of GPCR-initiated biochemical responses in each cellular and environmental context. Such systems level understanding may provide a molecular framework for the development of novel approaches for therapeutic intervention in some of the most prevalent human diseases.
Viral GPCRs
Early studies of virally-encoded oncogenes provided the foundation of our current understanding of cancer biology. At least seven human viruses, Epstein-Barr virus (EBV/HHV- 4), hepatitis B virus (HBV), hepatitis C virus (HCV), human papilloma virus (HPV), human T-cell lymphotropic virus (HTLV-1), and Kaposi's associated sarcoma herpes virus (KSHV/HHV- 8), and Merkel cell polyomavirus, contribute to 10-15% of cancers [48,49]. Surprisingly, many human viruses harbor open reading frames encoding GPCRs in their viral genomes, indicating that these signaling circuits are required for replicative success [50]. For example, EBV encodes one GPCR (BILF1), and human cytomegalovirus (HCMV/HHV-5) expresses at least four GPCRs (US28, US27, UL33 and UL78). KSHV encodes a receptor commonly known as KSHV vGPCR (or ORF74), that resembles CXCR1 and CXCR2, the receptors for CXCL8/IL-8 and CXCL1/Gro-α chemokines [51]. KSHV vGPCR is constitutively active due to the presence of a several structural changes, and contributes to KS development due to its potent transforming and pro-angiogenic properties. It promotes sarcomagenesis by increasing the activity of a complex signaling network, among which the activation of the PI3Kγ/AKT/mTOR pathway represents a clinically relevant target for KS treatment (Figure 2). Ultimately, KSHV vGPCR-expressing cells act in a paracrine fashion to stimulate the unrestricted growth of surrounding and distant endothelial cells, thereby representing an excellent example paracrine neoplasia.
GPCRs in migration, invasion and metastasis
One of the most serious challenges facing cancer treatment is metastasis, the spread of cancer cells through blood or lymphatic vessels to distant organs [52]. Rather than spreading randomly, cancer cells metastasize preferentially to specific organs, with a greater incidence than would be expected from the circulatory pattern between the primary tumor site and secondary organs [53]. The GPCR family of chemokine receptors is centrally linked to the organ-specific metastasis of a number of cancers, in line with their normal immune cell function of directing receptor-bearing leukocytes towards sites of chemokine production. Similarly, tumor cells aberrantly expressing chemokine receptors can co-opt the migratory activity of chemokines, facilitating metastasis to other organs [54]. Chemokines locally released into the tumor microenvironment can also enhance the motility and survival of cancer cells in an autocrine and paracrine fashion [54]. Chemokines direct cell movement by inducing changes in cytoskeletal structure and dynamics of receptor-bearing cells. Actin polymerization leads to formation of protrusions, or pseudopods, and with the help of integrins, form focal adhesions with the extracellular matrix (ECM) to help propel the cell forward [55].
CXCR4 represents one of the best established chemokine receptors driving cancer metastasis. Tumor cells frequently exhibit aberrant CXCR4 expression, which has proliferative, pro-survival, and pro-migratory effects; additionally, the organs that are the most frequent sites of metastasis, including lymph nodes, lungs, bone marrow and liver, express its chemokine ligand, CXCL12/SDF-1 [53]. Several factors may contribute to the observed overexpression of CXCR4 in many tumors. For example, the HER2/Neu oncogene, which occurs in ~30% of breast cancers, limits the degradation of CXCR4, leading to its increased expression [56]. Additionally, hypoxia through activation of hypoxia-inducible factor-1 (HIF-1α) induces transcription of CXCR4 [57]. As such, CXCR4 is highly expressed in breast cancer cells but not in normal breast tissues, and the inhibition of CXCR4 prevents the metastatic spread of breast cancer cells [53] and many other cancer types [52,54,58]. Although CXCR4 would represent an attractive target for therapeutic development, the use of CXCR4 inhibitors leads to the mobilization of stem cell progenitors from the bone marrow, thus limiting their use clinically for cancer treatment [59]. However, targeting molecules involved in the regulation of CXCR4 expression on cancer cells or their downstream signaling may offer alternative approaches for therapeutic intervention. In this regard, CXCR4 activates Rac1 through P-REX1, which plays a central role in metastasis in most breast cancer types [60]. CXCR4 can also couple to G12/13 in basal-like breast cancer cells, where Gα13 protein expression is highly up-regulated, thus driving metastasis through a Gα12/13-RhoA dependent manner [61], similarly to LPA and PAR-1 receptors (Figure 2), all of which can be considered potential targets for metastasis prevention and treatment.
Other chemokine receptors, including CCR7 and CCR10, have also been shown to play direct roles in metastatic homing of cancer cells types [62] and cancer cell survival and growth [63]. Local chemokine production in the tumor microenvironment can attract macrophages and leukocytes that may enhance the cytokine-rich microenvironment and induce the release of matrix metalloproteases (MMPs) that enable tumor cells to survive, proliferate and invade. In addition, recent functional screens demonstrated a role for GPR116, a member of the poorly characterized family of adhesion GPCRs, in invasion and migration of breast cancer cells, acting in a Gαq-RhoA/Rac1-dependent manner. Many new efforts are now focused on exploring the adhesion family of GPCRs, and their potential implications in tumor growth and metastasis [64].
GPCRs in tumor-induced angiogenesis
Solid tumors produce angiogenic factors promoting the migration and proliferation of endothelial cells, thus resulting in the formation of new vessels in response to the increasing nutrients and oxygen demands of the tumor cells. Many angiogenic factors act on GPCRs expressed on endothelial cells, including thrombin, prostaglandins, S1P, and chemokines [65-67]. Many chemokines, including CCL2, CCL5, and CXCL8/IL-8, recruit leukocytes and macrophages to the tumor site, which in turn can release VEGF and other angiogenic factors that contribute to the growth of new blood vessels [66]. In addition, inflammatory cytokines released in the tumor microenvironment promote the expression of COX-2 and the local release of prostaglandin E2 (PGE2), which increases the expression of pro-angiogenic VEGF, CXCL8 and CXCL5 by tumor and stromal cells [68].
Overall, GPCRs and their cognate ligands can promote angiogenesis directly by inducing proliferation of endothelial cells or indirectly by promoting release of VEGF and other angiogenic factors from stromal, immune, or cancer cells. Tumor vascularization provides nutrients to promote tumor outgrowth, and routes for invasion and metastasis.
Tumor microenvironment: Inflammation and immune cell evasion
Among the many mediators linking inflammation and cancer, the relationship between prostaglandin (PG) production and tumor progression is one of the best understood. PGs are a product of the cyclooxygenases COX-1 and COX-2, and their pro-inflammatory functions are initiated upon binding of PGs to their cognate GPCRs expressed in many cells. Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit COX-1/2 can reduce the risk and incidence of numerous cancer types [69,70]. For example, COX-2 inhibition with NSAIDs reduces the overall number and size of adenomas in patients genetically predisposed to colorectal cancer, and represents an effective chemopreventive strategy for colon cancer in healthy individuals [69,70]. The contribution of PGE2 and signaling through its cognate GPCRs, EP1-EP4, to tumor progression has been extensively investigated [71-73]. EP1 is a Gq-coupled GPCR, whereas EP2 and EP4, which play a more prominent role in colon cancer, are coupled to Gs and stimulate cAMP accumulation [71] (Figure 1). In colon cancer cells, PGE2 can stimulate multiple signaling pathways, including β-catenin [74,75] and peroxisome proliferator activated receptor δ (PPARδ), a nuclear hormone receptor.
Chemokines can also recruit macrophages to the site of the tumor. The role of CCL2 has been extensively studied for recruitment of CCR2-bearing tumor associated macrophages (TAMs), which play crucial roles in tumor vascularisation and growth. CCL5 has similarly been linked to macrophage recruitment [54,76]. On the other hand, some immune cells can facilitate killing of the tumor cells; in this case the tumor chemokine microenvironment may help evade the immune surveillance system, for example, by stimulating a less effective humoral response while inhibiting cell-mediated immune responses to tumor cells [54,76].
Widespread Mutations in GPCRs and G proteins in Cancer
Recent unbiased systematic approaches, including large-scale sequencing efforts, have highlighted an abundance of mutations in G proteins and GPCRs, and new studies are just beginning to explore the potential oncogenic effects of these mutations [77]. Initially, activating mutations in GNAS (encoding Gαs) were shown to promote hyperplasia of endocrine cells, occurring in 28% of growth hormone-secreting pituitary tumors and 5% of thyroid adenomas [78,79]. Overall, mutations in GNAS occur in ~4.4% of tumor samples in a variety of different cancers [77]. The vast majority of these mutations cluster around two hotspot residues, R201 and Q227, which reduce the rate of GTP hydrolysis of the active GTP-bound GαS, resulting in constitutive signaling activity [78,80,81]. Similar hotspot mutations, Q209 and R183, occur in the Gαq, family genes, GNAQ and GNA11 (reviewed in 77). These mutations are mutually exclusive and activate the same signaling cascades, such that over 5.6% of all tumors sequenced in the COSMIC (v62) database are disrupted. In particular, most ocular melanomas harbor mutations in GNAQ or GNA11, which are considered to act as driver oncogenes, and ~6% of cutaneous melanomas. Recently, characterization of the Gq/11 signaling in uveal melanoma demonstrated that Gαq activates the GEF, Trio, and its regulated Rho GTPase signaling to promote tumorigenesis through the activation of MAPKs [41] and the transcriptional co-activator, YAP (unpublished observations), rather than depending solely on the stimulation of the best known PLC-β and PKC pathway.
A surprising finding from recent mutation analyses of cancer genomes indicates that GPCRs are also mutated in approximately 20% of all cancers [77,82], including mutations in TSHR in thyroid cancer, and luteinizing hormone receptor (LHCGR) and follicle stimulating hormone receptor (FSHR) in breast, lung, and colon cancers [77]. One of the most frequently mutated seven transmembrane domain receptors in tumors is smoothened (SMO), which is negatively regulated by the twelve-transmembrane receptor Patched (PTCH) [83,84]. This inhibition of SMO is relieved when Hedgehog (HH) family members bind to PTCH and leads to downstream activation of the transcription factor GLI [83,84]. Mutations in PTCH and SMO have been linked to initiation of sporadic basal cell carcinoma [85,86]. Additionally, SMO is mutated in cancers arising in the colon and central nervous system among others. Frequent mutations are also observed in the family of GPCR adhesion receptors, the majority of which are still orphan, and the glutamate family of GPCRs. The adhesion receptors are thought to play roles in cell-to-cell and cell-to-matrix interactions, and includes GPR98 and brain-specific angiogenesis inhibitor (BAI) members [87]. Mutations in the glutamate receptors, GRM8, GRM1 and GRM3 have been implicated in squamous non-small cell lung cancer (NSCLC), NSCLC adenocarcinomas, and melanomas, respectively (reviewed in [77]). Yet more studies are needed in order to fully understand the molecular consequences of these mutations and their ultimate effects on tumor progression.
Tumor Suppressor functions of some GPCRs
Although this review is focused on the pro-tumorigenic capacity of GPCRs and G proteins, not all function as oncogenes. In certain malignancies, some GPCRs and G proteins may actually play tumor suppressive roles and mutations may reflect inactivation of the respective genes. For example, inactivating mutations in the melanocortin 1 receptor (MC1R), which is important for pigment production, increase the risk of melanoma development [88]. CXCR3 ligands can indirectly mediate anti-angiogenic effects to suppress tumor progression, while the cannabinoid receptors CB1 and CB2 display tumor suppressive roles in several cancers, including gliomas and breast, colorectal, and skin cancer [89]. Additionally, SIP2 receptor signaling through Gα13 in diffuse large B cell lymphoma (DLBCL) may exert tumor suppressive functions [90]. Although Gα13 signaling has implications in tumor progression and metastasis, in the case of DLBCL, reduced expression or inactivating mutations in S1P2 and/or Gα13 may instead enhance tumor progression. Lastly, GPR54/KiSS1-derived peptide receptor can function as a metastasis suppressor in melanoma and breast cancer cells [91]. Although GPR54 couples to Gq, the molecular basis of its anti-metastatic signaling mechanisms are still unknown. These are certainly not the only GPCR/G protein signaling pathways that may exhibit anti-tumorigenic effects in different cancers, and many yet are likely to be discovered in the future.
Concluding remarks
Activation of GPCRs elicits an array of signaling pathways including second messengers, GEFs, Ras and Rho GTPases, MAP kinases, PI3Ks, and their numerous downstream cytosolic and nuclear targets. These signaling pathways contribute to normal cell functions of growth, survival, differentiation, and migration; however, cancer cells can exploit these pathways through aberrant expression and regulation of GPCRs/G proteins and their ligands to enhance tumor growth, promote angiogenesis, invade and metastasize to distant sites, and evade the immune system (Figure 3). By directly targeting GPCRs or more selectively targeting particular downstream signaling components, there are many avenues for potential therapeutic development for cancer treatments.
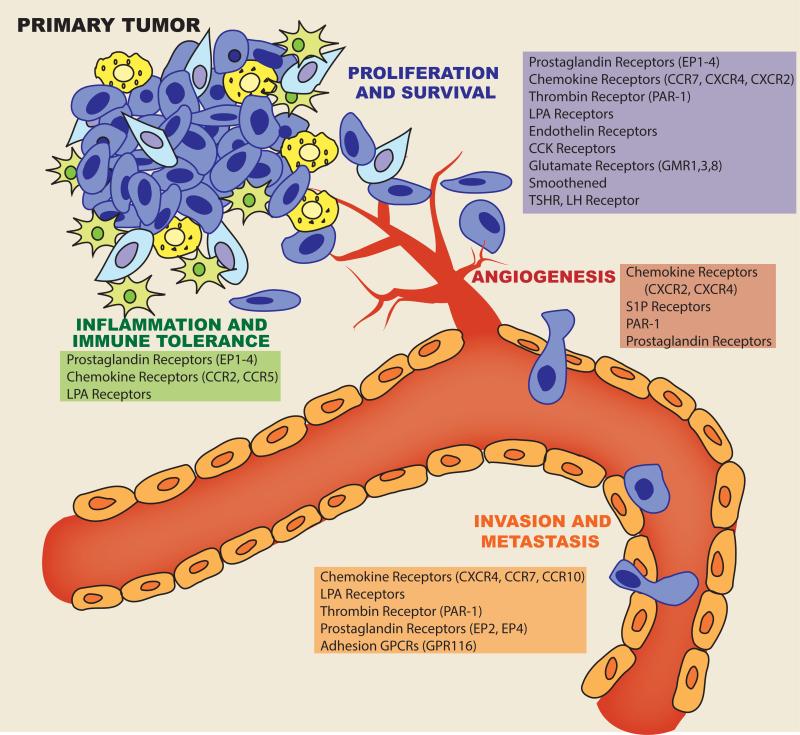
Figure 3. GPCRs contribute to multiple facets of cancer progression.
Cartoon depicting the involvement of GPCRs in cancer cell proliferation and survival, angiogenesis, invasion and metastasis, and inflammation and immune tolerance; short lists highlight some of the GPCRs involved in these processes.
Acknowledgements
We truly regret that we could not cite the seminal work of many of our colleagues due to space limitations. This work was supported by the Intramural Research Program of the US National Institutes of Health and the US National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [This seminal article highlights the emerging molecular understanding of GPCR structure and activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 3.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 2010;11:605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 5.Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol. 2012;165:1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 7.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 8.Ui M, Katada T. Bacterial toxins as probe for receptor-Gi coupling. Adv Second Messenger Phosphoprotein Res. 1990;24:63–69. [PubMed] [Google Scholar]
- 9**.Chambard JC, Paris S, G LA, Pouyssegur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987;326:800–803. doi: 10.1038/326800a0. [This paper demonstrated alternative mechanims for induction of DNA synthesis and cell proliferation existed besides tyrosine kinase growth factor receptors. This alternative pathway was found to be pertussis toxin sensitive, implicating the G protein, Gi, and its coupled receptors.] [DOI] [PubMed] [Google Scholar]
- 10.Pouyssegur J, Chambard JC, G LA, Magnaldo I, Seuwen K. Transmembrane signalling pathways initiating cell growth in fibroblasts. Philos Trans R Soc Lond B Biol Sci. 1988;320:427–436. doi: 10.1098/rstb.1988.0086. [DOI] [PubMed] [Google Scholar]
- 11*.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [This article demonstrated that LPA-induced cell proliferation was dependent on G proteins, and largely implicated Gi signaling since the mitogenic effects of LPA were blocked by pertussis toxin.] [DOI] [PubMed] [Google Scholar]
- 12.Moolenaar WH. G-protein-coupled receptors, phosphoinositide hydrolysis, and cell proliferation. Cell Growth Differ. 1991;2:359–364. [PubMed] [Google Scholar]
- 13.van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 14.Rozengurt E. Early signals in the mitogenic response. Science. 1986;234:161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- 15.Taussig R, Iniguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe AK. Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol. 2011;23:554–561. doi: 10.1016/j.ceb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 20.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 21.Julius D, Nathans J. Signaling by sensory receptors. Cold Spring Harb Perspect Biol. 2012;4:a005991. doi: 10.1101/cshperspect.a005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 24.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeldt H, Vazquez-Prado J, Gutkind JS. P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 2004;572:167–171. doi: 10.1016/j.febslet.2004.06.097. [DOI] [PubMed] [Google Scholar]
- 26.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 27*.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, et al. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–1927. doi: 10.1126/science.1147554. [This article structurally associates Gq and RhoA in a complex with p63-RhoGEF, and provides a link between activation of Gq and induction of RhoA activity.] [DOI] [PubMed] [Google Scholar]
- 28.Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H, et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem. 2013;288:12232–12243. doi: 10.1074/jbc.M112.428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 32.Costa C, Martin-Conte EL, Hirsch E. Phosphoinositide 3-kinase p110gamma in immunity. IUBMB Life. 2011;63:707–713. doi: 10.1002/iub.516. [DOI] [PubMed] [Google Scholar]
- 33.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 34.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [This recent article impicates GPCRs in activation of the Hippo pathway, important for controlling organ size and associated with tumorigenesis, which leads to activation of the transcriptional co-activators TAZ and YAP.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, Huebner K, Lehman D. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 40.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Vaque JP, Dorsam RT, Feng X, Iglesias-Bartolome R, Forsthoefel DJ, Chen Q, Debant A, Seeger MA, Ksander BR, Teramoto H, et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol Cell. 2013;49:94–108. doi: 10.1016/j.molcel.2012.10.018. [This article demonstrates molecular mechanisms by which activation of the G proteins, Gq and G11, leads to cell proliferation through activation of the GEF, Trio, and subsequent activation of Rho and Rac and downstream p38 and JNK MAPK pathways. It supports the emerging notion that cell growth promotion by GPCRs depends on a hard wired signaling network based on localized protein-protein interactions rather than solely on diffusible second messenger systems.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 43.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 44.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin- dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 47.Natarajan K, Berk BC. Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- 48.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin D, Gutkind JS. Oncogene. Vol. 27. Suppl 2: 2008. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. pp. S31–42. [DOI] [PubMed] [Google Scholar]
- 50*.Montaner S, Kufareva I, Abagyan R, Gutkind JS. Molecular Mechanisms Deployed by Virally Encoded G Protein-Coupled Receptors in Human Diseases. Annu Rev Pharmacol Toxicol. 2013;53:331–54. doi: 10.1146/annurev-pharmtox-010510-100608. [This review summarizes the currently known GPCRs encoded by different viruses and their implications in human disease, including cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation [see comments]. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [This article demonstrated that a virally encoded GPCR in Kaposi's sarcoma herpes virus tumors contributes to cellular proliferation.] [DOI] [PubMed] [Google Scholar]
- 52.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 53**.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [This paper demonstrated a direct link between the expression of the chemokine receptor, CXCR4, in tumor cells and organ specific metastasis to sites in which its ligand, CXCL12, is produced, with emphasis on breast cancer.] [DOI] [PubMed] [Google Scholar]
- 54.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 55.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 56.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 57.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 58.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 59.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, Benovic JL, Klein-Szanto A, Yagi H, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40:877–892. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS. A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal. 2011;4:ra60. doi: 10.1126/scisignal.2002221. [This paper implicates a link between the chemokine receptor, CXCR4, and Ga13 signaling to Rho and ROCK kinase, which can contribute to the metastasis of triple negative breast cancer cells that overexpress G13 a subunit.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 63.O'Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 64.Tang X, Jin R, Qu G, Wang X, Li Z, Yuan Z, Zhao C, Siwko S, Shi T, Wang P, et al. GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Galphaq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 2013;73:6206–6218. doi: 10.1158/0008-5472.CAN-13-1049. [DOI] [PubMed] [Google Scholar]
- 65.Moore BB, Keane MP, Addison CL, Arenberg DA, Strieter RM. CXC chemokine modulation of angiogenesis: the importance of balance between angiogenic and angiostatic members of the family. J Investig Med. 1998;46:113–120. [PubMed] [Google Scholar]
- 66.Richard DE, Vouret-Craviari V, Pouyssegur J. Angiogenesis and G-protein-coupled receptors: signals that bridge the gap. Oncogene. 2001;20:1556–1562. doi: 10.1038/sj.onc.1204193. [DOI] [PubMed] [Google Scholar]
- 67.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iniguez MA, Rodriguez A, Volpert OV, Fresno M, Redondo JM. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol Med. 2003;9:73–78. doi: 10.1016/s1471-4914(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 69.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 70.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 71.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3:1031–1039. [PubMed] [Google Scholar]
- 72.Hansen-Petrik MB, McEntee MF, Jull B, Shi H, Zemel MB, Whelan J. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res. 2002;62:403–408. [PubMed] [Google Scholar]
- 73.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 74*.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [This article characterizes the signaling pathways induced by inflammatory COX2 production of PGE2, leading to activation of the Gs-coupled EP2 receptor and downstream activation of AKT, axin and beta-catenin in colon cancer.] [DOI] [PubMed] [Google Scholar]
- 75.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 76.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 77**.O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [This bioinformatic analysis of large cancer sequencing data sets revealed an unexpected and surprisingly high incidence of G protein and GPCR mutations in some of the most prevalent human neoplastic diseases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 79.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome [see comments]. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 80.Drews RT, Gravel RA, Collu R. Identification of G protein alpha subunit mutations in human growth hormone (GH)- and GH/prolactin-secreting pituitary tumors by single-strand conformation polymorphism (SSCP) analysis. Mol Cell Endocrinol. 1992;87:125–129. doi: 10.1016/0303-7207(92)90240-7. [DOI] [PubMed] [Google Scholar]
- 81.Wilson CH, McIntyre RE, Arends MJ, Adams DJ. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene. 2010;29:4567–4575. doi: 10.1038/onc.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 83.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 85.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 86.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 87.Paavola KJ, Hall RA. Adhesion g protein-coupled receptors: signaling, pharmacology, and mechanisms of activation. Mol Pharmacol. 2012;82:777–783. doi: 10.1124/mol.112.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–444. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- 90.Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, Schmidt TH, Xu Y, Proia RL, Coughlin SR, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]