SUMMARY
Inflammatory activation of myeloid cells is accompanied by increased glycolysis, which is required for the surge in cytokine production. Although in vitro studies suggest that increased macrophage glucose metabolism is sufficient for cytokine induction, the pro-inflammatory effects of increased myeloid cell glucose flux in vivo and the impact on atherosclerosis, a major complication of diabetes, are unknown. We therefore tested the hypothesis that increased glucose uptake in myeloid cells stimulates cytokine production and atherosclerosis. Overexpression of the glucose transporter GLUT1 in myeloid cells caused increased glycolysis and flux through the pentose phosphate pathway, but did not induce cytokines. Moreover, myeloid cell-specific overexpression of GLUT1 in LDL receptor-deficient mice was ineffective in promoting atherosclerosis. Thus, increased glucose flux is insufficient for inflammatory myeloid cell activation and atherogenesis. If glucose promotes atherosclerosis by increasing cellular glucose flux, myeloid cells do not appear to be the key targets.
Keywords: Atherosclerosis, Diabetes, Glucose, GLUT1, Glycolysis, Lipopolysaccharide, Macrophage
INTRODUCTION
When a myeloid cell encounters inflammatory cues, such as the Gram-negative bacterial outer membrane component lipopolysaccharide (LPS) and cytokines governing innate and adaptive immunity, such as interferon-γ (IFN-γ), it undergoes inflammatory activation often referred to as classical (M1) activation. This process is associated with increased glucose flux through glycolysis and the pentose phosphate pathway (Vats et al., 2006; Krawczyk et al., 2010, Haschemi et al., 2012; O’Neill & Hardie, 2013) and reduced mitochondrial oxidation (Vats et al., 2006; O’Neill & Hardie 2013). Increased glycolysis in myeloid cells is not only a consequence of inflammatory activation, but is of critical importance for the ability of these cells to govern inflammatory processes (O’Neill & Hardie 2013; Tannahill et al., 2013). The shift to glycolysis is due, at least in part, to upregulation of the glucose transporter GLUT1 and enzymes in the glycolytic pathway, including the 6-phosphofructose-2-kinase isoform PFKFB3, and downregulation of TCA cycle enzymes (Tannahill et al., 2013), and to increased production of nitric oxide, which inhibits oxidative phosphorylation (Everts et al., 2012). Furthermore, the enzyme carbohydrate kinase-like protein CARKL, which is dramatically downregulated by LPS in macrophages and regulates flux through the non-oxidative arm of the pentose phosphate pathway, has recently been shown to inhibit the classical activation of these cells (Haschemi et al., 2012). Together, these findings indicate that increased flux of glucose through glycolysis and the pentose phosphate pathway relative to mitochondrial oxidation, is a key feature of inflammatory myeloid cells required for optimal inflammatory functions of these cells.
Increased glucose flux in myeloid cells might explain the increased inflammatory activity of these cells in diabetes, and by inference, might also explain complications of diabetes associated with increased myeloid cell activation. This is logical because diabetes is characterized by hyperglycemia and increased inflammatory activation of myeloid cells (Kanter et al., 2012; Nagareddy et al., 2013) as well as myelopoiesis (Nagareddy et al., 2013). A glucose-dependent increase in production of cytokines by macrophages in the artery wall in diabetic mice is associated with worsened atherosclerosis (Nagareddy et al., 2013), a major complication of diabetes leading to myocardial infarction and stroke (Bornfeldt & Tabas 2011). Blocking diabetes-induced inflammatory activation of myeloid cells prevents diabetes-accelerated atherosclerosis (Kanter et al., 2012). Furthermore, inhibition of glycolysis reduces monocyte adherence to the endothelium, a key event in initiation of lesions of atherosclerosis (Tsuruda et al., 2012). Finally, downregulation of GLUT1 has recently been shown to prevent proliferation of myeloid cell progenitors (Gautier et al., 2013), which is associated with worsened atherosclerosis and is stimulated by hyperglycemia in diabetic mice (Nagareddy et al., 2013). These findings have led to the hypothesis that hyperglycemia, in part through increased glucose flux in myeloid cells, contributes to diabetic vascular disease. This concept is in line with some clinical studies, while others have failed to show reduced cardiovascular disease following stricter blood glucose control (Brown et al., 2010). In fact, there is no evidence that the beneficial effects of stricter blood glucose control observed in some human studies are due to direct effects of glucose.
In this study, we tested whether increased glucose flux through glycolysis and the pentose phosphate pathway is sufficient for inflammatory activation of myeloid cells and atherosclerosis, by overexpressing GLUT1 - a glucose transporter which is maximally active at physiological glucose concentrations (Manolescu et al., 2007) - under control of the myeloid-selective CD68 promoter. Our results indicate that increased glucose flux in myeloid cells is insufficient to induce an inflammatory state in these cells and to promote atherosclerosis.
RESULTS AND DISCUSSION
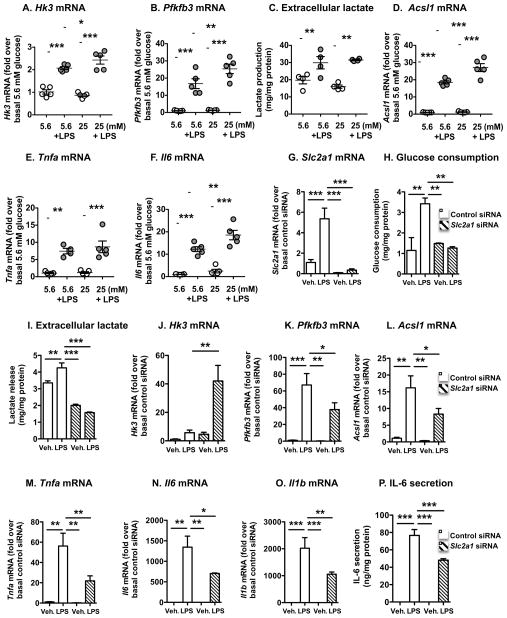
LPS-stimulated bone marrow-derived macrophages (BMDMs) from LDL receptor-deficient (Ldlr−/−) mice exhibited increased mRNA levels of hexokinase (Hk1, Hk2, and Hk3), the first enzyme in glycolysis, which converts glucose to glucose-6-phosphate through an irreversible reaction (Figures 1A and S1A-C). LPS also resulted in increased GLUT1 (Slc2a1) mRNA levels (Figure 1G). Glycolytic flux is controlled by 6-phosphofructo-1-kinase, with fructose 2,6-bisphosphate being its most powerful allosteric activator. The enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3) synthesizes fructose 2,6-bisphospate from fructose 6-phosphate and acts as a strong promoter of glycolysis (Ruiz-García et al., 2011). Accordingly, Pfkfb3 mRNA levels were dramatically upregulated in LPS-stimulated BMDMs (Figure 1B). The effects of LPS on Hk3 and Pfkfb3 were slightly exacerbated in BMDMs differentiated in the presence of 25 mM glucose as compared to those differentiated in normal 5.6 mM glucose. The phosphofructose kinase genes Pfkp and Pfkl were modestly upregulated by LPS, but enzymes downstream of these initial glycolytic steps were not increased (Figures S1D–G). Increased lactate release from LPS-stimulated BMDMs confirmed that LPS stimulates glycolysis (Figure 1C) both in normal glucose concentrations and the high glucose concentrations seen in diabetic mice with accelerated atherosclerosis (Renard et al., 2004). Carkl, which inhibits M1 activation of macrophages and acts to regulate flux through the non-oxidative branch of the pentose phosphate pathway was markedly downregulated by LPS in both normal and high glucose conditions (Figure S1H), consistent with the studies by Haschemi et al. (2012).
Figure 1. LPS-stimulated glycolysis and inflammatory activation are dependent on endogenous GLUT1 in macrophages.
BMDMs from Ldlr−/− mice were used to measure gene expression of enzymes involved in glycolysis and inflammation. (A–B, D–F) BMDMs differentiated in the presence of 5.6 mM or 25 mM glucose for 7 days were stimulated with LPS (5 ng/mL) or vehicle for 6 h. RNA was extracted and subjected to real-time PCR. (C) Lactate levels in conditioned media collected for the time-period 12–24 h after LPS stimulation were measured by a colorimetric kit. (G–P) GLUT1 expression was knocked down by siRNA in BMDMs maintained in 5.6 mM glucose. Changes in mRNA were measured 6 h after LPS stimulation, whereas glucose consumption, lactate and IL-6 secretion were measured 18 h after stimulation. Results are presented as mean ± SEM (n=3–5). Statistical analysis was performed by one-way ANOVA followed by Neuman Keuls post-hoc test. *p<0.05; **p<0.01; ***p<0.001 compared to indicated group. See also Figure S1
As expected, LPS increased expression of inflammatory mediators, such as acyl-CoA synthetase 1 (Acsl1) and the cytokines Tnfa and Il6 (Figures 1D–F). ACSL1 promotes an inflammatory phenotype of monocytes and macrophages, especially in the setting of diabetes, and myeloid ACSL1 is required for diabetes-accelerated atherosclerosis (Kanter et al., 2012). There was a modest stimulatory effect of high glucose on Acsl1 and Il6 mRNA in LPS stimulated macrophages. Thus, LPS-mediated activation of macrophages results in increased expression of key enzymes in glycolysis and inflammatory mediators, consistent with published studies (Vats et al., 2006; O’Neill & Hardie, 2013; Tannahill et al., 2013). There was no consistent synergistic effect of high extracellular glucose. Similar results were obtained in BMDMs differentiated in the presence of mouse recombinant M-CSF, in which the effect of high glucose was only observed for Pfkfb3 (Figures S1I–M).
Increased glycolysis is required for the inflammatory activation of myeloid cells (O’Neill & Hardie 2013; Tannahill et al., 2013), but the role of endogenous GLUT1 is unknown. We therefore knocked down endogenous GLUT1 in BMDMs by siRNA. The Slc2a1 siRNA effectively reduced Slc2a1 mRNA levels, glucose consumption and release of lactate in LPS-stimulated BMDMs (Figures 1G–I), showing that endogenous GLUT1 is required for LPS-induced glycolysis. Furthermore, Hk3 was increased by Slc2a1 siRNA in LPS stimulated BMDMs, while Pfkfb3 mRNA and mRNA levels of inflammatory mediators were reduced in GLUT1-deficient LPS-stimulated BMDMs, as compared to LPS-stimulated siRNA control-treated BMDMs (Figures 1J–O). Consistently, IL-6 secretion was reduced by GLUT1-deficiency (Figure 1P). Together, our results demonstrate that LPS stimulates glycolysis, and that endogenous GLUT1 is required for both the increased glycolysis and the inflammatory effects of LPS.
In order to ask whether GLUT1 and increased glucose flux are sufficient to induce an inflammatory macrophage phenotype, murine GLUT1 was stably overexpressed in the J774 macrophage cell line and in primary mouse BMDMs. GLUT1 has a Km for glucose of 3–7 mM and is therefore maximally active at physiological glucose levels (Manolescu et al., 2007). Because intracellular glucose is rapidly converted into early glycolytic intermediates, intracellular glucose concentrations are much lower (Cline et al., 1998) than that of the extracellular environment, and an increase in the number of GLUT1 molecules on the cell surface necessitates increased glucose uptake.
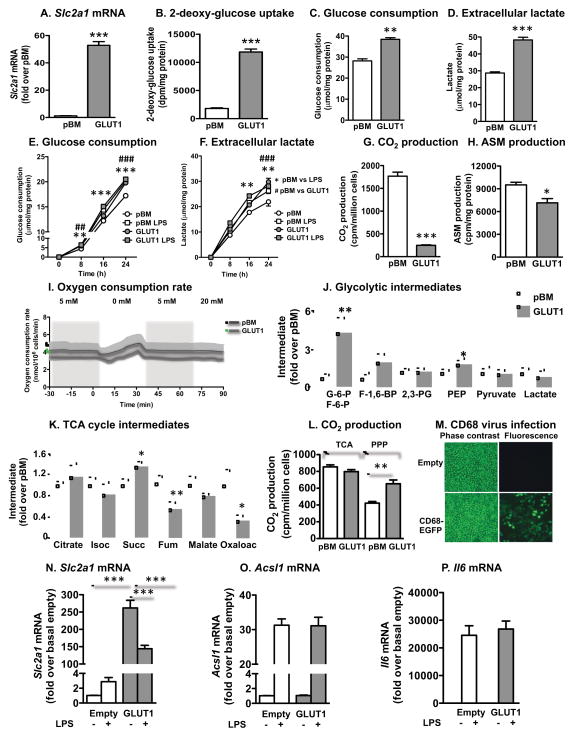
J774 macrophages transduced with the GLUT1 retrovirus exhibited a ~50-fold increase in Slc2a1 (Figure 2A) and increased [3H]-2-DOG uptake (Figure 2B), as compared with cells transduced with the empty pBM vector. The overexpressed GLUT1 is therefore functionally active. Increased glucose flux through glycolysis in GLUT1 overexpressing macrophages was reflected by an increased glucose consumption and lactate release (Figures 2C–D), which were similar to those of LPS-stimulated cells (Figures 2E–F). Thus, overexpression of GLUT1 mimics the increased glucose flux in LPS-stimulated macrophages.
Figure 2. Overexpression of GLUT1 in macrophages mimics the effect of LPS on glycolysis but not inflammation.
GLUT1 was overexpressed in J774 macrophages and BMDMs. (A) Slc2a1 levels were measured by real-time PCR in J774 cells transduced with empty pBM or a vector encoding GLUT1. (B) Glucose uptake was measured as [3H]-2-DOG uptake. (C–F) Glucose consumption and extracellular lactate were measured by colorimetric kits in the same samples. CO2 production (G) and acid-soluble metabolite (ASM) production (H) from [14C]palmitate as measures of β-oxidation. (I) Oxygen consumption was measured in real time under different glucose conditions. (J–K) Glycolytic and TCA cycle intermediates were measured in J774 macrophages by LC/ESI-MS/MS. (L) CO2 production from glucose utilized through the TCA cycle and the pentose phosphate pathway (PPP). (M) BMDMs were transduced with the CD68-EGFP vector or CD68-empty vector. The cells were visualized by phase contrast microscopy and fluorescence microscopy. BMDMs were transduced with CD68-GLUT1 or empty control vector, and levels of Slc2a1 mRNA (N) Acsl1 and Il6 mRNA (O–P) were measured by real-time PCR following 16 h stimulation with and without LPS in the presence of 5.6 mM glucose. Results are presented as mean ± SEM (n=3–9). Statistical analysis was performed by one-way ANOVA followed by Neuman Keuls post-hoc test or two-way ANOVA. *p<0.05; **p<0.01; ***p<0.001; ###p<0.001 compared to indicated group or control. Isoc, isocitrate; Succ, succinate; Fum, fumarate. See also Figure S2
Further analyses of metabolic changes in GLUT1 overexpressing J774 macrophages demonstrated a compensatory reduction in fatty acid β-oxidation (Figures 2G–H) but no significant change in overall oxygen consumption (Figure 2I). LC/ESI tandem mass spectrometric analyses revealed a large increase in the early glycolytic intermediates glucose-6-phosphate and/or fructose-6-phosphate, and a small increase in phosphoenolpyruvate (PEP) in GLUT1 overexpressing cells (Figure 2J). GLUT1 overexpressing macrophages showed no increase in glucose flux through the TCA cycle, but an increase in flux through the pentose phosphate pathway (Figure 2L), again consistent with the effects of LPS in macrophages (Haschemi et al., 2012). Accordingly, only minor differences were observed in TCA cycle intermediates, with a reduction in fumarate and oxaloacetate in GLUT1 overexpressing cells and a small increase in succinate (Figures 2K), the latter mimicking LPS-stimulated macrophages (Tannahill et al., 2013). Acylcarnitines were consistently reduced in GLUT1 overexpressing cells, indicative of reduced mitochondrial activity, whereas unsaturated acyl-CoAs were increased, probably due to reduced fatty acid oxidation (Figure S2A–B). GLUT1 overexpression was not deleterious to the cells; no significant changes in ATP/ADP ratios, ER stress markers, proliferation, or caspase 3 activity, a marker of apoptosis, were observed (Figures S2C–G).
Next, the inflammatory phenotype of GLUT1 overexpressing J774 macrophages and BMDMs was evaluated. GLUT1 was overexpressed using the CD68-GLUT1 vector in BMDMs, which resulted in a high transduction efficiency, as measured by expression of enhanced green fluorescent protein (EGFP) in macrophages transduced with CD68-EGFP (Figure 2M) and a large increase in Slc2a1 (Figure 2N). Increased glucose uptake (approximately 80-fold) was observed in BMDMs transduced with CD68-GLUT1 as compared to those transduced with the CD68-EGFP vector, similar to the J774 macrophages (Figure 2B). LPS induced Acsl1 and Il6 (Figures 2O–P), but GLUT1 overexpression did not affect levels of these mRNAs. Similar results were obtained in J774 macrophages overexpressing GLUT1 (Figures S2H–L). Thus, although GLUT1 overexpression mimics the effects of LPS on glucose flux through glycolysis and the pentose phosphate pathway, it does not mimic the inflammatory effects of LPS in J774 macrophages and mouse BMDMs. These results differ from a recently published study on the RAW264.7 macrophage cell line, in which overexpression of GLUT1 resulted in increased inflammatory activation in vitro (Freemerman et al., 2014). Different cell lines therefore appear to respond differently to GLUT1 overexpression, which makes studies on primary macrophages and myeloid cells in vivo especially important.
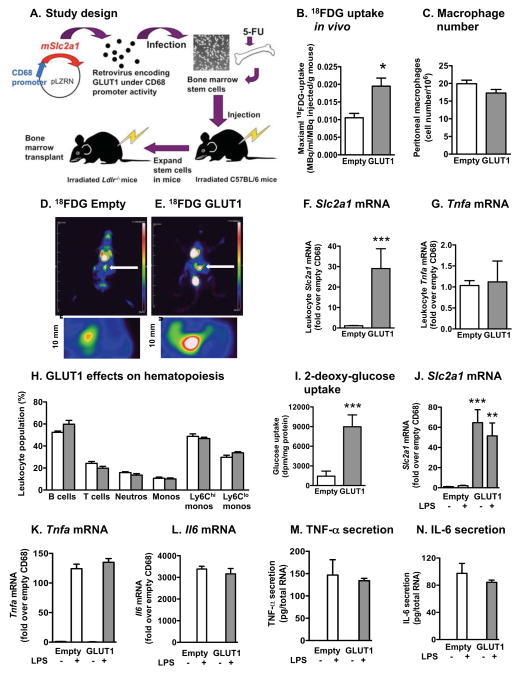
The effect of myeloid cell-specific overexpression of GLUT1 in vivo was evaluated next in Ldlr−/− mice, as shown in Figure 3A. Bone marrow transplants from congenic CD45.1 mice showed that the chimerism was high (>90%) in both the primary and secondary transplanted mice, and that myeloid cell GLUT1 expression did not affect chimerism (Figure S3A–B). The CD68 vector resulted in expression of its target gene in circulating monocytes and neutrophils as well as in macrophages (79.2 ± 4.1% of BMDMs isolated from mice carrying EGFP under control of the CD68 vector were EGFP positive), but not in B and T cells (Figure S3C–D).
Figure 3. Overexpression of GLUT1 in myeloid cells in vivo does not increase their inflammatory phenotype.
(A) Study design. (B–E) 18FDG PET analysis of peritoneal macrophages in Ldlr−/− mice fed the low-fat semipurified diet. Foci of peritoneal macrophages are indicated by white arrows and examples of the analyzed areas are shown in higher magnification under the whole mouse images. At the end of the 30 min image collection, peritoneal macrophages were fixed. The next day, the number of cells was determined by counting (C). Slc2a1 and Tnfa mRNA levels were measured by real-time PCR in leukocytes 22 weeks after bone marrow transplants (F–G). (H) Circulating leukocyte populations (% of total live cells) were analyzed by flow cytometry. Ly6Chi and Ly6Clo monocyte populations are expressed as % of total monocytes. At the end of the 22-week study, thioglycollate-elicited macrophages were collected and adherence purified for 1 h. (I) Glucose uptake was measured by [3H]-2-DOG uptake. (J–L) Slc2a1, Tnfa, and Il6 mRNA levels were measured by real-time PCR. (M–N) Macrophages were stimulated in the presence or absence of LPS for 24 h, and TNF-α and IL-6 secretion was measured by ELISA. Results are presented as mean ± SEM (n=3–5). Statistical analysis was performed by two-tailed unpaired Student’s t-test or one-way ANOVA followed by Neuman Keuls post-hoc test. *p<0.05; **p<0.01; ***p<0.001 compared to empty control virus. See also Figure S3
In order to verify that macrophage glucose uptake is increased by CD68-GLUT1 expression in vivo, 18F-flurodeoxyglucose (18FDG) positron emission tomography (PET) was used. Peritoneal macrophages were elicited by thioglycollate in mice with CD68-empty and CD68-GLUT1 bone marrow, and 18FDG uptake was measured in the peritoneal cavity 5 days later. Foci in the peritoneal cavity in CD68-GLUT1 mice showed significantly elevated 18FDG uptake, as compared to CD68-empty mice. The number of peritoneal macrophages was similar (Figures 3B–E). These results demonstrate that CD68-GLUT1 results in increased glucose uptake in macrophages in vivo.
At the end of the 22-week study, Slc2a1 mRNA levels in circulating myeloid cells were elevated by 30-fold, whereas Tnfa was not increased (Figures 3F–G). Myeloid GLUT1 overexpression also had no effect on hematopoiesis (Figure 3H). Furthermore, myeloid-targeted expression of GLUT1 did not affect blood cholesterol or glucose levels, glucose tolerance, or plasma levels of lactate, triglycerides or IL-6 (Figures S3E–J). Peritoneal macrophages from the same mice exhibited large increases in glucose uptake and Slc2a1 mRNA (Figures 3I–J), but no increase in TNF-α or IL-6 (Figures 3K–N). Thus, increased glucose flux due to GLUT1 overexpression is insufficient to promote an inflammatory myeloid cell phenotype.
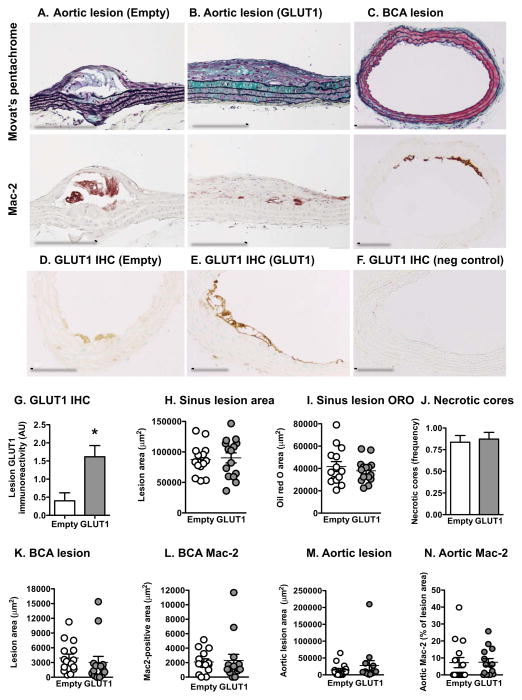
Consistent with the lack of effect of GLUT1 on inflammatory mediators, no effect of myeloid GLUT1 overexpression was observed on atherosclerotic lesion size in the aortic sinus, aorta or brachiocephalic artery (BCA). Lesion neutral lipid content, measured as oil red O staining, and macrophage accumulation, measured as Mac-2-positive lesion area, also were no different between the groups (Figures 4A–C, H–I, and K–N). Lesion severity was greatest in the aortic sinus, where the lesions contained necrotic cores, fibrous areas and cholesterol clefts (Figure S4A–C). Aortic lesions, especially in the lesser curvature of the arch were of an intermediate maturity, containing fibrous caps (Figure 4A–B). The BCA lesions were early fatty streak, consisting primarily of macrophages (Figure 4C). Myeloid cell GLUT1 expression had no effect on lesion severity in any of these sites, including necrotic cores and intraplaque hemorrhage (Figures 4J and S4D). Lesion macrophages from mice with GLUT1 overexpressing myeloid cells exhibited intense GLUT1 immunoreactivity whereas lesion macrophages from control mice had weak GLUT1 immunoreactivity (Figures 4D–G).
Figure 4. Overexpression of GLUT1 in myeloid cells does not increase atherosclerosis.
(A–B) The aorta was embedded longitudinally and sections were stained using a Movat’s pentachrome stain or an anti-Mac-2 antibody. Representative longitudinal sections are shown. (C) The maximal lesion site in the BCA was identified, and this site was used for Movat’s pentachrome stain and Mac-2 and GLUT1 immunohistochemitry. Representative cross sections from a CD68-GLUT1 mouse are shown. (D–G) GLUT1 immunoreactivity in lesion macrophages. (F) Negative controls showed no immunoreactivity. Sinus lesion area (H) and oil red O-positive area (I) were quantified on five cross sections and measured by image analysis. (J) The presence of necrotic cores in sinus lesions were scored in a blinded bi-modal fashion and expressed as mean frequency. (K) BCA lesion area was quantified at the maximal lesion area site. (L) Mac-2 positive area was quantified in sections adjacent to those in (K). (M–N) Aortic lesion area and Mac-2-positive lesion area were quantified. Results are presented as mean ± SEM (n=14–15). See also Figure S4
Together, our studies show that increased glucose flux selectively in myeloid cells is insufficient for increased inflammatory activity and atherosclerosis. These studies address the role of increased glucose flux in any specific cell population involved in atherosclerosis, and are of high relevance for our understanding of atherosclerosis associated with diabetes and other inflammatory states. Several hyperglycemic mouse models exhibit accelerated atherosclerosis (Bornfeldt & Tabas 2011) due to increased macrophage accumulation in the vessel wall (Renard et al., 2004). The role of increased glucose exposure of myeloid cells has been addressed in in vitro studies, many of which demonstrate that elevated glucose levels enhance production of cytokines (reviewed in Nishizawa & Bornfeldt 2012 and Bornfeldt & Tabas 2011). It has therefore long been hypothesized that increased glucose levels promote cytokine release from macrophages in vivo and as a consequence, promotes atherosclerosis. The present study clearly demonstrates that increased glucose flux in myeloid cells is insufficient to promote atherosclerosis, at least under the conditions used in this study. Although GLUT1 overexpression had no effect on lesions of different severity at three different sites, it is possible that other conditions, such as a high fat diet, might have revealed an effect. In addition, glucose might have important effects on other vascular cells, on other populations of bone marrow-derived cells, or extracellular glucose could exert pro-atherosclerotic effects, for example through formation of advanced glycation endproducts, because glucose lowering in diabetic mice prevents the detrimental effects of diabetes on atherosclerosis regression (Nagareddy et al., 2013) and GLUT1 overexpression in smooth muscle cells results in increased recruitment of neutrophils to sites of vascular injury (Adhikari et al., 2011).
One caveat is that our studies are based on mouse models. In patients with diabetes, multiple other metabolic arrangements independent of or indirectly related to glucose levels could contribute to an increased inflammatory myeloid cell phenotype and atherosclerosis. Indeed, in preliminary studies we noted that ACSL1 (a marker of inflammatory activation) gene expression was modestly increased in CD14+ monocytes from patients with type 1 diabetes as compared to matched controls (p<0.05; n=14), consistent with previous studies (Kanter et al., 2012). There was no correlation between ACSL1 mRNA and the glycolytic mediators PFKFB3 and SLC2A1 in these cells (p>0.10). Thus, there appears to be a disconnect between diabetes-induced myeloid cell inflammation and glycolysis in human and mouse myeloid cells. Further studies are needed to identify the mechanism of diabetes-induced activation of myeloid cells.
In summary, these studies demonstrate the role of increased glucose flux in a specific cell type with a key role in atherosclerosis, and set the stage for future studies to systematically investigate the role of glucose flux in cell types involved in this disease. Our findings suggest that increased glycolysis is required, but is not sufficient, for inflammatory activation of myeloid cells and atherosclerosis. These findings have important implications for studies on diabetic vascular disease; a multifactorial disease in which the role of glucose is still uncertain.
EXPERIMENTAL PROCEDURES
Generation of retroviral vectors for overexpression of GLUT1 targeted to myeloid cells
Murine GLUT1 cDNA was cloned into the pBM-IRES-PURO (pBM) vector or the pLZRS-SIN-CD68S (CD68) vector, as previously described (Gough & Raines, 2003). Phoenix ecotropic cells were used to generate retrovirus. The pLZRS-SIN-CD68S vector was used to overexpress GLUT1 or EGFP specifically in myeloid cells in vivo, following transduction of bone marrow stem cells.
Atherosclerosis studies
Myeloid cell-selective overexpression of GLUT1 or EGFP in Ldlr−/− mice was achieved by transducing bone marrow stem cells with the CD68-GLUT1 or CD68-EGFP or CD68-empty control) retroviral vectors. Briefly, the transduced cells were injected into irradiated 8–10 week-old male C57BL/6 mice. The mice were allowed to recover for 7 weeks to reconstitute bone marrow. Bone marrow cells were then harvested and transplanted to irradiated 8–10 week-old female Ldlr−/− mice (Jackson laboratory; Bar Harbor, ME). After a 7-week recovery period, the Ldlr−/− mice were fed a low-fat semipurified diet described previously (Kanter et al., 2012) for 22 weeks. At the end of the study, blood was collected and mice were perfusion-fixed under physiological pressure (Renard et al., 2004). A subset of mice was injected intraperitoneally with thioglycollate for isolation of peritoneal macrophages (Kanter et al., 2012). Atherosclerotic lesions in the aorta, BCA and aortic sinus were evaluated. The sinus was cryosectioned, and oil red O staining was used to evaluate lesion neutral lipid accumulation. The same sections were then used for Movat’s pentachrome stain. The BCA was paraffin embedded and serial sectioned (Kanter et al., 2012), and adjacent sections were used for Movat’s pentachrome stain and immunohistochemistry for Mac-2 or GLUT1. Aortas were sectioned longitudinally and were used for lesion analysis and Mac-2 immunohistochemistry. All measurements were done in a masked fashion.
Extended Methods can be found in Supplemental Materials.
Supplementary Material
HIGHLIGHTS.
Endogenous GLUT1 expression is required for inflammatory activation of macrophages
Increased glucose flux in myeloid cells is insufficient for inflammatory activation
Increased glucose flux in myeloid cells is insufficient for atherosclerosis
Acknowledgments
We thank Greg Garwin and Barbara Lewellen for help with PET imaging. Research reported in this publication was supported by the following NIH awards: R01HL062887, P01HL092969, R01HL097365 (KEB), P01HL018645, R01HL081795, R01HL067267 (EWR), U24DK097153 (SP), R33DK069801 (SD), by the Diabetes Research Center (P30DK017047) and the Nutrition Obesity Center (P30DK035816) at the University of Washington, and the Molecular Phenotyping Core, Michigan Nutrition and Obesity Center (P30DK089503). JEK was supported in part by The Dick and Julia McAbee Endowed Fellowship in Diabetes Research Fellowship from the DRC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z, Lehman U, Mullegama S, Weir EK, Hall JL. Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2011;31:86–94. doi: 10.1161/ATVBAHA.110.215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- Cline GW, Jucker BM, Trajanoski Z, Rennings AJ, Shulman GI. A novel 13C NMR method to assess intracellular glucose concentration in muscle, in vivo. Am J Physiol. 1998;274(2 Pt 1):E381–E389. doi: 10.1152/ajpendo.1998.274.2.E381. [DOI] [PubMed] [Google Scholar]
- Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: glucose transporter (GLUT1)-mediated glucose metabolism drives a pro- inflammatory phenotype. J Biol Chem. 2014 Feb 3; doi: 10.1074/jbc.M113.522037. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Westerterp M, Bhagwat N, Cremers S, Shih A, Abdel-Wahab O, Lütjohann D, Randolph GJ, Levine RL, Tall AR, et al. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med. 2013;210:339–353. doi: 10.1084/jem.20121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PJ, Raines EW. Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci USA. 2012;109:E715–E724. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology. 2007;22:234–240. doi: 10.1152/physiol.00011.2007. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T, Bornfeldt KE. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann Med. 2012;44:555–563. doi: 10.3109/07853890.2011.585346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-García A, Monsalve E, Novellasdemunt L, Navarro-Sabaté A, Manzano A, Rivero S, Castrillo A, Casado M, Laborda J, Bartrons R, et al. Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem. 2011;286:19247–19258. doi: 10.1074/jbc.M110.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruda T, Hatakeyama K, Nagamachi S, Sekita Y, Sakamoto S, Endo GJ, Nishimura M, Matsuyama M, Yoshimura K, Sato Y, et al. Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arterioscler Thromb Vasc Biol. 2012;32:1410–1417. doi: 10.1161/ATVBAHA.111.237065. [DOI] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.