Abstract
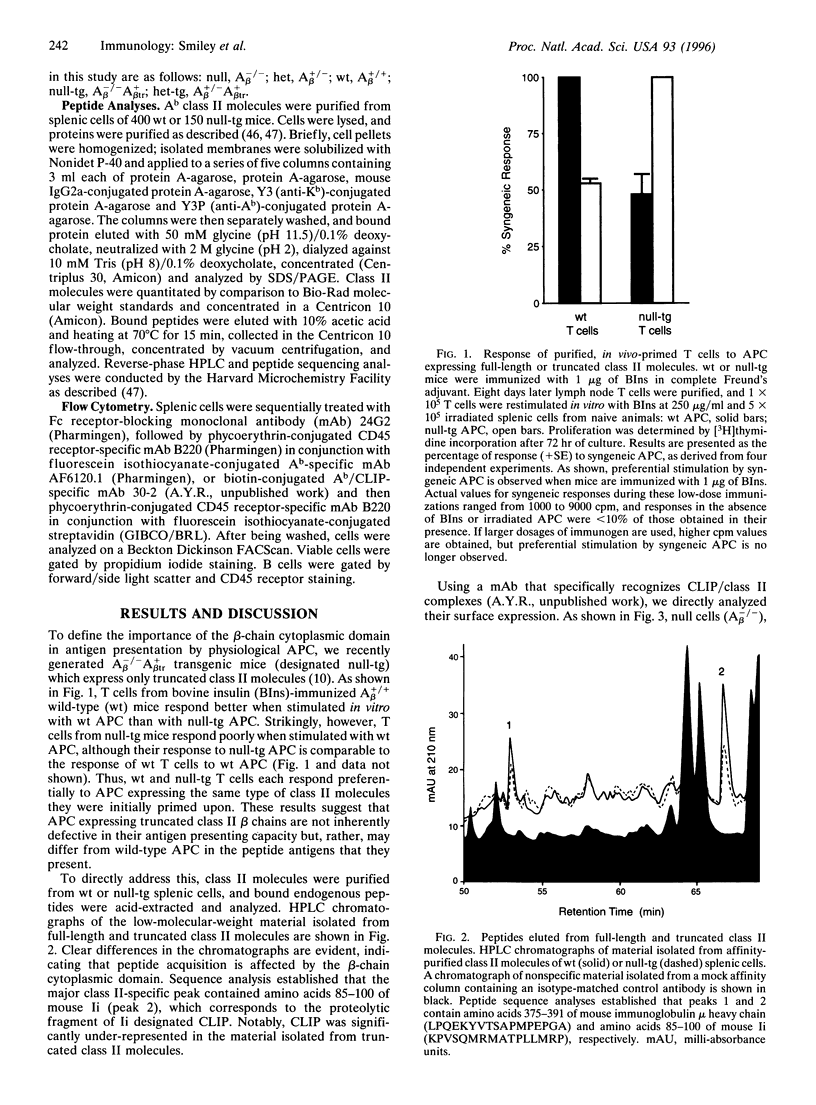
Previous studies have established that antigen presenting cells (APC) expressing major histocompatibility complex class II beta chains with truncated cytoplasmic domains are impaired in their capacity to activate T cells. While it had been widely accepted that this impairment is due to a defect in class II cytoplasmic domain-dependent signal transduction, we recently generated transgenic mice expressing only truncated class II beta chains, and functional analyses of APC from these mice revealed signaling-independent defects in antigen presentation. Here, we demonstrate that T cells primed on such transgenic APC respond better to stimulation by APC expressing truncated beta chains than by wild-type APC. This finding suggests that APC expressing truncated class II beta chains are not inherently defective in their antigen presenting capacity but, rather, may differ from wild-type APC in the peptide antigens that they present. Indeed, analysis of the peptides bound to class II molecules isolated from normal and transgenic spleen cells revealed clear differences. Most notably, the level of class II-associated invariant chain-derived peptides (CLIP) is significantly reduced in cells expressing only truncated beta chains. Prior studies have established that CLIP and antigenic peptides compete for binding to class II molecules. Thus, our results suggest that the cytoplasmic domain of the class II beta chain affects antigen presentation by influencing the level of CLIP/class II complexes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Amigorena S., Webster P., Drake J., Newcomb J., Cresswell P., Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995 May 1;181(5):1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. S., Miller J. Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2282–2286. doi: 10.1073/pnas.89.6.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990 Nov 16;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Barnes K. A., Mitchell R. N. Detection of functional class II-associated antigen: role of a low density endosomal compartment in antigen processing. J Exp Med. 1995 May 1;181(5):1715–1727. doi: 10.1084/jem.181.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar S., Ostrand-Rosenberg S., Nabavi N., Nadler L. M., Freeman G. J., Glimcher L. H. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F., Germain R. N. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995 Jan;2(1):73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Ceman S., Rudersdorf R., Long E. O., Demars R. MHC class II deletion mutant expresses normal levels of transgene encoded class II molecules that have abnormal conformation and impaired antigen presentation ability. J Immunol. 1992 Aug 1;149(3):754–761. [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Denzin L. K., Robbins N. F., Carboy-Newcomb C., Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994 Oct;1(7):595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Arp B., Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994 Apr 7;368(6471):554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- Freisewinkel I. M., Schenck K., Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9703–9706. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A. M., Pearson C., Quinn V., McDevitt H. O., Milburn P. J. Binding of an invariant-chain peptide, CLIP, to I-A major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):335–339. doi: 10.1073/pnas.92.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Harton J. A., Bishop G. A. Length and sequence requirements of the cytoplasmic domain of the A beta molecule for class II-mediated B cell signaling. J Immunol. 1993 Nov 15;151(10):5282–5289. [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Layet C., Germain R. N. Invariant chain promotes egress of poorly expressed, haplotype-mismatched class II major histocompatibility complex A alpha A beta dimers from the endoplasmic reticulum/cis-Golgi compartment. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2346–2350. doi: 10.1073/pnas.88.6.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992 Jun 26;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Malcherek G., Gnau V., Jung G., Rammensee H. G., Melms A. Supermotifs enable natural invariant chain-derived peptides to interact with many major histocompatibility complex-class II molecules. J Exp Med. 1995 Feb 1;181(2):527–536. doi: 10.1084/jem.181.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Yamamoto E. M., Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994 Aug;126(4):991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins E., Cameron P., Amaya M., Goodman S., Pious D., Smith L., Arp B. A mutant human histocompatibility leukocyte antigen DR molecule associated with invariant chain peptides. J Exp Med. 1994 Feb 1;179(2):541–549. doi: 10.1084/jem.179.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins E., Smith L., Arp B., Cotner T., Celis E., Pious D. Defective processing and presentation of exogenous antigens in mutants with normal HLA class II genes. Nature. 1990 Jan 4;343(6253):71–74. doi: 10.1038/343071a0. [DOI] [PubMed] [Google Scholar]
- Morris P., Shaman J., Attaya M., Amaya M., Goodman S., Bergman C., Monaco J. J., Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994 Apr 7;368(6471):551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Ghogawala Z., Myer A., Griffith I. J., Wade W. F., Chen Z. Z., McKean D. J., Glimcher L. H. Antigen presentation abrogated in cells expressing truncated Ia molecules. J Immunol. 1989 Mar 1;142(5):1444–1447. [PubMed] [Google Scholar]
- Odorizzi C. G., Trowbridge I. S., Xue L., Hopkins C. R., Davis C. D., Collawn J. F. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994 Jul;126(2):317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S., Roby C. A., Clements V. K. Abrogation of tumorigenicity by MHC class II antigen expression requires the cytoplasmic domain of the class II molecule. J Immunol. 1991 Oct 1;147(7):2419–2422. [PubMed] [Google Scholar]
- Pieters J., Bakke O., Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993 Nov;106(Pt 3):831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- Riberdy J. M., Avva R. R., Geuze H. J., Cresswell P. Transport and intracellular distribution of MHC class II molecules and associated invariant chain in normal and antigen-processing mutant cell lines. J Cell Biol. 1994 Jun;125(6):1225–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberdy J. M., Cresswell P. The antigen-processing mutant T2 suggests a role for MHC-linked genes in class II antigen presentation. J Immunol. 1992 Apr 15;148(8):2586–2590. [PubMed] [Google Scholar]
- Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992 Dec 3;360(6403):474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990 Jun 14;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Marks M. S., Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991 Dec 5;354(6352):392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rudensky A. Y., Maric M., Eastman S., Shoemaker L., DeRoos P. C., Blum J. S. Intracellular assembly and transport of endogenous peptide-MHC class II complexes. Immunity. 1994 Oct;1(7):585–594. doi: 10.1016/1074-7613(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sanderson F., Kleijmeer M. J., Kelly A., Verwoerd D., Tulp A., Neefjes J. J., Geuze H. J., Trowsdale J. Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science. 1994 Dec 2;266(5190):1566–1569. doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- Schafer P. H., Pierce S. K. Evidence for dimers of MHC class II molecules in B lymphocytes and their role in low affinity T cell responses. Immunity. 1994 Nov;1(8):699–707. doi: 10.1016/1074-7613(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Sette A., Ceman S., Kubo R. T., Sakaguchi K., Appella E., Hunt D. F., Davis T. A., Michel H., Shabanowitz J., Rudersdorf R. Invariant chain peptides in most HLA-DR molecules of an antigen-processing mutant. Science. 1992 Dec 11;258(5089):1801–1804. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- Sette A., Southwood S., Miller J., Appella E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J Exp Med. 1995 Feb 1;181(2):677–683. doi: 10.1084/jem.181.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Dunbrack R. L., Jr, Lee S., Strominger J. L. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J Biol Chem. 1991 Jun 5;266(16):10658–10665. [PubMed] [Google Scholar]
- Smiley S. T., Laufer T. M., Lo D., Glimcher L. H., Grusby M. J. Transgenic mice expressing MHC class II molecules with truncated A beta cytoplasmic domains reveal signaling-independent defects in antigen presentation. Int Immunol. 1995 Apr;7(4):665–677. doi: 10.1093/intimm/7.4.665. [DOI] [PubMed] [Google Scholar]
- St Pierre Y., Watts T. H. Characterization of the signaling function of MHC class II molecules during antigen presentation by B cells. J Immunol. 1991 Nov 1;147(9):2875–2882. [PubMed] [Google Scholar]
- St-Pierre Y., Nabavi N., Ghogawala Z., Glimcher L. H., Watts T. H. A functional role for signal transduction via the cytoplasmic domains of MHC class II proteins. J Immunol. 1989 Aug 1;143(3):808–812. [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H. L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994 May 12;369(6476):120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Wade W. F., Chen Z. Z., Maki R., McKercher S., Palmer E., Cambier J. C., Freed J. H. Altered I-A protein-mediated transmembrane signaling in B cells that express truncated I-Ak protein. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6297–6301. doi: 10.1073/pnas.86.16.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Alaverdi N., Wade W. F., Linsley P. S. Induction of costimulatory molecule B7 in M12 B lymphomas by cAMP or MHC-restricted T cell interaction. J Immunol. 1993 Mar 15;150(6):2192–2202. [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]



