Abstract
Background and Purpose
Plasminogen Activator Inhibitor-I (PAI-1), a ~50 kDa serine protease inhibitor, markedly reduces the extravascular toxicity of tissue-type plasminogen activator (tPA) in experimental hypoxic-ischemic (HI) brain injury of newborns. However, the current treatment with PAI-1 requires intracerebroventricle (ICV) injection to cross blood-brain-barrier (BBB), which is an invasive procedure of limited clinical potential. Thus, we tested whether intranasal administration of PAI-1 can bypass BBB and mitigate neonatal HI brain injury.
Method
Rat pups were subjected to HI, with or without lipopolysaccharide (LPS) pre-exposure, followed by intranasal delivery of a stable-mutant form of PAI-1 (CPAI).
Results
Immunoblotting showed that CPAI sequentially entered the olfactory bulbs and cerebral cortex following intranasal delivery and reduced ~75% of brain atrophy in HI or LPS-sensitized HI injury. Mechanistically, CPAI attenuated HI-induced plasminogen activators and LPS/HI-induced NF-κB signaling, neuroinflammation, and BBB permeability.
Conclusions
Intranasal delivery of CPAI is an effective treatment of experimental HI brain injury of newborns. Clinical application of this experimental therapy merits further investigation.
INTRODUCTION
High concentrations of tissue-type plasminogen activator (tPA) in the brain parenchyma has multiple detrimental effects, ranging from hemorrhagic transformation during thrombolysis therapy to tissue proteolysis in neonatal hypoxia-ischemia (HI).1-3 ICV-injection of CPAI, a stable-mutant form of PAI-1 with 72-fold longer half-life against tPA, reduces HI and lipopolysaccharide (LPS)-sensitized HI brain injury in neonatal rats.3-6 As such, CPAI is a potential therapeutics of neonatal encephalopathy. However, current administration of CPAI relies on ICV injection to bypass blood-brain-barrier (BBB) and avoid the adverse effect of anti-fibrinolysis in the blood. This invasive procedure is unsuited for broad clinical application. To overcome this obstacle, we tested whether CPAI can enter the brain by intranasal delivery and oppose neonatal HI injury. Intranasal delivery is a powerful method to deliver chemicals and peptides to the brain, but whether it supports larger protein across BBB remains unpredictable.7-9 Our results showed that intranasally administered CPAI enters the brain and mitigates HI injury with and without LPS-sensitization. These results suggest a novel therapy of neonatal encephalopathy and possible other conditions of tPA toxicity.
MATERIAL AND METHODS
Animal surgery and intranasal delivery
The Rice-Vannucci model of neonatal HI, with or without LPS sensitization, was performed in 7-day-old Wistar rats of both sexes in an approximately equal ratio as previously described.3,4,6 Intranasal delivery of CPAI (Molecular Innovations, Novi, Michigan) was performed at 30 or 120 min after HI as described.7,9 Briefly, rat pups were anesthetized and put on their backs on a heating pad (38°C). 2 μl CPAI (0.24 μg /μL) dissolved in PBS (with 4.2% endotoxin-free DMSO, Sigma D2650) was applied to both nares, alternating at 2 min-interval for a total 12 μL, via a Hamilton syringe connected with PE10 polyethylene tubing. Male and female pups showed no difference in response to HI injury or the CPAI treatment, and therefore the data were combined. Experimental procedures were approved by the Institutional Animal Care and Use Committee and in compliance with the ARRIVE guidelines (Animals in Research: Reporting In-vivo Experiments).
Biochemistry
Electrophoretic mobility shift assay (EMSA) for NF-κB was performed using a commercial kit (Lightshift chemiluminescent kit; Thermo Scientific, Waltham, MA). Plasminogen and gelatin zymograms were performed as described.3,4
RT-quantitative PCR
The mRNA extraction, cDNA preparation, and quantitative PCR were performed as described.10 The qPCR of rat IL-6, Ccl-2, and Tspo, cDNAs were detected based on SYBR green using the following primers after normalization to the level of β-actin. IL-6: 5′-GGAGAGGAGACTTCACAGAGGAT-3′, 5′-AGTGCATCATCGCTGTTCATAC-3′; Ccl-2: 5′-ACCACTATGCAGGTCTCTGTCAC-3′, 5′-GCTGCTGGTGATTCTCTTGTAGT-3′; Tspo: 5′-CTATGGTTCCCTTGGGTCTCTAC-3′, 5′-AGGCCAGGTAAGGATACAGCAAG-3′;
2,3,5-triphenyltetrazolium chloride (TTC) stain and BBB permeability assay
In-vivo TTC stain was performed as described.10 The BBB permeability was determined based on extravasation of 5% sodium fluorescence (NaF) as described.4
Statistical analysis
Values are represented as mean ± SD or mean ± SEM when n > 9. Quantitative data were compared using two-sample (unpaired) t-test assuming equal variance.
RESULTS
Intranasally applied CPAI enters the brain within 2 hours
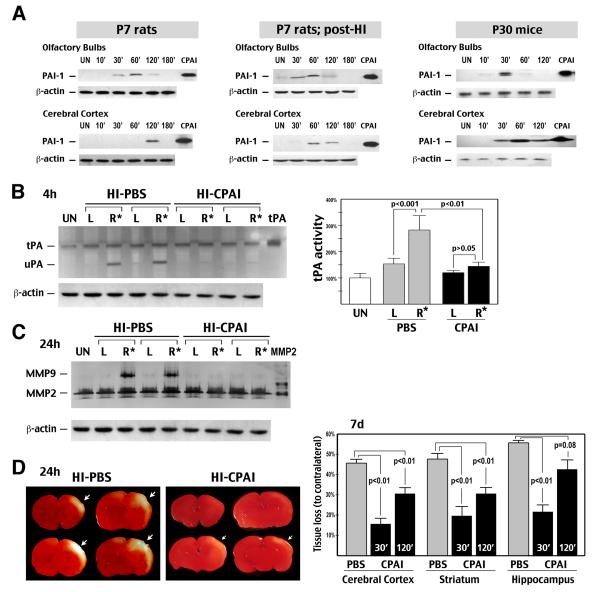
To assess the kinetics of brain-delivery, CPAI was applied intranasally to 7-day-old rats, with and without HI, or 30-day-old mice. Animals were sacrificed 10 to 180 min later for immunoblot detection of CPAI in the olfactory bulbs and cerebral cortex. In uninjured P7 rats, the presence of CPAI peaked around 60 min in the olfactory bulbs and 120 min in the cerebral cortex. In HI-injured P7 rats, the delivery of CPAI to the cerebral cortex was accelerated for an hour. P30 mice showed an even faster transport of CPAI to the olfactory bulbs (peak at 30 min) and cerebral cortex (starting at 30 min) (Figure 1A). Importantly, CPAI was undetectable (<12 pg/ml) in the blood following intranasal application. These results confirmed brain distribution of CPAI by intranasal delivery and suggested that this drug delivery method would not compromise the fibrinolytic capacity in blood.
Figure 1.
Intranasal delivery of CPAI mitigates experimental HI brain injury of newborns. A, Immunoblotting showed that CPAI sequentially entered the olfactory bulbs and cerebral cortex after intranasal delivery in unchallenged P7 rats (left), HI-injured P7 rats (middle), and P30 mice. UN: untouched animals. B, Plasminogen zymogram showed that intranasal administration of CPAI prevented HI-induced tPA and uPA activities at 4 h recovery. (Left) Two representative samples of 6 rats. (Right) Quantification of the tPA activity. R*: the HI-injured hemisphere. C, Zymography showed MMP-9 activation at 24 h post-HI was blocked by intranasal delivery of CPAI (n=6). D, (Left) Representative TTC-stained brain slices in animals that received intranasal delivery of saline (PBS with 4.2% DMSO) or CPAI treatment within 30 min post-HI and sacrificed at 24 h recovery. At this time-point, HI-induced infarction was incomplete and had not converted to tissue loss. (Right) Quantification at 7 d recovery showed reduction neural tissue loss in different brain regions by intranasal delivery of CPAI at 30 or 120 min post-HI (n=18 for PBS-treatment, n=9 for each CPAI treatment. Indicated were the p-value determined by t-test).
Intranasal delivery of CPAI inhibits plasminogen activators and HI brain damage
Next, we examined the effects of intranasal delivery of 2.85 μg CPAI in the Rice-Vannucci HI model in P7 rats. Compared to the saline (PBS with 4.2% DMSO) treatment, CPAI significantly decreased tPA and the urinary-type plasminogen activator (uPA) activities in the carotid-ligated hemisphere (the right side, R*) at 4 h, and blocked the induction of matrix metalloproteinase-9 (MMP-9) at 24 h recovery (Figure 1B, C, n=6 for each). Similarly, triphenyltetrazolium chloride (TTC) stain showed a larger infarcted area in animals receiving PBS treatment than those received CPAI treatment at 24 h recovery (Figure 1D; n=4). By 7 d post-HI, the PBS-treated rats developed 45% tissue loss in the cerebral cortex, 49% in the striatum, and 54% in the hippocampus (Fig. 1D, n=18). When intranasal delivery of CPAI was initiated within 30 min recovery, the extent of tissue loss dropped to 16% in the cerebral cortex, 19% in the striatum, and 22% in the hippocampus (all p <0.01; n=9). If intranasal CPAI was delivered at 2 h post-HI, tissue loss was 31% in the cerebral cortex (p<0.01), 31% in the striatum (p<0.01), and 45% in the hippocampus (p=0.08) (n=9). These results showed that intranasal delivery of CPAI mitigates HI-induced damage in the cerebral cortex and striatum with an at-least 2-hr therapeutic window.
Intranasally applied CPAI blocks LPS/HI-induced neuroinflammation and brain atrophy
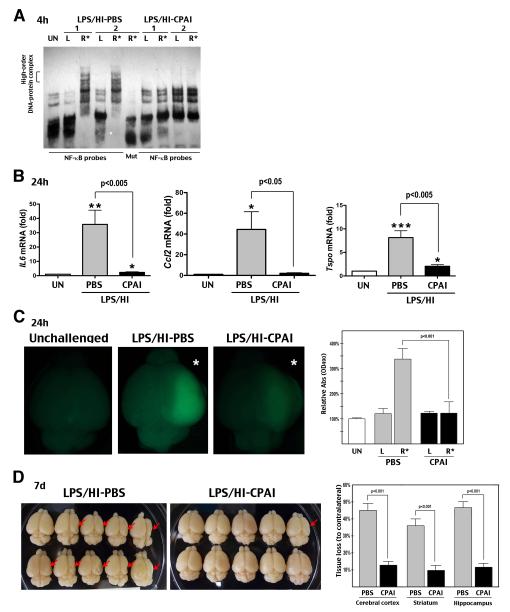
Previous studies have shown that 4 h pre-exposure to low-dose LPS (0.3 mg/kg, IP) attenuates HI-induced plasminogen activators but enhances NF-κB signaling and microglia activation.4 We found that intranasal delivery of 2.85 μg CPAI inhibited the LPS/HI-induced NF-κB activity at 4 h (Figure 2A, n > 6) and IL-6, Ccl-2 (also called Mcp-1), and Tspo (translocator protein of 18 kDa, a marker of activated microglia) transcripts at 24 h (Figure 2B, n=7-8). These results suggested a strong anti-neuroinflammatory effect by intranasal delivery of CPAI. Similarly, PBS-treated rats showed increased BBB permeability, as shown by extravasation of sodium fluorescein (NaF), in the LPS/HI-injured hemisphere at 24 h recovery, whereas animals receiving intranasal CPAI delivery exhibited much less NaF fluorescence (Figure 2C, n=5).
Figure 2.
Intranasal delivery of CPAI mitigates LPS-sensitized HI brain injury of newborns. A, EMSA showed that intranasal delivery of CPAI blocked the acute nuclear NF-κB activity at 4 h following LPS/HI injury. Mut: mutant NF-kB probe. B, RT-qPCR showed significant reduction of IL-6, Ccl-2 (Mcp-1), and Tspo mRNAs at 24 h recovery following intranasal delivery of CPAI (n=7-8). *: p< 0.05, **: p<0.01, ***: p<0.001 compared to the unchallenged group. C, (Left) Representative brain photographs of LPS/HI-injured rat pups receiving the indicated therapy and IP injection of sodium fluorescein (NaF) followed by saline perfusion at 24 h recovery. R*: the HI-challenged hemisphere. (Right) Quantification showed significant reduction of NaF fluorescence, an indicator of BBB permeability, by intranasal delivery of CPAI (n=5). D, (Left) Representative brain specimens at 7 d after LPS/HI insult in animals receiving intranasal delivery of saline (PBS) or CPAI. (Right) Quantification of regional brain atrophy showed significant reduction by intranasal application of CPAI over PBS (n=14-15 for each group).
Finally, we tested the effect of intranasal delivery of CPAI on LPS/HI-induced brain atrophy. At 7 d recovery, saline-treated rats developed 45% tissue loss in the cerebral cortex, 46% in the hippocampus, and 36% in the striatum. By contrast, intranasal delivery of 2.85 μg CPAI within 30 min after LPS/HI insult reduced tissue loss to 12% in the cerebral cortex, 10% in striatum, and 11% in hippocampus, indicating ~75% reduction of brain atrophy (Figure 2D, all p<0.001; n=14-15).
DISCUSSION
Hypoxic-ischemic encephalopathy (HIE) is an important neurological disorder of neonates. While therapeutic hypothermia is beneficial, there remains a need for better therapies of severe HIE and infection-sensitized HIE.11 In preclinical study, HI with pre-exposure to low-dose LPS mimics infection-sensitized HIE and produces greater brain damage.4,6 The stable-mutant form of PAI-1 (CPAI) is one of the few therapeutics that reduce both HI and LPS/HI brain injury.3-5 Furthermore, it inhibits multiple mechanisms of tPA neurotoxicity, ranging from tissue proteolysis to microglia activation.3,4 With such diverse mechanisms, CPAI is an attractive therapeutics of HIE and possibly other tPA-related pathologies.
Clinical application of CPAI in neurological disorders, however, is hampered by the need of ICV or subarachnoid injection in current delivery strategies. Due to its large molecular weight (43 kDa), CPAI fails to cross the BBB but would decrease the fibrinolytic capacity in blood following intravenous injection. In the present study, we showed that intranasal delivery of CPAI overcomes this obstacle and protects neonatal HI and LPS/HI injuries similar to the effect of ICV-injection.3,4 The efficient nose-to-brain drug delivery method, mediated in part by the continuous open channels formed by olfactory ensheathing cells that facilitate protein diffusion into the cerebrospinal fluid space, has been used to apply insulin to the brain in Alzheimer’s disease patients.8,12 In this study, we confirmed the entry of CPAI in the brain following intranasal delivery by immunoblots. Further, we showed that intranasal delivery of CPAI blocks HI-induced plasminogen activators, as well as, LPS/HI-induced NF-κB signaling, pro-inflammatory cytokine, and BBB disruption. Altogether, this therapeutic strategy confers up to 75% reduction of brain atrophy. These results suggest intranasal delivery of CPAI is an effective, non-invasive therapy of neonatal HI brain injury with and without infection sensitization.
Finally, limited by the scope of a brief report, our study only used 7-day histopathology as the endpoint for analysis. Future studies on the dose-response, extended therapeutic window, and long-term behavioral outcomes are warranted to assess clinical potential of this novel anti-tPA strategy.
ACKNOWLEDGMENTS/SOURCES OF FUNDING:
This work was supported by the National Institute of Health grants (NS074559 to C.K.; HL55374 and NS079639 to D.A.L.) and an American Heart Association fellowship (to D.Y.).
Footnotes
DISCLOSURES: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–30. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 3.Yang D, Nemkul N, Shereen A, Jone A, Dunn RS, Lawrence DA, et al. Therapeutic administration of plasminogen activator inhibitor-1 prevents hypoxic-ischemic brain injury in newborns. J Neurosci. 2009;29:8669–8674. doi: 10.1523/JNEUROSCI.1117-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang D, Sun YY, Nemkul N, Baumann JM, Shereen A, Dunn RS, et al. Plasminogen Activator Inhibitor-1 Mitigates Brain Injury in a Rat Model of Infection-Sensitized Neonatal Hypoxia-Ischemia. Cereb Cortex. 2013;23:1218–29. doi: 10.1093/cercor/bhs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkenpas MB, Lawrence DA, Ginsburg D. Molecular evolution of plasminogen activator inhibitor-1 functional stability. EMBO J. 1995;14:2969–2977. doi: 10.1002/j.1460-2075.1995.tb07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 7.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 8.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D, Sun YY, Lin X, Baumann JM, Dunn RS, Lindquist DM, et al. Intranasal delivery of cell-penetrating anti-NF-kB peptides (Tat-NBD) alleviates infection-sensitized hypoxic-ischemic brain injury. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.01.015. Epub ahead of print (PMID 23353638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun YY, Yang D, Kuan CY. Mannitol-facilitated perfusion staining with 2,3,5- triphenyltetrazolium chloride (TTC) for detection of experimental cerebral infarction and biochemical analysis. J Neurosci Methods. 2012;203:122–129. doi: 10.1016/j.jneumeth.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wintermark P, Boyd T, Gregas MC, Labrecque M, Hansen A. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am J Obstet Gynecol. 2010;203:579, e571–579. doi: 10.1016/j.ajog.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005;52:245–251. doi: 10.1002/glia.20241. [DOI] [PubMed] [Google Scholar]