Abstract
Mitochondria contain two enzymes, Cu, Zn superoxide dismutase (Sod1) and cytochrome c oxidase (CcO), that require copper as a cofactor for their biological activity. The copper used for their metallation originates from a conserved, bioactive pool contained within the mitochondrial matrix, the size of which changes in response to either genetic or pharmacological manipulation of cellular copper status. Its dynamic nature implies molecular mechanisms exist that functionally couple mitochondrial copper handling with other, extramitochondrial copper trafficking pathways. The recent finding that mitochondrial proteins with established roles in CcO assembly can also effect changes in cellular copper levels by modulating copper efflux from the cell supports a mechanistic link between organellar and cellular copper metabolism. However, the proteins and molecular mechanisms that link trafficking of copper to and from the organelle with other cellular copper trafficking pathways are unknown. This review documents our current understanding of copper trafficking to, and within, the mitochondrion for metallation of CcO and Sod1; the pathways by which the two copper centers in CcO are formed; and, the interconnections between mitochondrial function and the regulation of cellular copper homeostasis.
Keywords: Mitochondria, Superoxide dismutase, Cytochrome c oxidase, SCO proteins, Copper trafficking
1. Cellular copper distribution and homeostasis
Copper ions are cofactors for a number of metalloenzymes and, as such, are required for normal cell physiology. Copper deficiency and overload syndromes in humans that result in the decreased activity of these enzymes have severe clinical outcomes associated with the impaired function of many tissues, including the brain, liver and heart. In addition, copper has an established role in cell growth and proliferation that may be distinct from that it serves as a catalytic moiety in relevant cupro-proteins [1]. The properties that make copper an ideal biological cofactor also allow it to potentiate free radical generation when unbound within the cell. Therefore, cells have evolved mechanisms to balance global copper content, and to distribute copper to different subcellular compartments. These mechanisms are based primarily on the collective activity of copper transporters, chaperones and molecules that chelate copper in biologically inert complexes.
1.1. Copper homeostasis: lesson learned from unicellular model organisms
Many of the concepts that are fundamental to our mechanistic understanding of copper homeostasis have been developed in unicellular model organisms such as Saccharomyces cerevisiae, Enter-ococcus hirae and Escherichia coli. Here, we will focus our attention exclusively on the unicellular eukaryote S. cerevisiae. Copper enters yeast in part through the Ctr1 family of permeases [2]. The high affinity copper uptake system consists of two related permeases, Ctr1 and Ctr3, that transport cuprous ions and whose function therefore depends on the activity of metalloreductases, which reduce environmental Cu(II) to Cu(I). Ctr1 forms a trimeric channel complex, and its ectodomain has multiple MxxM sequence motifs that appear to function as Cu(I) scavenging modules, effectively gathering extra-cellular Cu ions for subsequent transport across the plasma membrane. In the absence of Ctr1, yeast cells are copper-deficient and exhibit impaired copper-dependent activities. These include mito-chondrial respiration, resistance to oxidative stress and high affinity iron uptake. In addition to Ctr1, yeast cells have low affinity Cu(I) permeases such as Fet4 and Smf1 [2]. Ctr2, a vacuolar copper exporter, also functions to mobilize copper in response to Cu-deficient conditions; however, it can also mislocalize to the plasma membrane and facilitate copper import into the cell [3].
Once in the cell, Cu(I) ions are transported to sites of utilization by two soluble Cu(I)-binding metallochaperones, Atx1 and Ccs1 [4], which facilitate Cu(I) insertion into target cupro-enzymes [5,6]. Ccs1 activates Sod1 [7] by inserting Cu(I) into the newly synthesized apo-protein and catalyzing the formation of an essential disulfide bond [8]. The metallochaperone Atx1 shuttles Cu(I) to Ccc2, a P-type ATPase transporter localized in the trans-Golgi network (TGN) [9]. P-type ATPases translocate cations against an electrochemical potential gradient by using the energy derived from ATP hydrolysis. TGN luminal Cu(I) ions are used to metallate Fet3 prior to its trafficking to the plasma membrane, where it functions in iron acquisition [10]. The eukaryotic Cu(I)-specific P-type ATPases contain multiple Cu(I) binding modules in their N-terminal domains. These modules structurally resemble Atx1 and serve as docking sites for metallochaperone binding and subsequent Cu(I) transfer via ligand exchange reactions [5,6].
Copper is required within the mitochondrion for the maturation of CcO and Sod1 [11,12] (Fig. 1). Two copper-binding subunits of CcO, Cox1 and Cox2, are encoded by the mitochondrial genome, and their metallation within the IMS depends on several CcO assembly factors, i.e. Cox17, Sco1 and Cox11, that facilitate the formation of the Cu centers contained within each structural subunit [13]. Metallation of IMS Sod1, which represents approximately 1–5% of the total cellular Sod1 pool [12], also occurs within the IMS, since both it and its chaperone Ccs1 are imported into the mitochondria as apo-molecules [14]. The fact that metallation of CcO and Sod1 occurs within the organelle necessitates that a specific pathway(s) exists for copper transport to the mitochon-drion. Biochemical experiments have identified a bioavailable copper pool within the matrix that is essential for these metallation reactions.
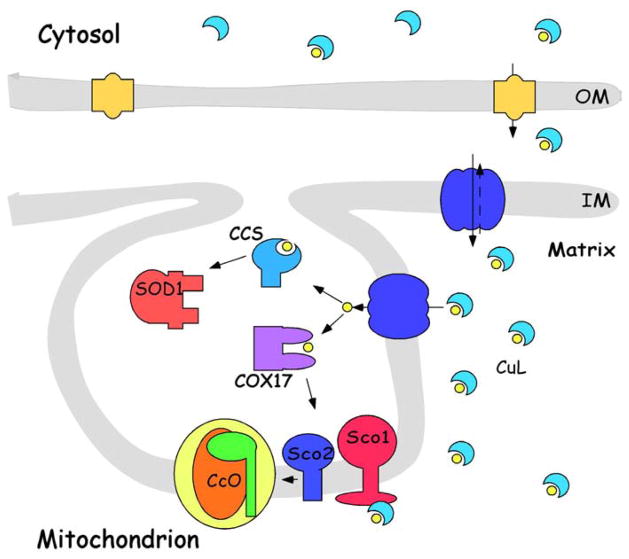
Fig. 1.
Mitochondrial copper trafficking pathways: Apo-ligand is predominantly distributed to the cytosol. Binding of copper results in its translocation/diffusion across the outer membrane (OM) (via open channels in orange), and its protein-mediated transport (blue) across the inner membrane (IM). The anionic ligand complex (CuL) is stored in the mitochondrial matrix in an inert form that is biologically accessible. It is mobilized in response to what remain as poorly understood signals/stimuli and transported across the IM by either the same transporter, or a second transporter, where it is transferred to COX17 or CCS for metallation of relevant targets (CcO and SOD1, respectively). CuL may also be ‘recycled’ through the transporter for eventual release to the cytosol (indicated by dashed arrow) in the absence of the copper chaperones or under high copper conditions.
In fungi, balancing the need for copper with its potential toxicity is regulated primarily at the level of gene transcription [15]. Cu-deficient growth conditions lead to an up-regulation of the high affinity Cu(I) permease family as well as the metalloreductase necessary for Cu(I) import. When grown in excess copper, toxicity is precluded by the induced transcription of Cu(I) buffering metallothioneins and post-transcriptional internalization and degradation of Ctr1 molecules localized to the plasma membrane [16]. Importantly, in the context of higher eukaryotes, yeast lack a defined copper export pathway. The only equivalent is import of copper into the vacuole, which actually forms an additional cellular store that can be accessed in a Ctr2-dependent manner [3,17].
1.2. Multicellular eukaryotes: added complexities of copper homeostasis
In contrast to unicellular organisms, copper homeostasis in mammals requires that its levels and distribution be appropriately balanced at the cellular, tissue and systemic levels. Nutritional copper is taken up in the diet and distributed throughout the body in serum, bound to proteins such as ceruloplasmin or albumin. Albumin-bound copper is chelated by an unidentified, kinetically labile copper complex [18,19]. The dynamics of its systemic transport will not be discussed here.
Known mechanisms of cellular copper uptake and distribution are largely similar to those described above for yeast. Eukaryotic cells have a Ctr1 homolog that is expressed in all tissues, and mice that are heterozygous at the CTR1 locus are growth impaired and show developmental defects when compared with their wild-type litter-mates, while a CTR1 null is embryonic lethal [20,21]. Animals in which CTR1 has been conditionally knocked out in intestinal epithelial cells exhibit a systemic copper deficiency, and all organs except for the kidney are severely copper-deficient. Partial rescue of the resultant hypertrophic cardiomyopathy in these conditional mice is achieved by a single intravenous injection of copper [22]. This observation reinforces the fact that mammals possess a hierarchical regulatory network that allows for the relative prioritization of copper at a whole organism level.
After crossing the plasma membrane into cells, copper is distributed by the human orthologs of Atx1 and Ccs1; ATOX1 and CCS, respectively. ATOX1 has a well established role in transporting Cu(I) to the Menkes and Wilson P-type ATPases (ATP7A and ATP7B, respectively), and was recently shown to have a novel role in shuttling Cu(I) ions to the nucleus where it is involved in the transcriptional activation of cyclin D1 gene expression [1]. ATP7A and ATP7B are two homologous proteins with the analogous function but with essentially non-overlapping expression patterns across tissues [23–26]. ATP7A translocates Cu(I) into the lumen of the TGN for incorporation into secreted cupro-enzymes, including tyrosinase, dopamine β monoox-ygenase, peptidylglycine α-amidating monooxygenase and super-oxide dismutase-3 [27]. However, it also trafficks to the cell periphery to efflux Cu(I) ions in response to high cellular Cu concentrations and, accordingly, an increase in ATP7A copy number in CHO cells confers a hyper-resistant phenotype to growth in elevated copper salts [28]. In contrast, loss-of-function mutations in the ATP7A gene cause Menkes disease, a disorder in which defective copper transport from en-terocytes into the general circulation produces a severe copper deficiency in several tissues, in particular the brain [29]. Mutations in ATP7B result in Wilson’s disease, a condition in which patients suffer from copper overload in the liver due to an inability to export copper into the bile, the main pathway for its excretion from the body. ATP7B also transports Cu(I) into the lumen of the hepatic TGN for its incorporation into ceruloplasmin. Thus, the function of both ATP7A and ATP7B is critical not only to the metallation of cupro-enzymes within the secretory pathway but also to the removal of excess copper from the cell.
The amyloid precursor protein (APP), a member of a family of related proteins, APLP1 and APLP2, has been proposed to modulate copper efflux from the liver and cerebral cortex [30], and may therefore represent yet another copper trafficking pathway crucial to the regulation of cellular copper homeostasis. However, the mechanism of APP-mediated copper export from the cell has yet to be resolved, although APP contains a copper-binding site within its extracellular domain [31].
Copper homeostasis in mammalian cells is achieved in part by several distinct post-transcriptional regulatory processes. First, copper uptake by Ctr1 is responsive to copper status. Elevated copper levels result in diminished abundance of Ctr1 at the plasma membrane through its endocytosis and subsequent degradation [32]. In adult mice, its predominant localization to intracellular vesicles suggests that, at least in this organism, copper levels are such that significant changes in its high affinity uptake are not generally required under normal physiological conditions. Second, the subcellular localization of ATP7A and ATP7B is sensitive to cellular copper status, with each ATPase trafficking from the TGN to the cell periphery or cytoplasmic vesicles, respectively, in response to elevated copper levels [33]. The differential Cu-regulated trafficking of each ATPase to either apical or basolateral surfaces is likely mediated by accessory proteins and multiple sorting signals within their C-terminal tails [34]. Third, the abundance of several proteins is tightly coupled to cellular copper status. Elevated copper levels, for example, induce the degradation of CCS in a process that couples changes in cellular copper content with the activation of Sod1 [35,36]. Copper can also control the activity and abundance of the E3 ubiquitin ligase XIAP, X-linked inhibitor of apoptosis [37,38]. Copper binding to XIAP induces a conformational change that results in its inactivation and proteosomal degradation. These changes in XIAP levels in turn affect the abundance of COMMD1, a multifunctional, cytosolic protein implicated in controlling copper export from the cell through ATP7B [39].
Traditionally, copper trafficking pathways have been studied in isolation; however, experimental evidence is beginning to accumulate that supports the idea that dynamic communication between individual copper trafficking pathways is critical to how global decisions are made about cellular copper handling and distribution. One such connection linking two discrete copper trafficking pathways involves APP and Sod1. The formation of a 40–42 residue Aβ peptide derived from APP, the potential copper export molecule, is initially catalyzed by the aspartic protease BACE1. BACE1 can bind Cu(I) and this leads to an interaction with Ccs1 which may in turn serve to modulate the Ccs1-dependent activation of Sod1 [40]. A second example of such an interconnection links the functions of Sco1 and Sco2, two proteins with established functions in CcO assembly, with the regulation of one or more extramitochondrial pathways that modulate copper efflux from the cell [41] (see section 4).
2. Copper in the mitochondria: CuA and CuB site formation
The essential nature of copper in the mitochondria is derived from the role it plays in the utilization of oxygen (CcO) and the protection it affords against oxidative stress (Sod1). CcO contains multiple cofactors critical to its catalytic competence, and their delivery and insertion into the nascent holoenzyme complex require multiple ancillary proteins [13]. The subunits of CcO that form the catalytic core of the enzyme, Cox1–Cox3, are encoded by the mitochondrial genome [42]. Two heme a moieties and three copper ions are contained within conserved domains of Cox1 (a, a3, CuB) and Cox2 (CuA) [11]. The mononuclear CuB site in Cox1 interacts with heme a3 to form a heterobimetallic heme a3–CuB center, while the CuA site contained within Cox2 exists as a cysteine-bridged, binuclear, mixed valent center. The catalytic core is surrounded by nuclear-encoded structural subunits, which confer stability to the holoenzyme and provide sites for the allosteric regulation of its activity [43]. The fully assembled holoenzyme is embedded within the inner mitochondrial membrane (IM), and is further organized into higher order structures termed supercomplexes in yeast and mammals [44].
The insertion of copper into Cox1 and Cox2 in the IMS appears to mirror the copper chaperone paradigm from the cytosol. That is, copper is inserted by copper-binding chaperones and co-chaperones at specific stages of holoenzyme assembly, which in mammalian cells proceeds in a modular fashion [45]. Cox17 acts as the copper chaperone that delivers copper to both Sco1 and Cox11 in yeast, donating Cu(I) to each molecule through transient interactions that are mediated by distinct structural interfaces [46]. Its ability to load human SCO1 in vitro, along with the observation that COX17 null mice are embryonic lethal, is consistent with the conservation of its function in higher eukaryotes [47]. Although Cox17 readily adopts multiple oligomeric states and is capable of forming distinct Cu(I) conformers, it now appears that the bioactive conformer consists of a single Cu(I) ion coordinated to a monomeric protein that is stabilized by two disulfide bonds [48]. Cox17 has two additional conserved Cys residues upstream of the first Cys of the twin Cx9C motif, and these vicinal Cys residues coordinate Cu(I) in a bent, two coordinate complex [49]. The redox couple of the double disulfide configuration to the fully reduced state has a midpoint potential of −340 mV, consistent with the dual disulfide molecule being a likely species in vivo [50]. Mutational analyses further reveal that Cu(I) coordination and Cox17 function are not dependent on the presence of two disulfide crosslinks [51].
In yeast, Sco1 and Sco2 were first identified as high copy suppressors of a COX17 null [52]. Yeast lacking Sco1 fail to grow on a non-fermentable carbon source, and thorough genetic and biochemical analyses clearly implicate Sco1 in the formation of the mixed valent CuA site in Cox2 [53]. Deletion of SCO2 does not result in a respiratory defect, and to date, its function in yeast remains unknown. In contrast, both SCO1 and SCO2 are required in humans for viability [54]. Mutations in either gene produce a severe, isolated CcO deficiency that results in early onset disease with a fatal clinical outcome; however, SCO2 patients present with neonatal encephalocardiomyo-pathy, whereas SCO1 patients exhibit neonatal hepatic failure. The distinctive clinical presentation is not a result of tissue-specific expression of the two genes, as SCO1 and SCO2 are ubiquitously expressed and exhibit a similar expression pattern in different human tissues. Studies with immortalized fibroblasts derived from SCO1 and SCO2 patients suggest that SCO1 and SCO2 have non-overlapping but cooperative functions in CcO assembly [54].
Sco1 and Sco2 are tethered to the IM by a single transmembrane helix with the C-terminal globular domains protruding into the IMS [55,56]. A single Cu(I) binding site exists within the globular domain of Sco1 and Sco2, consisting of two cysteinyl residues within a Cx3C motif and a conserved histidyl residue. Mutation of either the Cys or His residues in Sco1 or Sco2 abrogates Cu(I) binding and results in decreased CcO activity [55,56]. Studies in organisms with a single Sco, such as Bacillus subtilis, suggest that the copper in Sco1 is transferred to CcO [57]. The Cu(I) ion coordinated by Sco1 is solvent-exposed and poised for ligand exchange transfer reactions. The structures of the metal-free Sco1 and Cu1Sco1 complex are similar, with only one loop showing significant rearrangements [58]. The movement of this loop orients the Cu(I) binding His residue in the proper orientation for Cu binding [58]. The structural dynamics of loop 8 suggest that it may be an important interface for interactions with Cox17 and/or Cox2 [59]. In addition to binding Cu(I), Sco proteins bind Cu(II) [60]; however, it is not clear whether Sco1 transfers both Cu(I) and Cu(II) ions to build the mixed valent, binuclear CuA site in Cox2. The human SCO2 conformer resembles human SCO1, although it exhibits greater conformational dynamics [61]. While copper binding is also required for its function, whether the copper is transferred to CcO or is necessary for SCO2 to facilitate an aspect of SCO1 function during CcO assembly remains equivocal.
Cu(I) binding to Cox17, Sco1 and Sco2 necessitates that the Cu(I)-binding cysteines be maintained in the reduced state during the Cu(I) transfer reactions. Mutations that alter the redox state or Cu(I) binding capacity of any of these proteins are expected to attenuate CcO assembly. Although no mutations in human COX17 have been described, missense mutations identified in SCO1 (P174L) and SCO2 patients (C133S, E140K and S240F) are located within or adjacent to the conserved Cx3C and essential His residues. The P174L substitution in human SCO1 does not affect the ability of the protein to bind and retain either Cu(I) or Cu(II) [62], yet its function in vivo is severely compromised as evidenced by the pronounced CcO assembly defect in SCO1 patient tissues and fibroblasts [54]. Further study of the P174L mutant SCO1 using both in vitro and in vivo assays revealed that the amino acid substitution impairs its ability to be metallated by Cox17 [62], and this likely represents the molecular basis for the inefficient assembly of the CcO complex in this genetic background. In contrast, it appears that mutations in SCO2 largely affect protein dynamics, altering the quaternary structure of the protein in such a way that promotes its degradation and ultimately leads to very low, residual levels of the mutant protein in the patient background. Appreciable residual function of the common E140K SCO2 mutant is corroborated by the observations that the recombinant protein retains the ability to bind copper, albeit less stably [63], and that its overexpression in SCO2 patient cell lines functionally complements the CcO deficiency [54].
Like Sco1 and Sco2, IM-tethered Cox11 has a single transmembrane helix with a C-terminal domain that contains its cysteinyl copper-binding residues protruding into the IMS. It is thought to be required for CuB site formation, given that yeast cells lacking Cox11 have impaired CcO activity and lower levels of Cox1, but still maintain the ability to transiently form the heme a3 site [64,65]. Cysteinyl residues within its C-terminus are important for Cu(I)-binding and correlate with in vivo function such that, when mutated, Cu(I) binding is abrogated and CcO activity is attenuated [66,67]. A specific role for Cox11 in the biogenesis of the CuB site is also supported by the observation that CcO isolated from Rhodobacter sphaeroides cox11Δ cells lacked CuB but contained both hemes. Cu(I) transfer from Cox11 to Cox1 to form the CuB site likely occurs concurrently with the insertion of heme a3 as nascent Cox1 chains are being inserted into the IM, since the heterobimetallic site lies 13 Å below the membrane surface in the mature holoenzyme [67,68].
3. Source of copper for CcO and Sod1 assembly
Copper ions used in the metallation of CcO and Sod1 are derived from a low molecular weight copper complex within the mitochon-drial matrix whose biochemical characteristics are conserved from yeast to humans [69], arguing that its functional importance arose early on in evolution and has since been retained. Although the structure of the ligand has yet to be elucidated, it stably binds Cu(I) in an anionic complex that can be depleted by the targeted expression of two heterologous Cu(I) binding molecules, human SOD1 or yeast Crs5 metallothionein [70]. The functional consequence of attenuating the CuL pool is apparent in Cu-limited cells, where the presence of either competitor molecule within the matrix diminishes CcO activity and, as a result, impairs growth on non-fermentable carbon sources. Matrix targeted Crs5 expression also reduces Sod1 protein levels within the IMS and impairs activity of an IM-tethered human SOD1. Supplementation of the cultures with exogenous Cu salts causes a significant expansion in the matrix CuL pool and reverses the observed phenotypes, arguing that it is the source of copper used in the metallation of CcO and Sod1 within the IMS.
The matrix CuL complex appears to be a storage depot for Cu(I) ions destined for CcO and Sod1 metallation reactions in the IMS (Fig. 1). How Cu(I) is trafficked to the mitochondria and subsequently translocated to the matrix for its storage remains an open question. Although the presence of Ccs1 and Cox17 in both the cytosol and IMS made them attractive candidates as mitochondrial Cu(I) shuttles, yeast lacking Ccs1 or Cox17 retain normal or near wild-type levels of mitochondrial copper, respectively [69]. Ccs1-depleted yeast also have wild-type CcO activity and the CcO deficiency in cox17Δ cells is functionally complemented by restricting Cox17 localization to the IMS using a heterologous IM membrane-binding domain [71]. Thus, neither protein participates in Cu(I) transport and delivery to the organelle. Cox19 and Cox23 are two additional cysteine-rich, soluble proteins which are also present in both the cytosol and IMS; however, like Cox17, their deletion has modest effects on mitochondrial copper levels [69].
While the CuL complex is localized largely to the mitochondrial matrix, the Cu-free ligand is abundant within the cytosol [70] (Cobine, Leary, Winge and Shoubridge, unpublished results) (Fig. 1). Cu(I) binding to the apo-ligand yields an anionic complex that shares the known biochemical characteristics of the authentic matrix Cu(I) complex. We therefore postulate that Cu(I) binding to the ligand within the cytosol triggers the translocation of the anionic CuL complex to the mitochondrion. This model is a novel paradigm of copper trafficking distinct from that of the metallochaperone systems in the cytosol. The facile expansion of the matrix CuL pool upon supplementation of cells with exogenous copper is consistent with the CuL translocation model, and with the high level of apo-ligand in the cytosol. Yeast cells depleted of Cup1, which serves to buffer against changes in cytosolic copper levels, exhibit an expansion of the matrix CuL pool over wild-type yeast which is dramatically enhanced upon their growth in copper salts. Thus, trafficking of copper to the mitochondria is more facile in cells unable to buffer copper in the cytosol, suggesting some competition between the ligand and other cytosolic fates for copper. The CuL complex translocation model predicts that transport occurs both into and out of the matrix. Therefore, either two transporters or a single bi-directional transporter exists within the IM. We postulate that once the CuL diffuses through channels in the OM, a transporter functions to move it from the IMS into the matrix. Its regulated release by a transporter would then result in the channeling of either Cu(I) or the CuL complex from the matrix back to the IMS for subsequent use by Cox17 and Ccs1 in downstream metallation reactions. The protein-mediated regulation of transporter activity is supported by the observation that the deletion of Coa1 and Shy1, two CcO assembly factors with roles in Cox1 maturation, attenuates matrix copper levels [72]. The regulation of the export step by a subset of proteins that participate either in CcO assembly or Sod1 activation would effectively limit the amount of free copper within IMS, and may in fact have been the driving force that necessitated the existence of the Cox17 copper chaperone pathway in the IMS.
4. Mitochondrial regulation of cellular copper homeostasis
While most of the proteins responsible for trafficking copper to, within and from mitochondria have yet to be identified, it is clear that the organelle contains the requisite machinery to acquire, maintain and mobilize a bioactive pool of copper within its matrix. Copper is present in vast molar excess of that required for the metallation of these proteins, suggesting that the matrix copper pool fulfills at least one additional function. Its ability to either expand or contract in response to changes in total cellular copper levels [69] (Cobine, Leary, Winge and Shoubridge, unpublished data) further suggests that the matrix copper pool could be a dynamic rheostat that responds to changes in cellular copper status. Such a rheostatic role would presumably afford the organelle an opportunity to ensure the presence of both an adequate supply and reserve of copper within mitochondria for the metallation of its targets under all physiological conditions. The fact that the pool can be expanded to a much greater extent than it can be depleted [69] (Cobine, Leary, Winge and Shoubridge, unpublished data) supports the idea that the organelle’s relative priority is to retain sufficient copper to metallate CcO and IMS Sod1.
Given that the mitochondrial inner membrane is impermeable, the ability of the matrix copper pool to act as a rheostat is contingent upon a signaling pathway that communicates information derived from various mitochondrial compartments to relevant extramitochondrial targets (Fig. 2). This requires inner membrane proteins with functional domains in both the matrix and IMS that are capable of sensing relevant stimuli and transducing appropriate signals. It also depends on either proteins or second messengers that monitor and report on the functional state of these inner membrane proteins. Subsequent transduction of the original signal could then be achieved by release of the relevant molecule from the IMS to the cytosol, where it would interact with effectors responsible for regulating the activity of other cellular copper trafficking pathways. Alternatively, the same signal could be transduced by translocation of the protein or second messenger to, and its interaction with, the IMS side of an outer membrane protein complex to which cytosolic effectors are either docked (and released) or recruited (Fig. 2).
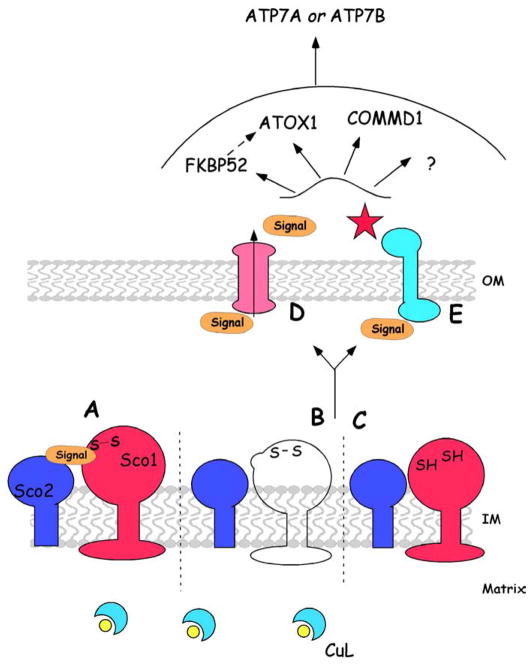
Fig. 2.
Proposed mechanism of SCO-dependent regulation of cellular copper efflux: The redox state of SCO1 (red) and SCO2’s (blue) cysteine thiols exists in a dynamic equilibrium between oxidized and reduced forms that is sensitive to cellular/mito-chondrial copper status (A). Genetic interactions between SCO1 and SCO2 that are relevant to both CcO assembly and the regulation of cellular copper homeostasis have been demonstrated in human cells [54,41]. The P174L mutation (B, unfilled) or a disproportionate shift in SCO1’s cysteine sulfurs towards a reduced state (C), like that observed in a SCO2 background, initiates a signaling cascade by abrogating an interaction with a molecule/sensing protein. Release of either factor then transduces the signal, by either translocating from the IMS to the cytosol (D) or interacting with an outer membrane (OM) protein complex to which relevant effectors are docked or recruited (E). The trafficking of one of several potential cytosolic effectors, including FKBP52, ATOX1, COMMD1 or an unknown factor(s), may in turn be sensitive to such a signal, and through interactions with ATP7A or ATP7B, alter the rates of copper efflux from the cell.
Attractive candidates to function in such a signaling pathway are copper-binding proteins, given their ability to bind the substrate in question. The copper-binding sites of 3 integral inner membrane (Cox11, Sco1 and Sco2) and 4 soluble, cysteine-rich (Cox17, Cox19, Cox23 and Pet191) CcO assembly factors are localized to the IMS. Changes in their abundance, cysteine thiol redox state or metallation state are all therefore potential mechanisms whereby mitochondrial signals relevant to either intra-organellar communication of copper status or the activity of other copper trafficking pathways within the cell could be both generated and fine tuned. The importance of redox chemistry within the IMS to mitochondrial biology is an emerging theme in the field, and has been linked to protein import [73] and the regulation of electron transport chain activity [74]. Thus, a role for any of these proteins in a redox-dependent signaling pathway is not without precedence.
4.1. SCO-dependent copper efflux
Consistent with a role for CcO assembly factors in the regulation of cellular copper homeostasis, we recently demonstrated that affected tissues from SCO patient backgrounds exhibit a copper deficiency phenotype that likely contributes to the etiology of the associated diseases [41]. A clear genotype–phenotype relationship was observed in the SCO2 background, with the copper deficiency being most severe in fibroblasts derived from those patients expressing a single missense allele. Significant reductions in copper levels are also observed in multiple COX15 patient fibroblast lines. In all three patient backgrounds, the CcO and copper deficiency phenotypes are dissociable, and overexpression of SCO2 increases cellular copper levels; however, only a partial rescue of the latter phenotype is observed in the SCO1 background, due to active signaling by the P174L variant. Kinetic labeling studies with 64Cu established that reduced cellular copper levels in both SCO1 and SCO2 patient fibroblasts are not caused by an inability to acquire copper via its high affinity uptake, but rather are attributable to a defect in its cellular retention. Collectively, the findings of this study argue that SCO1 and SCO2 are bifunctional molecules, with SCO2 acting to modify an aspect of SCO1 function that is crucial to the generation of a signal that modulates the rate of copper efflux from the cell.
How might changes in the functional state of the SCO proteins effect signals that are relevant to the regulation of cellular copper home-ostasis? One mechanistic possibility is that signaling is sensitive to changes in either their abundance or their relative ratio; however, the cellular copper content in control fibroblasts is unresponsive to several fold changes in the abundance of either SCO protein [41], suggesting that this is unlikely to represent a viable strategy with which to modulate signaling in response to physiological cues in vivo. A more likely mechanistic scenario by which SCO-dependent signaling could be rapidly modulated is through changes in the redox state of SCO1’s cysteine thiols [41] (Fig. 2). Accordingly, we observe that; the cysteine thiols of SCO1, but not those of other CcO assembly factors, are disproportionately reduced in copper-deficient COX10, COX15 and SCO2 patient backgrounds relative to controls; and, the ratio of its reduced to oxidized cysteinyl sulfurs explains a significant amount of the variation in copper levels across these different genetic backgrounds (Leary, Cobine, Winge and Shoubridge, unpublished data). While the cysteine thiols of P174L SCO1 are entirely oxidized in SCO1 patient fibroblasts (Leary, Cobine, Winge and Shoubridge, unpublished data), we favor a model in which this mutant protein elicits a signal equivalent to the wild-type, copper-loaded SCO1 conformer, thus signaling a state of cellular copper overload [75]. A causal role for the redox state of SCO1’s cysteine thiols in the aberrant signaling that results in a copper deficiency phenotype in these patient backgrounds is supported by two additional observations (Leary, Cobine, Winge and Shoubridge, unpublished data); first, the cysteine thiols of SCO1 are also disproportionately reduced in ATP7A patient fibroblasts, a genetic context in which cellular copper levels are several fold higher than those in controls [76]; and second, the cysteine thiols of SCO1 are shifted towards an oxidized state in control fibroblasts in which cellular copper levels have been pharmacologically depleted, a response which is significantly attenuated in a SCO2 background. Although the proteins) responsible for maintaining or altering the redox state of the cysteine thiols of either human SCO protein remains unknown, a recent study reported that the Rhodobacter ortholog PrrC, a protein identified as a regulator of photosynthesis, exhibits thiol-disulphide oxidore-ductase activity [77]. Our most recent data suggest that human SCO2 also functions in such a capacity to modify the redox state of SCO1’s cysteine thiols, and that a shift in the total population of SCO1’s cysteine thiols towards a reduced state results in the generation of a signal that increases the rate of copper efflux from the cell. The potential contribution of disproportionate metallation of these reduced cysteine thiols to the generation of this SCO-dependent regulatory signal is being actively investigated.
At the organellar level, the mechanism(s) that senses and transduces a SCO-dependent signal based on the redox state of SCO1’s cysteine thiols is completely unknown. Several soluble IMS proteins with roles in either Sod1 activation (Ccs1) or CcO assembly (Cox19, Cox23 and Pet191) represent potential candidates. CCS may impact upon SCO-dependent signaling by competing for copper that is mobilized from the matrix to metallate CcO within the IMS, effectively titrating out substrate availability. This postulate is consistent with the recent finding that transgenic mice overexpressing CCS and mutant G93A SOD1 exhibit an isolated CcO deficiency in spinal cord [78]; however, additional studies are required that directly evaluate the potential for crosstalk between IMS proteins that deliver copper to Sod1 and CcO under more physiologically relevant conditions.
One or more of the aforementioned CcO assembly factors may also play a role in regulating copper availability, either at the level of its trafficking within the IMS or its routing from the organelle. This may in fact explain why there are so many cysteine-rich, soluble CcO assembly factors within the IMS with poorly defined roles in holoenzyme biogenesis. In yeast, mitochondrial copper levels are unaffected by the single deletion of any of these genes, and the double deletion of COX17 and COX19; however, the inability to alter the mitochondrial copper content in these mutants may reflect the fact that there is considerable buffering capacity within the system, such that knockout of all redundant components is required to observe a phenotype. Whether total cellular copper content is affected in any of these genetic backgrounds has not been considered, and at present it is unclear how much of a percent change in the total mitochondrial copper pool is required to impact upon cellular copper status. Alternatively, the transduction of a SCO-dependent signal may rely on other cytosolic copper chaperones that do not participate directly in IMS Sod1 activation or CcO assembly, such as COMMD1 or ATOX1 (Fig. 2). Systematic genetic and biochemical analysis of the potential involvement of all candidates is required.
We have previously shown that SCO-dependent copper efflux from the cell is not attributable to the altered abundance of known extra-mitochondrial copper chaperones [41]. Indirect immunofluorescence studies of ATP7A localization in control and SCO patient fibroblasts also failed to reveal gross mislocalization of the protein to the cell periphery in the patient backgrounds. If, however, only a small percentage of the total cellular pool of ATP7A is required to effect changes in the rate of copper efflux from the cell [79], this methodology may not be sufficiently sensitive to detect subtle but biologically meaningful changes in its cellular distribution. Indeed, two subsequent genetic experiments implicate ATP7A in a SCO-dependent signaling pathway that regulates copper efflux from fibroblasts (Leary, Cobine, Winge, and Shoubridge, unpublished data). First, cellular copper levels in ATP7A patient fibroblasts are unchanged upon overexpression of the P174L SCO1 mutant following the knockdown of wild-type SCO1 below immunologically detectable levels. Second, ATP7A knockdown in control and SCO patient fibroblasts results in a disproportionate increase in cellular copper levels in the patient backgrounds. Whether the pathway being mapped in fibroblasts is conserved in other cell types remains an open question. It is possible that the basic architecture of the efflux pathway is conserved, but that different proteins fulfill the identical role in different cell types (Fig. 2). For example, in the severely copper-deficient liver SCO1 of a patient [41], enhanced rates of copper efflux may be achieved by the inappropriate activation of ATP7B, the homologue of ATP7A. Stimulation of copper export by either ATPase may be achieved by interactions with either COMMD1 or ATOX1. Alternatively, other mechanisms such as the immunophilin-dependent regulation of copper efflux from the cell by FKBP52 may be relevant [80]. An evaluation of the tissue-specificity of copper efflux pathways, and their potential to explain the tissue-specific etiology of disease observed in SCO patient backgrounds awaits the generation of relevant transgenic models.
5. Perspectives and future outlook
Individual copper trafficking pathways collectively regulate the cellular concentration of copper ions within a fairly narrow range. This ensures its appropriate distribution to key cupro-enzymes and minimizes its potential toxicity. While these pathways have been studied in isolation, the interplay between them has not been thoroughly investigated. Here, we have focused our attention on SCO proteins, whose functions lead to the intersection of pathways relevant to both the assembly of the mitochondrial cupro-enzyme CcO and cellular copper export.
Mitochondria have a vested interest in how cellular copper is prioritized, based on the need to metallate CcO to sustain aerobic life, and, as a major source of reactive oxygen species, to activate IMS SOD1 and control the levels of free copper within the cell. Organellar signaling for the redistribution of copper in response to metabolic need is not without precedence. In Chlamydomonas grown under copper-limiting conditions, copper is reprioritized from the chloroplast to the mitochondria [81], demonstrating the existence of mechanisms that allow for redistribution of the total cellular copper quota through dynamic signaling between an organelle and other cellular compartments.
How do SCOs modulate the generation of such a signal? The simplest explanation is that their interactions with a protein/molecule in the IMS occur in a redox-dependent manner that stimulates its translocation to the cytosol where it in turn regulates copper export, analogous to the role of cytochrome c in regulating apoptosis. Cytochrome c binds to APAF-1, causing structural rearrangements that eventually lead to the formation of the apoptosome, a multi-subunit complex that recruits and activates caspases, ultimately causing cell death [82]. APAF-1 is found in a monomeric “locked” state in the absence of cytochrome c. Binding of cytochrome c triggers the first structural rearrangement in APAF-1 to a ‘semi-open’, auto-inhibited form. Its transition to the “open” form through nucleotide exchange precedes the assembly of the multi-subunit complex that leads to the eventual activation of the caspase by proteolysis. This pathway represents a multi-layered mechanism that protects the cell in response to a suicide signal. The major uncertainty with respect to the mitochondrial regulation of cellular copper export remains the identification of the effector molecule whose activity is ultimately sensitive to SCO-dependent signaling. At present, ATP7A and ATP7B are the primary copper exporters, and these proteins have an extended amino-terminus that has been implicated in binding to a variety of different cellular proteins (e.g. COMMD1, glutaredoxin, dynactin subunit p62). The SCO-mediated signaling pathway could affect any of these interactions in a multi-layer response that stimulates copper export.
A role for the SCO1 transmembrane domain in regulating the signal [41] suggests either that it mediates an interaction with an unknown regulatory factor or that SCO1 is somehow monitoring mitochondrial copper content with its matrix-localized, N-terminal tail. Matrix copper levels appear to be largely independent of the energetic status of the cell and the abundance of CcO and IMS Sod1. However, a link between mitochondrial copper and CcO assembly factors has been observed in yeast [72]. Shy1 is the yeast homolog of human SURF1, and mutations in SURF1 are a common cause of Leigh’s syndrome. CcO assembly factor 1 (Coa1) and Shy1 interact in multiple protein complexes that are required for the assembly of an early Cox1 intermediate, and may function to promote a Cox1 conformation that is suitable for the insertion of the heme a3:CuB site [72]. Yeast lacking either Coa1 or Shy1 exhibit attenuated matrix copper pools and a partial rescue of respiratory growth can be achieved by supplementing the mutant cells with high levels of exogenous copper. We propose that one of the functions of a Coa1/Shy1 complex is to regulate IM copper transport, thereby linking CcO biogenesis with matrix copper pool dynamics. The dynamic regulation could be achieved by the equilibrium of the different protein complexes containing Coa1 and Shy1. This, like the role of Sco1, is yet another example of CcO assembly proteins regulating a homeostatic intersection. It also adds to the growing list of proteins with multiple functions, one of which involves a critical regulatory role in a given aspect of copper homeostasis.
Why would the organelle have evolved such an elaborate strategy for the trafficking and storage of copper? The strategy is quite similar to the one employed by prokaryotes. Escherichia coli has limited requirements for copper in the cytoplasm yet takes copper into the cytoplasm where it is sensed directly by a transcriptional repressor. The repressor dissociates from an operon encoding a copper exporter and a periplasmic copper-binding protein required for copper tolerance. In eukaryotes, mitochondrial copper is retained in a storage form and regulated release is required for efficient metallation of IMS enzymes. The storage and regulation of export probably arose from the limited copper availability in the eukaryotic cytosol and the cell’s need for an immediate source of copper for CcO assembly. As a consequence, its chelation in a biologically inert ligand was critical because mitochondrial respiration is a major source of superoxide production. The superoxide may in turn drive the generation of more harmful reactive oxygen species [83]. In most organisms, the bulk of superoxide anion produced on a daily basis by the respiratory chain is derived from the ubisemiquinone of coenzyme Q (CoQ) [84]. The CoQ semiquinone generated during the Q-cycle of complex III’s catalytic cycle can react with oxygen to produce superoxide anions in both the IMS and matrix [85,86]. Superoxide anions can in turn be converted by free Cu(I) to the highly reactive hydroxyl radical, or dismutated to yield hydrogen peroxide by either IMS Sod1 or matrix-localized Sod2. Thus, the need to complex Cu(I) with a ligand for its storage within the matrix, and to use protein-mediated mechanisms to chaperone Cu(I) during its subsequent mobilization and trafficking to the IMS may reflect the importance of minimizing Cu-catalyzed production of deleterious free radicals. The highly regulated trafficking of copper at the organellar level may also provide a robust mechanism to couple Cu (I) transport from the matrix to the IMS with the biogenesis of CcO and activation of Sod1 under physiological conditions in which respiratory demands are elevated.
In this review, we highlighted the emerging role of mitochondrial proteins in the regulation of cellular copper export. Future studies that identify the mechanisms by which interdependent signals allow for dynamic communication between individual copper trafficking pathways will be invaluable to our understanding of the complex, hierarchical nature of the regulation of cellular copper homeostasis. Such studies also will ultimately provide further insight into how global decisions are made about the fate of cellular copper, under both normal conditions and in response to physiological stimuli.
Acknowledgments
P.A.C., D.R.W. and S.C.L. are supported respectively by grants-in-aid of research from the United Mitochondrial Disease Foundation (#07-130), the National Institutes of Environmental Health Sciences, NIH (ES 03817) and the Muscular Dystrophy Association (#4181).
References
- 1.Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (atox1) as a copper dependent transcription factor involved in cell proliferation. J biol Chem. 2008 doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 3.Rees EM, Lee J, Thiele DJ. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem. 2004;279:54221–54229. doi: 10.1074/jbc.M411669200. [DOI] [PubMed] [Google Scholar]
- 4.Huffman DL, O’Halloran TV. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 5.Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 6.Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, Hadjiliadis N, Pierattelli R, Rosato A, Voulgaris P. The Atx1–Ccc2 complex is a metal-mediated protein–protein interaction. Nat Chem Biol. 2006;2:367–368. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
- 7.O’Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa Y, Torres AS, O’Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004;23:2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SJ, Pufahl RA, Dancis A, O’Halloran TV, Culotta VC. A role for the Sac-charomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J Biol Chem. 1997;272:9215–9220. [PubMed] [Google Scholar]
- 10.Yuan DS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD. The Menkes/ Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Hakashima R, Yaono R, Yoshikawa S. Science. Vol. 269. New York, N.Y: 1995. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A; pp. 1069–1074. [DOI] [PubMed] [Google Scholar]
- 12.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermem-brane space of mitochondria. a physiological role for Sod1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 13.Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim Biophys Acta (Mol Cell Res) 2006;1763:759–772. doi: 10.1016/j.bbamcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Field LS, Furukawa Y, O’Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278:28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 15.Winge DR, Jensen LT, Srinivasan C. Metal-ion regulation of gene expression in yeast. Curr Opin Chem Biol. 1998;2:216–221. doi: 10.1016/s1367-5931(98)80063-x. [DOI] [PubMed] [Google Scholar]
- 16.Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- 17.Rees EM, Thiele DJ. Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem. 2007;282:21629–21638. doi: 10.1074/jbc.M703397200. [DOI] [PubMed] [Google Scholar]
- 18.Gubler CJ, Lahey ME, Cartwright GE, Wintrobe MM. Studies on copper metabolism. IX. The transportation of copper in blood. J Clin Invest. 1953;32:405–414. doi: 10.1172/JCI102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahey ME, Gubler CJ, Brown DM, Smith EL, Jager BV, Cartwright GE, Wintrobe MM. Studies on copper metabolism. VIII. The correlation between the serum copper level and various serum protein fractions. J Lab Clin Med. 1953;41:829–835. [PubMed] [Google Scholar]
- 20.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 22.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metabolism. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Wernimont AK, Huffman DL, Lamb AL, O’Halloran TV, Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/ Wilson disease proteins. Nat Struct Biol. 2000;7:766–771. doi: 10.1038/78999. [DOI] [PubMed] [Google Scholar]
- 24.Hamza I, Schaefer M, Klomp LWJ, Gitlin JD. Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci U S A. 1999;96:13363–13368. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yatsunyk LA, Rosenzweig AC. Copper(I) binding and transfer by the N-terminus of the Wilson disease protein. J Biol Chem. 2007;282:8622–8631. doi: 10.1074/jbc.M609533200. [DOI] [PubMed] [Google Scholar]
- 26.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 27.Qin Z, Reszka KJ, Fukai T, Weintraub NL. Extracellular superoxide dismutase (ecSOD) in vascular biology: an update on exogenous gene transfer and endogenous regulators of ecSOD. Transl Res. 2008;151:68–78. doi: 10.1016/j.trsl.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camakaris J, Petris MJ, Bailey L, Shen P, Lockhart P, Glover TW, Barcroft C, Patton J, Mercer JF. Gene amplification of the Menkes (MNK: ATP7a) P-type ATPase gene of CHO cells is associated with copper resistance and enhanced copper efflux. Hum Mol Genet. 1995;4:2117–2123. doi: 10.1093/hmg/4.11.2117. [DOI] [PubMed] [Google Scholar]
- 29.de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treiber C, Simons A, Strauss M, Hafner M, Cappai R, Bayer TA, Multhaup G. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of Alzheimer’s disease. J Biol Chem. 2004;279:51958–51964. doi: 10.1074/jbc.M407410200. [DOI] [PubMed] [Google Scholar]
- 31.Kong GK, Miles LA, Crespi GA, Morton CJ, Ng HL, Barnham KJ, McKinstry WJ, Cappai R, Parker MW. Copper binding to the Alzheimer’s disease amyloid precursor protein. Eur Biophys J. 2008;37:269–279. doi: 10.1007/s00249-007-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 33.La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Petris MJ, Mercer JFB. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Human Mol Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- 35.Bertinato J, Iskandar M, L’Abbe MR. Copper deficiency induces the upregulation of the copper chaperone for Cu/Zn superoxide dismutase in weanling male rats. J Nutr. 2003;133:28–31. doi: 10.1093/jn/133.1.28. [DOI] [PubMed] [Google Scholar]
- 36.Bertinato J, L’Abbe MR. Copper modulates the degradation of copper chaperone for Cu,Zn superoxide dismutase by the 26 S proteosome. J Biol Chem. 2003;278:35071–35078. doi: 10.1074/jbc.M302242200. [DOI] [PubMed] [Google Scholar]
- 37.Mufti AR, Burstein E, Csomos RA, Graf PC, Wilkinson JC, Dick RD, Challa M, Son JK, Bratton SB, Su GL, Brewer GJ, Jakob U, Duckett CS. XIAP Is a copper binding protein deregulated in Wilson’s disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Mufti AR, Burstein E, Duckett CS. XIAP: cell death regulation meets copper homeostasis. Arch Biochem Biophys. 2007;463:168–174. doi: 10.1016/j.abb.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burstein E, Ganesh L, Dick RD, van De Sluis B, Wilkinson JC, Klomp LW, Wijmenga C, Brewer GJ, Nabel GJ, Duckett CS. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244–254. doi: 10.1038/sj.emboj.7600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, Miller CC, Lau KF, Tennant ME, Dennison C, Robinson NJ, Dingwall C. BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem. 2005;280:17930–17937. doi: 10.1074/jbc.M412034200. [DOI] [PubMed] [Google Scholar]
- 41.Leary SC, Cobine PA, Kaufman BA, Guercin GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R, Shoubridge EA. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, Tzagoloff A. Cytochrome oxidase in health and disease. Gene. 2006;286:53–63. doi: 10.1016/s0378-1119(01)00803-4. [DOI] [PubMed] [Google Scholar]
- 43.Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic Biol Med. 2000;29:211–221. doi: 10.1016/s0891-5849(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 44.Heinemeyer J, Braun HP, Boekema EJ, Kouril R. A structural model of the cytochrome c reductase/oxidase supercomplex from yeast mitochondria. J Biol Chem. 2007;282:12240–12248. doi: 10.1074/jbc.M610545200. [DOI] [PubMed] [Google Scholar]
- 45.Nijtmans LG, Taanman JW, Muijsers AO, Speijer D, Van den Bogert C. Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem. 1998;254:389–394. doi: 10.1046/j.1432-1327.1998.2540389.x. [DOI] [PubMed] [Google Scholar]
- 46.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J Biol Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi Y, Kako K, Kashiwabara SI, Takehara A, Inada Y, Arai H, Nakada K, Kodama H, Hayashi JI, Baba T, Munekata E. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome c oxidase and embryonic development. Mol Biol Cell. 2002;22:7614–7621. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abajian C, Yatsunyk LA, Ramirez BE, Rosenzweig AC. Yeast Cox17 solution structure and copper(I) binding. J Biol Chem. 2004;279:53584–53592. doi: 10.1074/jbc.M408099200. [DOI] [PubMed] [Google Scholar]
- 49.Banci L, Bertini I, Ciofi-Baffoni S, Janicka A, Martinelli M, Kozlowski H, Palumaa P. A structural–dynamical characterization of human Cox17. J Biol Chem. 2007 doi: 10.1074/jbc.M708016200. [DOI] [PubMed] [Google Scholar]
- 50.Voronova A, Meyer-Klaucke W, Meyer T, Rompel A, Krebs B, Kazantseva J, Sillard R, Palumaa P. Oxidative switches in functioning of mammalian copper chaperone Cox17. Biochem J. 2007;408:139–148. doi: 10.1042/BJ20070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heaton D, Nittis T, Srinivasan C, Winge DR. Mutational analysis of the mitochondrial copper metallochaperone Cox17. J Biol Chem. 2000;275:37582–37587. doi: 10.1074/jbc.M006639200. [DOI] [PubMed] [Google Scholar]
- 52.Glerum DM, Shtanko A, Tzagoloff A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem. 1996;271:20531–20535. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 53.Schulze M, Rodel G. SCO1, a yeast nuclear gene essential for accumulation of mitochondrrial cytochrome c oxidase subunit II. Mol Gen Genet. 1988;211:492–498. doi: 10.1007/BF00425706. [DOI] [PubMed] [Google Scholar]
- 54.Leary SC, Kaufman BA, Pellechia G, Gguercin GH, Mattman A, Jaksch M, Shoubridge EA. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 55.Rentzsch N, Krummeck-WeiB G, Hofer A, Bartuschka A, Ostermann K, Rodel G. Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr Genet. 1999;35:103–108. doi: 10.1007/s002940050438. [DOI] [PubMed] [Google Scholar]
- 56.Nittis T, George GN, Winge DR. Yeast Sco1, a protein essential for cytochrome c Oxidase function is a Cu(I)-binding protein. J Biol Chem. 2001;276:42520–42526. doi: 10.1074/jbc.M107077200. [DOI] [PubMed] [Google Scholar]
- 57.Mattatall NR, Jazairi J, Hill BC. Characterization of YpmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J Biol Chem. 2000;275:28802–28809. doi: 10.1074/jbc.M002741200. [DOI] [PubMed] [Google Scholar]
- 58.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, Marinelli M, Palumma P, Wang S. A hint for the function of human Sco1 from different structures. Proc Natl Acad Sci USA. 2006;103:8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, Martinelli M, Palumaa P, Wang S. A hint for the function of human Sco1 from different structures. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horng YC, Leary SC, Cobine PA, Young FB, George GN, Shoubridge EA, Winge DR. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 61.Banci L, Bertini I, Ciofi-Baffoni S, Gerothanassis IP, Leontari I, Martinelli M, Wang S. A structural characterization of human SCO2. Structure. 2007;15:1132–1140. doi: 10.1016/j.str.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Cobine PA, Pierrel F, Leary SC, Sasarman F, Horng YC, Shoubridge EA, Winge DR. The P174L mutation in human Sco1 severely compromises Cox17-dependent metallation but does not impair copper binding. J Biol Chem. 2006;281:12270–12276. doi: 10.1074/jbc.M600496200. [DOI] [PubMed] [Google Scholar]
- 63.Foltopoulou PF, Zachariadis GA, Politou AS, Tsiftsoglou AS, Papadopoulou LC. Human recombinant mutated forms of the mitochondrial COX assembly Sco2 protein differ from wild-type in physical state and copper binding capacity. Mol Genet Metab. 2004;81:225–236. doi: 10.1016/j.ymgme.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Tzagoloff A, Capitanio N, Nobrega MP, Gatti D. Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J. 1990;9:2759–2764. doi: 10.1002/j.1460-2075.1990.tb07463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khalimonchuk O, Bird A, Winge DR. Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J Biol Chem. 2007;282:17442–17449. doi: 10.1074/jbc.M702379200. [DOI] [PubMed] [Google Scholar]
- 66.Carr HS, George GN, Winge DR. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I) binding protein. J Biol Chem. 2002;277:31237–31242. doi: 10.1074/jbc.M204854200. [DOI] [PubMed] [Google Scholar]
- 67.Khalimonchuk O, Rodel G. Biogenesis of cytochrome c oxidase. Mitochondrion. 2005;5:363–388. doi: 10.1016/j.mito.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Carr HS, Winge DR. Assembly of cytochrome c oxidase within the mitochon-drion. Acc Chem Res. 2003;36:309–316. doi: 10.1021/ar0200807. [DOI] [PubMed] [Google Scholar]
- 69.Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 70.Cobine PA, Pierrel F, Bestwick ML, Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 71.Maxfield AB, Heaton DN, Winge DR. Cox17 is functional when tethered to the mitochondrial inner membrane. J Biol Chem. 2004;279:5072–5080. doi: 10.1074/jbc.M311772200. [DOI] [PubMed] [Google Scholar]
- 72.Pierrel F, Bestwick ML, Cobine PA, Khalimonchuk O, Cricco JA, Winge DR. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 2007;26:4335–4346. doi: 10.1038/sj.emboj.7601861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005:1059–1069. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Bihlmaier K, Mesecke N, Terziyska N, Bien M, Hell K, Herrmann JM. The disulfide relay system of mitochondria is connected to the respiratory chain. J cell Biol. 2007;179:389–395. doi: 10.1083/jcb.200707123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochimica et Biophysica Acta. 2006;1763:759–772. doi: 10.1016/j.bbamcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Goka TJ, Stevenson RE, Hefferan PM, Howell RR. Menkes disease: a biochemical abnormality in cultured human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:604–606. doi: 10.1073/pnas.73.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Badrick AC, Hamilton AJ, Bernhardt PV, Jones CE, Kappler U, Jennings MP, McEwan AG. PrrC, a Sco homologue from Rhodobacter sphaeroides, possesses thiol-disulfide oxidoreductase activity. FEBS Lett. 2007;581:4663–4667. doi: 10.1016/j.febslet.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 78.Son M, Leary SC, Romain N, Pierrel F, Winge DR, Haller RG, Elliott JL. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice over-expressing CCS protein. J Biol Chem. 2008 doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pase L, Voskoboinik I, Greenough M, Camakaris J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J. 2004;378:1031–1037. doi: 10.1042/BJ20031181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanokawa-Akakura R, Dai H, Akakura S, Weinstein D, Fajardo JE, Lang SE, Wadsworth S, Siekierka J, Birge RB. A novel role for the immunophilin FKBP52 in copper transport. J Biol Chem. 2004;279:27845–27848. doi: 10.1074/jbc.C400118200. [DOI] [PubMed] [Google Scholar]
- 81.Merchant SS, Allen MD, Kropat J, Moseley JL, Long JC, Tottey S, Terauchi AM. Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochimica et Biophysica Acta. 2006;1763:578–594. doi: 10.1016/j.bbamcr.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 83.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 84.Maxwell DP, Wang Y, McIntosh L. The alternate oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 86.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]