Abstract
Recently developed neuroimaging and electrophysiological techniques are allowing us to answer fundamental questions about how behavioral states regulate our perception of the external environment. Studies using these techniques have yielded surprising insights into how sensory processing is affected at the earliest stages by attention and motivation, and how new sensory information received during wakefulness (e.g., during learning) continues to affect sensory brain circuits (leading to plastic changes) during subsequent sleep. This review aims to describe how brain states affect sensory response properties among neurons in primary and secondary sensory cortices, and how this relates to psychophysical detection thresholds and performance on sensory discrimination tasks. This is not intended to serve as a comprehensive overview of all brain states, or all sensory systems, but instead as an illustrative description of how three specific state variables (attention, motivation, and vigilance [i.e., sleep vs. wakefulness]) affect sensory systems in which they have been best studied.
1. Introduction: What determines the brain’s state?
“The universe is an intelligence test.”
– Timothy Leary (Wilson, 1977)
In 1964, psychologist Timothy Leary described sensory experience (in this case, channeled through the lens of psychedelic drug use) as a function of “set and setting” (Leary et al., 1995). The term “set” refers to the internal state of the individual; “setting” refers to external stimuli. The question of how neurons and neural circuits respond to sensory stimuli has received extensive study. A relatively unexplored question is how one’s “set”, or internal state, determines how these neurons and circuits process sensory information. Ultimately, how we experience the outside world can be seen as a function of both sensory input (our setting) and the state of our internal sensory processing systems (our set).
How is an organism’s “set” set? The term “state” can refer to motivation, emotion, attention, consciousness, or arousal. All of these brain states can be altered by either external context (e.g., in the case of attention, by distracting stimuli) or internal factors (e.g., fatigue, hormones, and neuromodulator levels). Certain internal factors are (relatively speaking) immutable: one obvious example is genetics (Bendesky and Bargmann, 2011; Plomin and Craig, 1997). Other internal factors can be modulated in a dynamic way over the lifespan, through behavioral, environmental, and pharmacological manipulations. This review is focused on describing neural mechanisms that affect sensory function during states of attention, motivation, and vigilance (sleep and wakefulness). These states affect both how incoming sensory information is received (i.e., how neurons respond to sensory input), and how neuronal responses are altered over time by changing sensory input (i.e., sensory plasticity).
2. Attention
Effects of attention on responses to specific, task-relevant stimuli have been described for all sensory modalities, and even across modalities (Eimer et al., 2002). In general, attention serves to decrease sensory detection and discrimination thresholds for a subset of attended stimuli, while at the same time increasing sensory thresholds for other, non-attended stimuli. Two brain state-specific features are generally associated with attention at the early stages of sensory processing: changes in neuromodulator tone - including localized, cortical circuit-specific release of norepinephrine and acetylcholine (Lee and Dan, 2012) - and changes in network activity - including circuit-specific increases in oscillatory activity (Fontanini and Katz, 2008).
2.1 Attention and the visual system
The effects of attention on early stages of information processing in the visual system have been studied using psychophysics, functional neuroimaging, and single-neuron recording, and have been described at the level of stimulus detection, response selectivity, and response magnitude. Numerous human studies have taken advantage of the retinotopic maps within primary and secondary visual cortices to assess local effects of attention directed to a specific retinotopic area of visual space. During various visual discrimination tasks (i.e., orientation, contrast, color, texture, or object), discrimination thresholds specifically decrease for visual stimuli presented in the attended area of visual space, and increase for those presented in a non-attended area. Similarly, BOLD fMRI signal during task performance specifically increases in the corresponding retinotopic region of visual cortex when stimuli are presented in an attended area, and decreases when they are presented in a non-attended area (Gutnisky et al., 2009; Jehee et al., 2011; Pessoa et al., 2003; Watanabe et al., 2011). Attention also modulates fMRI responses to visual stimuli within the lateral geniculate nucleus of the thalamus, suggesting effects on visual processing at an even earlier stage (O'Connor et al., 2002).
The underlying electrophysiological events associated with attention-mediated BOLD activity changes have been studied extensively in primate visual cortex (Figure 1). At the earliest level of cortical processing (the primary visual cortex, V1), attention enhances firing rate responses of simple cells to visual stimuli presented within their receptive field (McAdams and Reid, 2005). Similar increases in firing have been seen in higher visual cortical areas such as V4 (i.e., at later stages of visual processing) in response to attended receptive field stimulation (Fries et al., 2001b). Importantly, however, not all neuronal responses are uniformly affected by attention, but instead are altered as a function of cell type and spatial location of attention. Specifically, attention specifically enhances firing rate responses for stimuli presented within the receptive field of V1 or V4 interneurons, and specifically decreases firing rate responses for stimuli presented outside the receptive field of V1 or V4 excitatory (principal) neurons (Chen et al., 2008; Mitchell et al., 2007). Both mechanisms contribute to inhibiting responses to distracting stimuli presented outside a given neuron’s receptive field, leading to center-surround interactions that optimize response specificity (Reynolds, 2008; Sundberg et al., 2009).
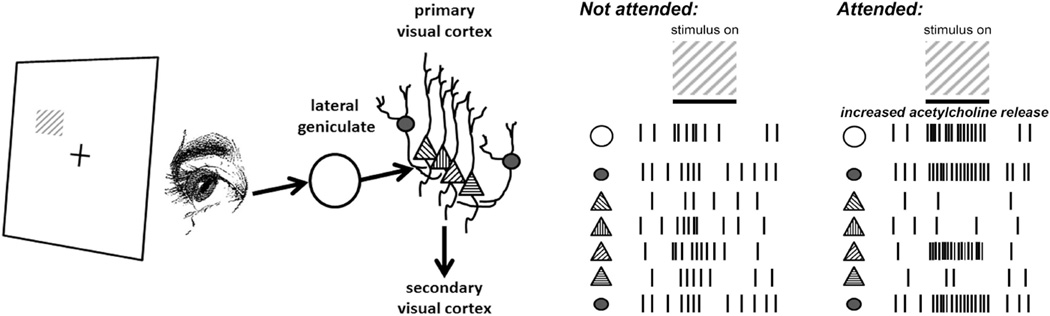
Figure 1. Improved feature detection during spatially-selective attention.
A visual stimulus presented to a specific area of the visual field evokes responses within corresponding retinotopic areas of the thalamic lateral geniculate (LGN) and primary visual cortex (V1). The stimulus is composed of an oriented grating; preferred orientations are indicated for individual V1 principal neurons (triangles), and V1 interneurons are shown as filled circles. A low-contrast grating stimulus evokes modest firing responses in the LGN and cortex, which are only slightly greater than background firing rates. Within V1, attention to the visual field area enhances stimulus-evoked interneuron activity. During stimulus presentation, LGN responses to the attended visual stimulus are enhanced. Against a background of increased inhibition in V1, incoming LGN input strongly and selectively activates only those neurons that are most selective for the stimulus orientation.
Attended stimulus presentation also increases firing synchrony with respect to ongoing gamma (30–80 Hz) oscillatory activity in corresponding retinotopic areas of V4 (Fries et al., 2001b). Gamma oscillations are specifically enhanced within V4 as a function of attention, driven by feedforward input from attended areas in V1 (Bosman et al., 2012). This gamma-frequency oscillatory drive synchronizes V4 neuronal firing to oscillatory input from V1 areas with overlapping receptive fields (Bosman et al., 2012; Grothe et al., 2012). Because the receptive fields in V4 are much larger than those in V1 (corresponding to the combined fields of multiple V1 neurons), this selective synchronization appears to serve as a mechanism underlying selective attention (Borgers et al., 2008). Intriguingly, inter-areal gamma synchrony may also be used at even higher levels of visual processing - recent data suggest that gamma synchrony is a correlate of visual attention during visual working memory tasks, synchronizing activity between higher visual cortical areas (e.g., area MT, which plays an important role in motion detection) and areas mediating executive control (e.g., prefrontal cortex) (Ardid et al., 2010).
Available data suggest that neuromodulation mediates the many of the electrophysiological changes seen in the visual system during selective attention (Figure 1). Acetylcholine can enhance visual responses to stimuli in excitatory neurons within the lateral geniculate nucleus (Hartveit and Heggelund, 1993; Soma et al., 2012). Acetylcholine release from the basal forebrain appears to modulate visual responses in V1 by enhancing interneuron activity (Alitto and Dan, 2012; Disney et al., 2007). It is also an essential mediator of visual stimulus-evoked gamma oscillations in the cortex (Rodriguez et al., 2004). It is likely that acetylcholine improves the precision and reliability of cortical neuronal responses to visual stimulation with regard to both spike number (i.e., response magnitude) and spike timing (Rodriguez et al., 2010) through this enhancement of gamma-frequency oscillations (Fries et al., 2001a). While the function of gamma oscillations within visual cortical circuits is still under investigation, it is clear that in addition to a potential role in signal selection and propagation between cortical areas (Borgers et al., 2008), these oscillations may play an important role in initiating cortical plasticity in response to changing visual input (Grossberg and Versace, 2008). In vitro, timing of excitatory neuronal input with respect to ongoing gamma oscillations in the visual cortex determines the sign of plastic changes between pre- and post-synaptic neurons - with excitatory potentials timed to the peak of oscillations leading to synaptic strengthening, and those timed to the trough leading to synaptic weakening (Wespatat et al., 2004). Timing-dependent strengthening, but not weakening, during gamma oscillations is dependent on acetylcholine receptor activation (Wespatat et al., 2004).
Acetylcholine release is modulated as a function of both attention (Dalley et al., 2001) and visual stimulation (Fournier et al., 2004; Laplante et al., 2005), and behavioral studies have demonstrated that acetylcholine is necessary for both visual attention and cortical visual response plasticity. If the basal forebrain (which sends cholinergic projections throughout the cortex) is lesioned or transiently inhibited, performance on visually-mediated serial reaction time tasks or visual navigation tasks, which require visuospatial attention, are impaired (Dotigny et al., 2008; Muir et al., 1992). Performance on these tasks can be rescued in basal forebrain-lesioned animals by administration of an acetylcholine receptor agonist (Muir et al., 1995). In human subjects, naturally-occurring visual response plasticity can be induced by brief visual deprivation, resulting in reduced visual response thresholds. This type of response plasticity can be blocked pharmacologically by antagonizing cholinergic receptors (Boroojerdi et al., 2001). This acetylcholine-mediated functional plasticity may be expressed within the cortex itself (i.e., through strengthening of intracortical synapses, as described for gamma-mediated plasticity above), or through long-term potentiation (LTP) of lateral geniculate synapses within primary visual cortex, which occurs in an acetylcholine-dependent manner in vivo (Dringenberg et al., 2007).
Since the 1960s, experimental data have shown that general arousal and motivational state (see below) regulate the effects of attention on visual responses. For example, Eason and colleagues found that while spatially selective attention augments cortical visually-evoked potentials in human subjects, the threat of shock for errors on a visual detection task augments cortical responses for attended stimuli even further, and reduces reaction times for reporting detected stimuli (Eason et al., 1969). More recently, beneficial effects of non-spatial “alerting” stimuli on subsequent psychophysical performance during spatial visual attention tasks have been reported (Posner and Cohen, 1984); in monkeys, these “alerting” effects can be reduced by blocking norepinephrine synaptic release. The level at which norepinephrine affects the visual system during attentional tasks is unclear. Norepinephrine can have direct actions on responses of visual cortex neurons; however, these have only been described in anesthetized animals (Waterhouse et al., 1990) or ex vivo in cortical slices (Kobayashi et al., 2000) - not during attentive behavior in vivo. Alternatively, norepinephrine may act indirectly by activating cholinergic neurons in the basal forebrain (Berridge and Waterhouse, 2003). It is worth noting that in a recent study in which the authors simultaneously measured both norepinephrine and acetylcholine release in the cortex during a visual attention task, attentive behavior resulted in far greater increases in the acetylcholine, while norepinephrine release seemed to be related to reward presentation, regardless of task-related attention to the stimulus (Dalley et al., 2001). Two things are clear. First, as the tonic firing rate of noradrenergic locus coeruleus neurons increases, so does the level of behavioral arousal (Carter et al., 2010). Second, sensory stimuli (of all modalities) that are behaviorally arousing increase locus coeruleus neuronal firing (Sara and Bouret, 2012). Insofar as behavioral arousal is a prerequisite for and modulator of attention, it appears likely that these functions are served by locus coeruleus release of norepinephrine.
2.2 Attention and the somatosensory system
As is true for visual processing, attention has been shown to improve psychophysical performance in somatosensory discrimination (e.g., stimulus detection, localization, and intensity or frequency discrimination) tasks in human subjects (Haegens et al., 2011; van Ede et al., 2011). And as is true for visual processing, effects of attention can be seen at the level of BOLD fMRI responses evoked by somatosensory stimuli, within primary and secondary somatosensory cortices (Albanese et al., 2009; Chen et al., 2010; Johanson-Berg et al., 2000; Schubert et al., 2008; Sterr et al., 2007). These changes are specific to areas of the somatotopic cortical map corresponding to the attended part of the body (for human studies, usually the right or left hand). Primate electrophysiological studies suggest that the neural correlates of increased BOLD signal activity in somatosensory cortex with attention include modest increases in neuronal firing rate, but profound increases in neuronal firing synchrony (Steinmetz et al., 2000). Thus attended tactile stimuli activate a larger number of somatosensory cortical neurons simultaneously than non-attended stimuli. One possibility is that this attention-dependent enhancement of spike synchrony among somatosensory neurons is mediated via the same mechanisms associated with attentional modulation of visual responses, i.e., through oscillatory synchrony among neurons. Recordings from human subjects during tactile discrimination tasks have shown that performance improvements correlate with attention-dependent reduction of alpha (8–14 Hz) and beta (15–20 Hz) oscillatory activity, which is expressed in cortical areas representing the predicted site of stimulation, in anticipation of the stimulus (Haegens et al., 2011; Jones et al., 2010; van Ede et al., 2011). Outside of these anticipatory attentional effects, attended stimulation elicits even greater reductions in alpha and beta oscillations, and increased gamma oscillatory activity (Bauer et al., 2006; Dockstader et al., 2010). Thus gamma may provide a crucial timing signal to promote synchrony of neuronal firing within and between somatosensory cortical areas as a function of attention, as it seems to do in the visual system.
Additional data have been provided by recordings from the rodent barrel cortex, the area of primary somatosensory cortex representing the facial whiskers. Rodents use their whiskers to explore the world in much the same way that humans use their hands. Thus, as is true of human hands, a relatively large proportion of the somatotopic map in the rodent somatosensory cortex is dedicated to representing the whiskers, making this area easily accessible for experimental manipulation and recording. Like tactile stimulation in human studies, attended whisker stimulation appears to decrease alpha and beta activity, and increase gamma oscillations, in the corresponding “barrels” within the barrel cortex (Hamada et al., 1999; Sobolewski et al., 2011). Intriguingly, recordings from the barrel cortex during optogenetically-induced gamma oscillations have demonstrated clearly that the timing of whisker stimulation with respect to these oscillations plays a critical role in gating cortical neurons’ response to whisker deflection (Cardin et al., 2009). Available data indicate that barrel cortex acetylcholine release is specifically modulated as a function of both tactile stimulation (Fournier et al., 2004) and attention (Alenda and Nunez, 2007). Basal forebrain stimulation decreases barrel cortex alpha and beta oscillations and increases higher-frequency oscillations in an acetylcholine-dependent manner, similar to attentional effects reported in human studies (Kuo et al., 2009). In addition, acetylcholine release from the basal forebrain (Nunez et al., 2012) and norepinephrine release from the locus coeruleus (Devilbiss and Waterhouse, 2004) both enhance firing rate responses of barrel cortex neurons to whisker stimulation, and may also increase the reliability of spike timing in response to stimulation (Devilbiss and Waterhouse, 2004; Lecas, 2004).
In addition to acute effects on sensory encoding, attentional mechanisms may play a role in somatotopic map plasticity. Human studies suggest that focusing on a manual sensorimotor task can lead to alterations in the functional spatial representation of the fingers and the hand within the primary somatosensory cortex (Braun et al., 2002; Schaefer et al., 2005). Acetylcholine release from the basal forebrain has itself been shown to promote experience-dependent plasticity in the barrel cortex. Repeated stimulation of a single whisker leads to progressive, whisker-specific response enhancement among primary somatosensory cortex neurons when the basal forebrain is stimulated in vivo, and this plasticity can be blocked by antagonizing acetylcholine receptors (Kuo et al., 2009).
3. Motivation and reinforcement
While neural mechanisms underlying behavioral reinforcement have been studied for decades with respect to motivation, only recently have these mechanisms been studied with respect to sensory processing and plasticity. Recent findings suggest that both rewarding and punishing behavioral feedback can affect sensory processes. Available data indicate that reward- or punishment-associated changes in release of dopamine, norepinephrine, and acetylcholine (Schultz and Dickinson, 2000) can profoundly impact how incoming sensory information is initially received, and can drive long-term plastic changes in sensory circuits.
3.1 Reinforcement and the visual system
Psychophysical studies of human subjects have demonstrated that the reward structure associated with performance of visual discrimination tasks (e.g., orientation discrimination) affects discrimination thresholds, with greater reward (not surprisingly) leading to improved performance (Baldassi and Simoncini, 2011; Weil et al., 2010). This improvement in performance is associated with increased BOLD fMRI response strength in early visual cortical areas (e.g., V1 and V4), both during post-discrimination reward presentation (Weil et al., 2010), and during presentation of visual stimuli (i.e., during discrimination task performance) when reward expectation is high (Serences, 2008). Little is known about the electrophysiological events which mediate these reward-associated activity changes. However, two recent studies have attempted to characterize neural correlates of reward expectation in V1. The first assessed the effects of high-value vs. low-value rewards on V1 activity during a visual discrimination task in monkeys. The authors found that (as is true in human subjects) high-value rewards led to improved task performance, which was associated with both enhanced gamma oscillatory activity, and enhanced neuronal firing in V1 during stimulus presentation (Lima et al., 2011) (Figure 2). The second study, characterizing V1 response properties in mice, found that visual cues associated with pending reward availability can generate large firing rate responses (primarily increases in firing rate) in V1 neurons, which are timed with respect to both the visual cue itself, and to reward delivery (Schuler and Bear, 2006). The proportion of recorded V1 neurons which were affected by reward timing (~40%) was impressive, and of these, 50% showed a steady increase in firing from visual cue to reward presentation, while another ~30% continued to show increased firing throughout the reward delivery period (Figure 2).
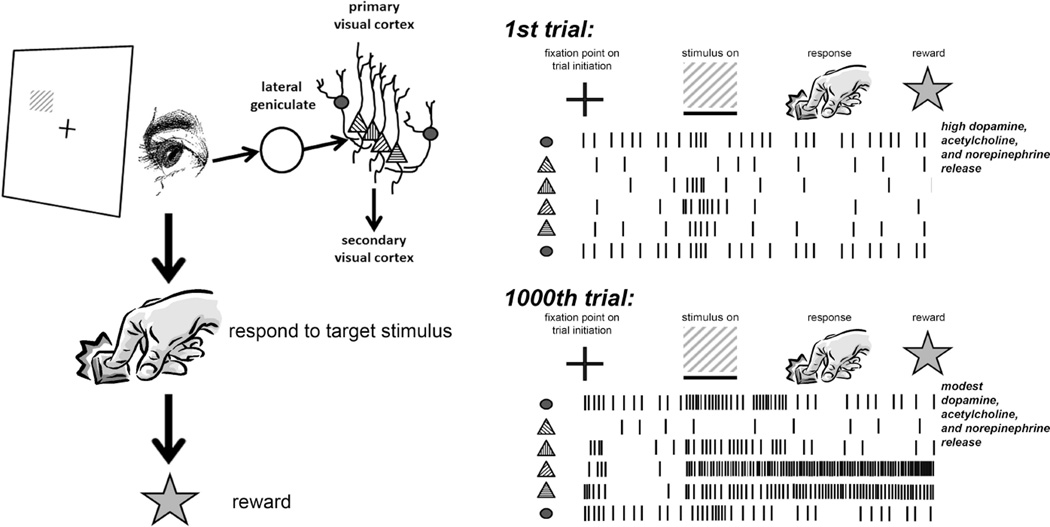
Figure 2. Augmented stimulus- and task-associated responses following reinforcement learning.
Behavioral responses to specific target visual stimulus (a grating with a 45° orientation) are rewarded after a brief interval. On the first trial (prior to reward association), V1 neurons respond normally to the grating stimulus; upon reward presentation, neuromodulators (dopamine, acetylcholine, and norepinephrine) are released at high levels. After several trials, V1 neurons show greater firing rate responses to the rewarded grating stimulus. Some may change their orientation preference in favor of the rewarded stimulus. A large proportion show prolonged firing rate increases (from the time of stimulus presentation to the time of reward presentation). Some V1 neurons develop selective responses to other stimuli associated with task performance, such as presentation of the fixation point that signals trial initiation. Over repeated trials, reward presentation evokes more modest neuromodulator release.
Based on presently-available data, it remains unclear whether these reward-related changes in visual cortex electrophysiology require neuromodulation (i.e., reinforcement-associated release of dopamine, norepinephrine, and acetylcholine) (Schultz and Dickinson, 2000; van Ooyen and Roelfsema, 2006). Available data suggest that dopaminergic, cholinergic, and noradrenergic neurons all respond strongly to unpredicted rewards during visual discrimination task performance, less strongly during repeated rewarded task performance, and negligibly to highly predictable rewards (Schultz and Dickinson, 2000) (Figure 2). Activity in dopaminergic neurons is also actively suppressed during reward omission following visual discrimination task errors (Hollerman and Schultz, 1998). This suggests that neuromodulator release could act as a “salience tag” for sensory events specifically associated with new reward contingencies. In support of this idea, locus coeruleus noradrenergic neurons can respond selectively to one specific visual stimulus, and then to another, as reward contingencies change; this change in responsiveness occurs many trials in advance of behavioral changes in task performance (Aston-Jones et al., 1997; Aston-Jones et al., 1994). Thus changes in cortical neuromodulator release (occurring rapidly as task demands change), over time, could drive adaptive circuit-level plasticity (occurring on the timescale of hours to days) that promotes visual discrimination learning.
3.2 Reinforcement and the auditory system
Relatively few studies have examined the effects of reinforcement on psychophysical measures of auditory function in human subjects. Recently, classical conditioning was used in human subjects to test the effects of pairing pleasant and noxious stimuli with a pure tone (Resnik et al., 2011), with the authors finding that pleasant stimuli decrease thresholds for auditory discrimination, while noxious stimuli increase thresholds and decrease discrimination ability. In contrast to the relative dearth of behavioral data in humans, numerous primate and rodent studies have found effects of reinforcement on auditory-driven task performance and underlying sensory response changes in the auditory system. For example, rodents will learn to press a lever in response to a pure tone cue when rewarded with water; their performance of the task is frequency-specific, and directly proportional to their level of prior water deprivation (Rutkowski and Weinberger, 2005). This improvement in task performance is associated with plasticity of the tonotopic map in the primary auditory cortex (A1), with greater cortical territory dedicated to the frequency of the cue tone. This auditory-guided behavior can be extinguished within a few training sessions if water rewards are withheld; a correlate of behavioral extinction is a contraction of A1 territory representing the frequency of the cue tone back to baseline levels (Bieszczad and Weinberger, 2012). Spectrotemporal tuning profiles of individual rat A1 neurons were also recently recorded under conditions in which target tones became associated with either reward (water) or punishment (foot shock). The authors found that association with reward and punishment led to differential changes to the tuning profile of neurons responsive to the target tone, with reward leading to a general decrease in neuronal responses to the target, and punishment leading to an increase (David et al., 2012). While it is unclear how these cellular changes drive A1 tonotopic map remodelling and auditory-guided behavior in response to training, these data clearly demonstrate that behavioral valence (reward vs. punishment) differentially modify sensory responses at an early stage of processing. Additional recent studies carried out in primates suggest that A1 neurons encode much more detailed information about reward structure during auditory task processing than valence alone. For example, when reward size and delivery are variable, A1 neuron firing varies according to expected reward size, and according to whether or not the expected reward is received (Brosch et al., 2011). In well-trained monkeys carrying out a familiar rewarded auditory task, A1 neurons also can respond with great specificity to other features of task performance - including reward-associated cues in other modalities (e.g., visual cues) and actions (e.g., lever pressing and lever releasing) involved in reporting auditory discrimination (Brosch et al., 2005). These effects are consistent with reinforcement-related plasticity between A1 and other brain areas (e.g., other sensory cortical areas)(Harley, 2003; Schultz, 2002).
Dopamine and norepinephrine release in the rat cortex are both known to increase in response to tones that are paired with a reward. During subsequent extinction training where the same tone goes unrewarded, tone-evoked dopamine (but not norepinephrine) release ceases (Mingote et al., 2004). Tone-paired stimulation of dopaminergic neurons in the ventral tegmental area is sufficient to improve discrimination of tone cues; intriguingly, this reinforcement regimen also leads to tonotopic map plasticity in A1 (Hui et al., 2009). Critically, stimulating dopaminergic neurons just after presenting a tone is sufficient to enhance the tone’s representation in A1 (Bao et al., 2001), while stimulating them before presenting the tone shrinks the tone’s representation (Bao et al., 2003). These data, and the recent finding that dopamine receptor antagonism within A1 leads to impairment of sound sequence discrimination learning (Kudoh and Shibuki, 2006), suggest that dopamine is a critical mediator of reinforcement-induced A1 map plasticity which accompanies auditory learning.
4. Sleep
Sensory experiences in wakefulness encode the information necessary for adaptive plastic changes in sensory circuits. However, available data suggest that many forms of sensory plasticity are only consolidated in the minutes and hours after sensory learning, and some appear to only be consolidated during sleep (Aton et al., 2009b). It is tempting to speculate that since plasticity sometimes requires sleep, this implies that either certain features of wakefulness are incompatible with plasticity mechanisms, or certain features of sleep promote these mechanisms. The features of brain function which differ between sleep and wakefulness are numerous - so numerous that a comprehensive description would be far beyond the scope of this review. However, several brain-wide changes have been characterized during sleep which may play a critical role in sensory plasticity (Figure 3).
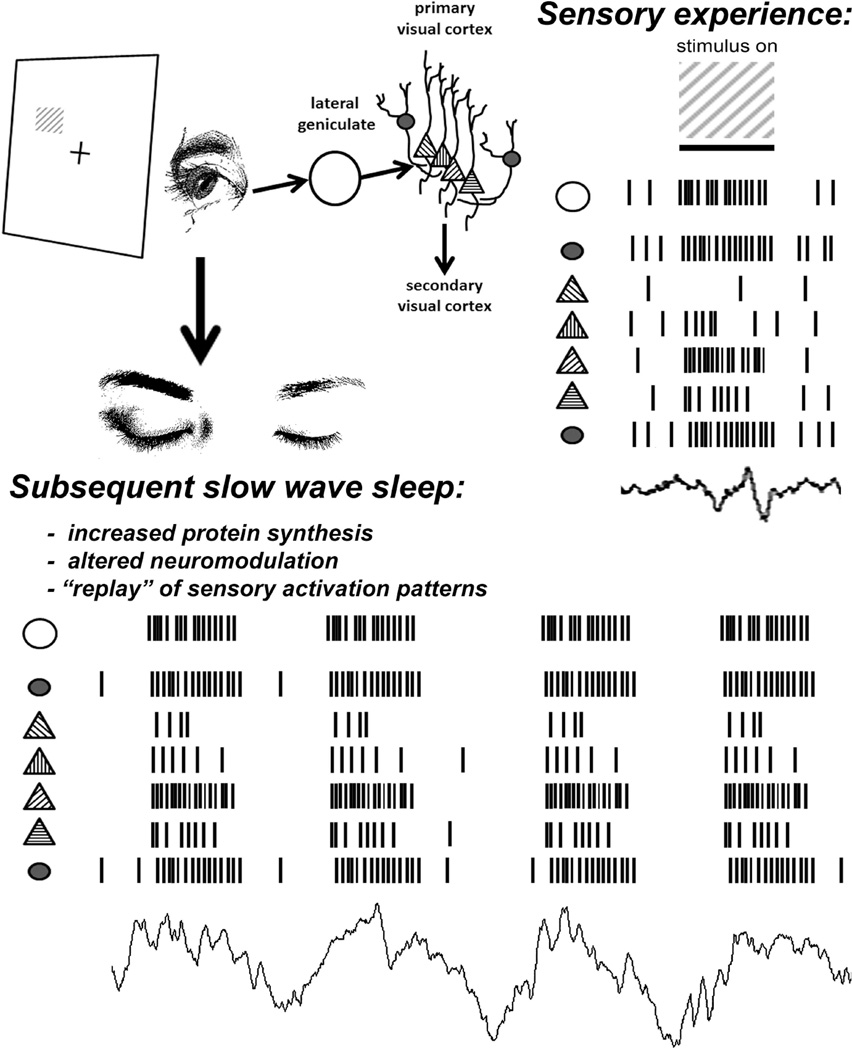
Figure 3. Sleep-associated changes in thalamocortical circuits following sensory experience.
During wakefulness, subjects are on a visual task (e.g., orientation discrimination), in which V1 neurons with a specific orientation preference (45°) are specifically activated. During subsequent sleep, numerous changes occur within LGN-V1 circuits that might be conducive to response plasticity and learning. Among these are the synchronous bursts of activity among LGN and V1 neurons that give rise to slow wave sleep oscillations, alterations in neuromodulator tone, alterations in transcription and translation, and “replay” of activity patterns associated with sensory experience. In this case, replay would consist of selective reactivation of neurons preferentially activated by oriented gratings displayed during the previous task.
One obvious state-dependent change which is likely to contribute to plasticity mechanisms is alteration of neuromodulator tone. The average firing rates of noradrenergic, cholinergic, and dopaminergic neurons are all reduced during slow wave sleep relative to wakefulness. During subsequent rapid eye movement (REM) sleep, catecholamine-producing neurons remain quiescent, while cholinergic neurons fire at their highest rates (Espana and Scammell, 2011). Because norepinephrine and dopamine seem to play an essential role in encoding new sensory information and de novo synaptic plasticity, it is possible that stabilization of cortical changes initiated by a new sensory experience requires a period of time during which catecholamine levels are reduced, to minimize further synaptic changes. In the case of norepinephrine, however, available data do not support this hypothesis. One recent study demonstrated that following training on an sensory recognition task, pharmacological manipulations which enhance norepinephrine levels during sleep lead to improved next-day task performance; manipulations that reduce norepinephrine levels impair performance (Gais et al., 2011). Electrophysiological recordings from rats also suggest that after new learning experiences in wakefulness, firing rates in locus coeruleus neurons are enhanced during slow wave sleep, and noradrenergic neuron spike timing with respect to slow waves is altered to optimize cortical plasticity (Eschenko et al., 2012; Eschenko and Sara, 2008). On the other hand, available data strongly support the idea that after learning, reduced cholinergic neurotransmission during sleep is critical for next-day improvements in performance (Gais and Born, 2004; Rasch et al., 2006).
Another obvious change which occurs specifically during slow wave sleep is the emergence of the slow waves of thalamocoritical activity which give the state its name (Figure 3). These waves are generated from bursts of neuronal activity occurring intermittently within the cortex, driven by thalamic input, and synchronized by corticothalamic feedback (Steriade, 2000, 2005, 2006). This dramatic change in network activity is likely to alter thalamocorical communication significantly, and is widely hypothesized to be a major contributor to sleep-dependent plasticity, through various mechanisms (Aton et al., 2009b; Sejnowski and Destexhe, 2000; Tononi and Cirelli, 2003). Recent work has used long-term recording of neuronal activity within the well-studied circuitry of mammalian sensory cortices to probe the role of sleep in neural function. Recordings from the somatosensory cortex of freely behaving rats have characterized how activity in these circuits changes as a function of prior sleep/wake history (Vyazovskiy et al., 2009). The authors found that bursts of activity among cortical neurons are more synchronized immediately after a period of extended wakefulness (vs. after a prior interval of sleep), yielding the higher-amplitude slow wave oscillations that characterize early sleep. They also found that firing rates in different classes of cortical neurons are differentially affected by sleep-wake history. While principal neurons show little change in firing after extended wakefulness, fast-spiking interneurons are exquisitely sensitive to state, and fire much more rapidly after wakefulness than after sleep (Vyazovskiy et al., 2009). Based on these findings, it is tempting to speculate that in sensory cortical circuits, the balance of excitatory and inhibitory signaling gradually decreases across wakefulness, but increases across sleep. These effects may be related to a more recent finding, which demonstrated that evoked potentials in the somatosensory cortex were enhanced after a period of slow wave sleep, through the same cellular mechanisms that mediate LTP of glutamatergic synapses (Chauvette et al., 2012).
Finally, a major change in the sleeping brain is an alteration in biosynthetic pathways; this could play a critical role in regulating sensory plasticity. Both transcription and translation (Vecsey et al., 2012) are profoundly affected by sleep states. At the transcriptional level, mRNAs that are up-regulated in the brain during sleep encode proteins involved in macromolecule biosynthesis, including those involved in protein translation, metabolism, and vesicular transport (Mackiewicz et al., 2007; Vecsey et al., 2012). While proteomic analysis of the sleeping brain, to date, has been limited (Cirelli et al., 2009), overall protein synthesis is higher throughout the brain during slow wave sleep than in any other state (Ramm and Smith, 1990) (Figure 3). Thus one possibility is that sleep facilitates sensory plasticity at a very fundamental level, by promoting synthesis of structural components required to build new synapses and circuits. In vivo imaging of synaptic structure in sensory cortices has recently been used to study how sleep and wakefulness affect neuronal cytoarchitecture. Imaging studies of somatosensory or sensorimotor cortex in non-anesthetized juvenile mice (during a developmental period in which dendritic spine turnover, and particularly spine loss, is expected to be high) have shown that while spine formation occurs to a similar extent across intervals of wakefulness or sleep, spine loss occurs preferentially during sleep (Maret et al., 2011; Yang and Gan, 2012). Interestingly, however, similar studies in adult mice have shown that, if anything, the opposite is true; spine loss appears to occur at a slightly higher rate across wakefulness, while spine formation occurs at a slightly higher rate across sleep (Maret et al., 2011).
4.1 Sleep and the visual system
Several examples of sleep facilitation of sensory discrimination after training have been described in human subjects, for visual texture discrimination (Gais et al., 2000; Mednick et al., 2003), visual contour integration (Gervan and Kovacs, 2010), and orientation discrimination (Matarazzo et al., 2008) tasks. Available data suggest that improvements on these tasks are associated with plasticity at an early visual processing stage (i.e., within the primary visual cortex), and that they are specific to the features of the training stimulus (e.g., to a specific area in the retinotopic map). After post-training sleep, improved perceptual performance is associated with increased activation of the trained retinotopic area of visual cortex during subsequent task performance (Schwartz et al., 2002). Indeed, during post-training sleep, increased fMRI activity is seen within the trained retinotopic area of the visual cortex (Yotsumoto et al., 2009). The cellular mechanisms underlying these plastic changes are still poorly defined, but they can be blocked by ionotropic glutamate receptor antagonism (Gais et al., 2008).
Recent work using animal models to study sensory plasticity has improved our understanding of how sleep contributes to changes in these processes at the cellular level. One example is sleep-dependent consolidation of response plasticity in the cat visual cortex. Ocular dominance plasticity occurs in vivo following brief monocular visual experience during a critical developmental window (Hubel and Wiesel, 2004). First described fifty years ago (Wiesel and Hubel, 1963), it has since become a canonical model for studying nervous system remodeling in response to changes in sensory input. Monocular vision results in both depression of cortical responses to stimuli presented to the previously closed eye, and enhancement of cortical responses to stimuli presented to the open eye (Frenkel and Bear, 2004; Mioche and Singer, 1989). We recently demonstrated that closed eye responses are depressed across waking visual experience, while open eye responses are preferentially enhanced during subsequent sleep; the latter, sleep-dependent process is mediated by intracellular signaling pathways associated with LTP (Aton et al., 2009a). Sleep-dependent ocular dominance shifts also require activation of protein synthesis machinery, and translation of synaptic proteins required for LTP (Seibt et al., 2012). These intracellular changes, like functional plasticity in the cortex, can be blocked by sleep deprivation in the hours after visual experience.
What happens in the visual cortex to bring about these cellular and functional changes, and why does it happen uniquely during sleep? Among the many changes occurring simultaneously in the brain during sleep are dramatic changes in thalamocortical activity patterns (Steriade, 2000) (Figure 3). We recently carried out long-term recordings of neuronal activity in freely-behaving cats, across periods of waking visual experience and subsequent sleep, to quantify features of network activity associated with sleep-dependent cortical remodeling (Aton et al., 2013). We found that after monocular visual experience, suppression of fast-spiking interneuron activity (and disinhibition between fast-spiking interneurons and principal neurons) occurs specifically in areas of cortex representing the open eye. These changes appear to be critical for subsequent plasticity of cortical visual responses. Importantly, administration of zolpidem - a hypnotic which specifically activates GABAergic receptors at synapses between fast-spiking interneurons and principal neurons (Bacci et al., 2003) - impairs ocular dominance plasticity during sleep (Seibt et al., 2008). Suppression of fast-spiking interneuron activity is present in all vigilance states (wakefulness, REM sleep, and slow wave sleep), so this change alone cannot explain why sleep is necessary for promoting ocular dominance plasticity. The most obvious feature of slow wave sleep, that makes it unique from other states, is the presence of slow waves of thalamocortical activity. We also found that following monocular visual experience, principal neurons show greater firing coherence with slow wave oscillations. The relative timing of firing between principal neurons and neighboring fast-spiking interneurons during slow wave oscillations also changes, resulting from a relative delay in firing among principal neurons (Aton et al., 2013). This interneuron-before-principal neuron firing relationship may allow fast spiking interneurons, whose overall activity is selectively reduced in open eye-biased areas of cortex, to selectively inhibit neighboring principal neurons in closed-eye biased areas of cortex during slow wave oscillations. From these data we conclude that in the case of sleep-dependent ocular dominance plasticity, waking sensory experience reconfigures cortical circuitry in a way that leads to altered spike timing, entrained by thalamocortical slow waves, in subsequent sleep. We hypothesize that such spike timing changes directly drive synaptic plasticity in the visual cortex.
4.2 Sleep and sensorimotor systems: audition and vocalization
Recent human studies have shown that central auditory processing, like many other forms of sensory processing, is adversely affected by sleep deprivation (Liberalesso et al., 2012). Auditory tone discrimination (Gaab et al., 2004) and linguistic perceptual learning (Fenn et al., 2003) appear to benefit from a night of sleep following auditory task training. As is true for the visual system, little is known about the cellular or circuit-level processes that mediate these sleep-dependent phenomena. Available data from quantification of auditory evoked potentials suggest that some changes in auditory processing due to post-training sleep are associated with more precise and consistent timing of brain activity (Atienza et al., 2005; Atienza et al., 2004). However, it is unclear whether neural changes associated with improved task performance are due to synaptic plasticity in the cortex, changes in neuronal excitability, or some other process. It is also unclear what features of sleep physiology bring such changes about.
Changes in neuronal and network activity have been described in other animal model systems in which a brief interval of sleep affects neuronal responses to sound and corresponding behavior. One example is in the avian song-producing sensorimotor system. Sleep has been shown to play an essential role in both song discrimination learning in adult songbirds (Brawn et al., 2010b) and in initial learning of a tutor’s song in juvenile birds (Shank and Margoliash, 2009). Several sleep-dependent changes in network activity have been noted within the sensorimotor nuclei associated with song production. Specifically, during sleep (but not wakefulness), neurons in the robust nucleus of the archipallium (RA; a forebrain motor nucleus) respond selectively to the sound of the bird’s own song (Dave et al., 1998). While during wakefulness, RA neurons show a tonic pattern of activity that is not altered by auditory stimuli, during sleep they respond with bursts of firing in response to individual song syllables. The mode of firing in RA is similarly altered during sleep immediately following song tutoring, with an increased propensity to bursts of firing, rather than tonic firing (Dave et al., 1998; Shank and Margoliash, 2009). The sleep-associated, “auditory-like” song responses in RA are similar to those normally seen in wakefulness in auditory-responsive neurons which project to RA from the HVC (high vocal center) nucleus (Dave and Margoliash, 2000; Dave et al., 1998). During sleep, HVC neurons burst in a temporal pattern which mimics the pattern associated with the sound of the bird’s own song (Chi et al., 2003). The gating of HVC auditory input to RA during wakefulness is mediated by noradrenergic and cholinergic signaling within HVC; local agonist application within HVC, or basal forebrain stimulation leads to suppression of auditory responses in RA, but not HVC (Dave et al., 1998; Rauske et al., 2003). Because auditory feedback is essential for song learning in juveniles, and song maintenance in adults (Tschida and Mooney, 2012), one hypothesis is that the “auditory-like” input to RA during sleep drives a template-matching process, wherein premotor sequences for song production are compared with auditory representations of tutor song, or adult birds’ own songs. Intriguingly, as song tutoring goes on, spontaneous sequences of activity (not driven by auditory stimuli) emerge within RA during sleep which mimic premotor activity during song production (Dave and Margoliash, 2000). It has been hypothesized that appropriately-timed replay of auditory and premotor activity sequences during sleep drives synaptic plasticity in the song system. HVC neurons undergo significant anatomical plasticity following initial exposure to a tutor’s song, with the degree of in vivo dendritic spine remodeling predicting song learning (Roberts et al., 2010). That this plasticity may occur during sleep is suggested by greater variability of premotor activity sequences during singing in RA immediately after a night of sleep (Rauske et al., 2010), as well as variability in song production immediately after sleep (Shank and Margoliash, 2009).
4.3 Sleep and sensorimotor systems: learning visually-guided and sequential movements
A number of recent studies have demonstrated that for human subjects, performance of newly-learned motor tasks benefit from post-training sleep. For the most part these tasks fall into one of two categories: motor tasks guided by visual cues or visual feedback (Huber et al., 2004; Maquet et al., 2003; Tamaki et al., 2008), and complex motor sequence learning (guided by proprioceptive feedback) (Brawn et al., 2010a; Debas et al., 2010; Korman et al., 2007; Kuriyama et al., 2004). Some advances in our understanding of the underlying mechanisms have come from functional imaging studies of these sleep-dependent motor skill gains. Over a period of days, post-training sleep seems to promote a process akin to “systems consolidation” (Aton et al., 2009b) of sensorimotor memory traces - performance improvements resulting from post-training sleep are associated with “re-mapping” of task-associated brain activity to new cortical areas. For example, sleep immediately after training leads to improvement in a sequential finger movement task several days later; this sleep-dependent change is associated with a shift in brain activation during task performance, with decreasing activation of areas involved in task acquisition (i.e., premotor and motor cortices), and increasing activation of other cortical areas (Fischer et al., 2005). Certain features of brain activity during post-training sleep itself are also associated with performance improvements, including local increases in spindle (7–15 Hz) and slow wave (0.5–4 Hz) oscillations, which occur specifically in cortical areas engaged during task acquisition (Huber et al., 2004; Nishida and Walker, 2007).
As is true for other forms of sleep-dependent sensory plasticity, attempts have been made to develop animal models in order to study the underlying cellular and network mechanisms. In rodents, performance of a novel skilled reaching task improves after a post-training interval which includes sleep (Hanlon et al., 2009). Although it is unclear whether sleep is required for improvements in performance of this task, in this model system, like motor task learning in humans, post-training sleep is characterized by increases in slow wave activity which are proportional to learning improvements.
5. Conclusions
Recent studies using an integrative neurobiology approach have yielded fundamental insights into mechanisms by which an organism’s “set”, or internal state, shapes how it experiences the external world, even at the earliest stages of sensory information processing. To date, much of our understanding of sensory system function has come from electrophysiological studies carried out in anesthetized animals. Such studies led to fundamental insights into how sensory information is encoded at the level of individual neurons. However, new recording techniques and experimental designs are allowing neuroscientists to examine how naturally-occurring behavioral states affect processes as fundamental as sensory processing and experience-dependent plasticity. These studies should improve our understanding of how state-dependent brain changes (e.g., in network activity and neuromodulator tone) affect how information about the world - one’s setting - is encoded, processed, stored for future use, and integrated with past experiences.
Review highlights:
-
-
Effects of three behavioral state features (attention, reinforcement, vigilance) are described in specific sensory systems.
-
-
Attention decreases discrimination thresholds for attended stimuli, and increases thresholds for nonattended stimuli.
-
-
Various reinforcement parameters and reward cues can be encoded at the level of primary sensory cortices.
-
-
Changes in neuromodulation and network activity during sleep are associated with plasticity in sensory systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- Albanese MC, Duerden EG, Bohotin V, Rainville P, Duncan GH. Differential effects of cognitive demand on human cortical activation associated with vibrotactile stimulation. J Neurophysiol. 2009;102:1623–1631. doi: 10.1152/jn.91295.2008. [DOI] [PubMed] [Google Scholar]
- Alenda A, Nunez A. Cholinergic modulation of sensory interference in rat primary somatosensory cortical neurons. Brain Res. 2007;1133:158–167. doi: 10.1016/j.brainres.2006.11.092. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci. 2012;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardid S, Wang XJ, Gomez-Cabrero D, Compte A. Reconciling coherent oscillation with modulation of irregular spiking activity in selective attention: gamma-range synchronization between sensory and executive cortical areas. J Neurosci. 2010;30:2856–2870. doi: 10.1523/JNEUROSCI.4222-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza M, Cantero JL, Quiroga RQ. Precise timing accounts for posttraining sleep-dependent enhancements of the auditory mismatch negativity. NeuroImage. 2005;26:628–634. doi: 10.1016/j.neuroimage.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL, Stickgold R. Posttraining sleep enhances automaticity in perceptual discrimination. Journal of Cognitive Neuroscience. 2004;16:53–64. doi: 10.1162/089892904322755557. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci U S A. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009a;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Frank MG. In Encyclopedia of Life Science. Chichester: John Wiley and Sons, Ltd; 2009b. Sleep and memory. [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassi S, Simoncini C. Reward sharpens orientation coding independently of attention. Frontiers in Neuroscience. 2011;5:13. doi: 10.3389/fnins.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Zhang LI, Merzenich MM. Suppression of cortical representation through backward conditioning. Proc Natl Acad Sci U S A. 2003;100:1405–1408. doi: 10.1073/pnas.0337527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendesky A, Bargmann CI. Genetic contributions to behavioural diversity at the gene-environment interface. Nat Rev Genet. 2011;12:809–820. doi: 10.1038/nrg3065. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleu-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci. 2012;35:598–613. doi: 10.1111/j.1460-9568.2011.07974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell N. Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc Natl Acad Sci U S A. 2008;105:18023c–18028c. doi: 10.1073/pnas.0809511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms underlying rapid experience-dependent plasticity in the human visual cortex. Proc Natl Acad Sci U S A. 2001;98:14698–14701. doi: 10.1073/pnas.251357198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Haug M, Wiech K, Birbaumer N, Elbert N, Roberts LE. Functional organization of primary somatosensory cortex depends on the focus of attention. NeuroImage. 2002;17:1451–1458. doi: 10.1006/nimg.2002.1277. [DOI] [PubMed] [Google Scholar]
- Brawn TP, Fenn KM, Nusbaum HC, Margoliash D. Consolidating the effects of waking and sleep on motor-sequence learning. J Neurosci. 2010a;30:13977–13982. doi: 10.1523/JNEUROSCI.3295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn TP, Nusbaum HC, Margoliash D. Sleep-dependent consolidation of auditory discrimination learning in adult starlings. J Neurosci. 2010b;30:609–613. doi: 10.1523/JNEUROSCI.4237-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25:6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Representation of reward feedback in primate auditory cortex. Front Syst Neurosci. 2011;7:5. doi: 10.3389/fnsys.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TL, Babiloni C, Ferretti A, Perrucci MG, Romani GL, Rossini PM, Tartaro A, Del Gratta C. Effects of somatosensory stimulation and attention on human somatosensory cortex: an fMRI study. NeuroImage. 2010;53:181–188. doi: 10.1016/j.neuroimage.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11:974–982. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Rauske PL, Margoliash D. Detection of spike patterns using pattern filtering, with applications to sleep replay in birdsong. Neurocomputing. 2003:52–54. 19–24. [Google Scholar]
- Cirelli C, Pfister-Genskow M, McCarthy D, Woodbury R, Tononi G. Proteomic profiling of the rat cerebral cortex in sleep and waking. Arch Ital Biol. 2009;147:59–68. [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules of sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci U S A. 2012;109:2144–2149. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockstader C, Cheyne D, Tannock R. Cortical dynamics of selective attention to somatosensory events. NeuroImage. 2010;49:1777–1785. doi: 10.1016/j.neuroimage.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Dotigny F, A Y, B A, Burke M, Vaucher E. Neuromodulatory role of acetylcholine in visually-induced cortical activation: behavioral and neuroanatomical correlates. Neuroscience. 2008;154:1607–1618. doi: 10.1016/j.neuroscience.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Hamze B, Wilson A, Speechley W, Kupo MC. Heterosynaptic facilitation of in vivo thalamocortical long-term potentiation in the adult rat visual cortex by acetylcholine. CerebCortex. 2007;17:839–848. doi: 10.1093/cercor/bhk038. [DOI] [PubMed] [Google Scholar]
- Eason RG, Harter MR, White CT. Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiol Behav. 1969;4:283–289. [Google Scholar]
- Eimer M, van Velzen J, Driver J. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Magri C, Panzeri S, Sara SJ. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb Cortex. 2012;22:426–435. doi: 10.1093/cercor/bhr121. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem-cortex interplay for memory consolidation? Cereb Cortex. 2008;18:2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- Espana RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep. 2011;34:845–858. doi: 10.5665/SLEEP.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier GN, Semba K, Rasmusson DD. Modality- and region-specific acetylcholine release in the rat neocortex. Neuroscience. 2004;126:257–262. doi: 10.1016/j.neuroscience.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001a;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001b;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gaab N, Paetzold M, Becker M, Walker MP, Schlaug G. The influencce of sleep on auditory learning: a behavioral study. Neuroreport. 2004;15:731–734. doi: 10.1097/00001756-200403220-00032. [DOI] [PubMed] [Google Scholar]
- Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. PNAS. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. NatNeurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gais S, Rasch B, Dahmen JC, Sara S, Born J. The memory function of noradrenergic activity in non-REM sleep. J Cogn Neurosci. 2011;23:2582–2592. doi: 10.1162/jocn.2011.21622. [DOI] [PubMed] [Google Scholar]
- Gais S, Rasch B, Wagner U, Born J. Visual-procedural memory consolidation during sleep blocked by glutamatergic receptor antagonists. J Neurosci. 2008;28:5513–5518. doi: 10.1523/JNEUROSCI.5374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervan P, Kovacs I. Two phases of offline learning in contour integration. J Vis. 2010:10. doi: 10.1167/10.6.24. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Versace M. Spikes, synchrony, and attentive learning by laminar thalamocortical circuits. Brain Res. 2008;1218:278–312. doi: 10.1016/j.brainres.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Grothe I, Neitzel SD, Mandon S, Kreiter AK. Switching neuronal inputs by differential modulations of gamma-band phase-coherence. J Neurosci. 2012;32:16172–16180. doi: 10.1523/JNEUROSCI.0890-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposure-based learning. Curr Biol. 2009;19:555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Miyashita E, Tanaka H. Gamma-band oscillations in the "barrel cortex" precede rat's exploratory whisking. Neuroscience. 1999;88:667–671. doi: 10.1016/s0306-4522(98)00468-0. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2003;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E, Heggelund P. The effect of acetylcholine on the visual response of lagged cells in the cat dorsal lateral geniculate nucleus. Exp Brain Res. 1993;95:443–449. doi: 10.1007/BF00227137. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Brain and visual perception: the story of a 25-year collaboration. Oxford University Press; 2004. [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Hui GK, Wong KL, Chavez CM, Leon MI, Robin KM, Weinberger NM. Conditioned tone control of brain reward behavior produces highly specific representational gain in the primary auditory cortex. Neurobiol Learn Mem. 2009;92:27–34. doi: 10.1016/j.nlm.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehee JF, Brady DK, Tong F. Attention improves encoding of task-relevant features in the human visual cortex. J Neurosci. 2011;31:8210–8219. doi: 10.1523/JNEUROSCI.6153-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson-Berg H, Christensen V, Woolrich M, Matthews PM. Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport. 2000;11:1237–1241. doi: 10.1097/00001756-200004270-00019. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hamalainen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Imamura K, Sugai T, Onoda N, Yamamoto M, Komai S, Watanabe Y. Selective suppression of horizontal propagation in rat visual cortex by norepinephrine. Eur J Neurosci. 2000;12:264–272. doi: 10.1046/j.1460-9568.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- Kudoh M, Shibuki K. Sound sequence discrimination learning motivated by reward requires dopaminergic D2 receptor activation in the rat auditory cortex. Learn Mem. 2006;13:690–698. doi: 10.1101/lm.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MC, Rasmusson DD, Dringenberg HC. Input-selective potentiation and rebalancing of primary sensory cortex afferents by endogenous acetylcholine. Neuroscience. 2009;163:430–441. doi: 10.1016/j.neuroscience.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante F, Morin Y, Quirion R, Vaucher E. Acetylcholine release is elicited in the visual cortex, but not in the prefrontal cortex, by patterned visual stimulation: A dual in vivo microdialysis study with functional correlates in the rat brain. Neuroscience. 2005;132:501–510. doi: 10.1016/j.neuroscience.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Leary T, Metzner R, Alpert R. The Psychedelic Experience. Citadel Press; 1995. [Google Scholar]
- Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. European Journal of Neuroscience. 2004;19:2519–2530. doi: 10.1111/j.0953-816X.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberalesso PB, D'Andrea KF, Cordeiro ML, Zeigelboim BS, Marques JM, Jurkiewicz AL. Effects of sleep deprivation on central auditory processing. BMC Neurosci. 2012:13. doi: 10.1186/1471-2202-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Neuenschwander S. Gamma responses correlate with temporal expectation in monkey primary visual cortex. J Neurosci. 2011;31:15919–15931. doi: 10.1523/JNEUROSCI.0957-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00275.2006. 00275.02006. [DOI] [PubMed] [Google Scholar]
- Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. JNeurosci. 2003;23:1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarazzo L, Franko E, Maquet P, Vogels R. Offline processing of memories induced by perceptual visual learning during subsequent wakefulness and sleep: A behavioral study. J Vis. 2008:8. doi: 10.1167/8.4.7. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mingote S, de Bruin JP, Feenstra MG. Noradrenaline and dopamine efflux in the prefrontal cortex in relation to appetitive classical conditioning. J Neurosci. 2004;24:2475–2480. doi: 10.1523/JNEUROSCI.4547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. Journal of Neurophysiology. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. Reversal of visual attentional dysfunction following lesions of the cholinergic basal forebrain by physostigmine and nicotine but not by the 5-HT3 receptor antagonist, ondansetron. Psychopharmacology. 1995;118:82–92. doi: 10.1007/BF02245253. [DOI] [PubMed] [Google Scholar]
- Muir JL, Robbins TW, Everitt BJ. Disruptive effects of muscimol infused into the basal forebrain on conditional discrimination and visual attention: differential interactions with cholinergic mechanisms. Psychopharmacology. 1992;107:541–550. doi: 10.1007/BF02245269. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A, Dominguez S, Buno W, Fernandez de Sevilla D. Cholinergic-mediated response enhancement in barrel cortex layer V pyramidal neurons. J Neurophysiol. 2012;108:1656–1668. doi: 10.1152/jn.00156.2012. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Craig I. Human behavioural genetics of cognitive abilities and disabilities. BioEssays. 1997;19:1117–1124. doi: 10.1002/bies.950191211. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of attention. In: Bouma H, Bowhuis D, editors. In Attention and performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow-wave sleep in the rat. PhysiolBehav. 1990;48:749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- Rasch BH, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. Journal of Cognitive Neuroscience. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
- Rauske PL, Chi Z, Dave AS, Margoliash D. Neuronal stability and drift across periods of sleep: premotor activity patterns in a vocal control nucleus of adult zebra finches. J Neurosci. 2010;30:2783–2794. doi: 10.1523/JNEUROSCI.3112-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and Neuronal Class-Dependent Reconfiguration in the Avian Song System. J Neurophysiol. 2003;89:1688–1701. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat Neurosci. 2011;14:791–796. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- Reynolds JH. Mapping the microcircuitry of attention. Nat Neurosci. 2008;11:861–862. doi: 10.1038/nn0808-861. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioral learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Stabilization of visual responses through cholinergic activation. Neuroscience. 2010;165:994–954. doi: 10.1016/j.neuroscience.2009.10.059. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze HJ, Rotte M. Task-relevant modulation of primary somatosensory cortex suggests a prefrontal-cortical sensory gating system. NeuroImage. 2005;27:130–135. doi: 10.1016/j.neuroimage.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Schubert R, Ritter P, Wüstenberg T, Preuschhof C, Curio G, Sommer W, Villringer A. Spatial attention related SEP amplitude modulations covary with BOLD signal in S1--a simultaneous EEG--fMRI study. Cereb Cortex. 2008;18:2686–2700. doi: 10.1093/cercor/bhn029. [DOI] [PubMed] [Google Scholar]
- Schuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal about dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci U S A. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt J, Aton S, Jha SK, Dimoulin M, Coleman C, Frank MG. The non-benzodiazepine hypnotic Zolpidem impairs sleep-dependent cortical plasticity. SLEEP. 2008 In Press. [PMC free article] [PubMed] [Google Scholar]
- Seibt J, Dumoulin M, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Destexhe A. Why do we sleep? Brain Research. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–77. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski A, Swiejkowski DA, Worobel A, Kublik E. The 5–12 Hz oscillations in the barrel cortex of awake rats - Sustained attention during behavioral idling? Clinical Neurophysiology. 2011;122:483–489. doi: 10.1016/j.clinph.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Soma S, Shimegi S, Osaki H, Sato H. The effect of acetylcholine on the visual response of lagged cells in the cat dorsal lateral geniculate nucleus. J Neurophysiol. 2012;107:283–291. [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Steriade M. Brain electrical activity and sensory processing during waking and sleep states. In: Kryger MH, Roth T, Dement WC, editors. In Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005. pp. 101–119. [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sterr A, Shen S, Zaman A, Roberts N, Szameitat A. Activation of SI is modulated by attention: a random effects fMRI study using mechanical stimuli. Neuroreport. 2007;18:607–611. doi: 10.1097/WNR.0b013e3280b07c34. [DOI] [PubMed] [Google Scholar]
- Sundberg KA, Mitchell JF, Reynolds JH. Spatial attention modulates center-surround interactions in macaque visual area V4. Neuron. 2009;61:952–953. doi: 10.1016/j.neuron.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tschida K, Mooney R. The role of auditory feedback in vocal learning and maintenance. Curr Opin Neurobiol. 2012;22:320–327. doi: 10.1016/j.conb.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, de Lange FP, Maris E. Attentional cues affect accuracy and reaction time via different cognitive and neural processes. J Neurosci. 2011;32:10408–10412. doi: 10.1523/JNEUROSCI.1337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A, Roelfsema PR. Envisioning the reward. Neuron. 2006;311:188–190. doi: 10.1016/j.neuron.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olscese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Cheng K, Murayama Y, Ueno K, Asamizuya T, Tanaka K, Logothetis N. Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science. 2011;334:829–831. doi: 10.1126/science.1203161. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RS, Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 1990;514:276–292. doi: 10.1016/0006-8993(90)91422-d. [DOI] [PubMed] [Google Scholar]
- Weil RS, Furl N, Ruff CC, Symmonds M, Flandin G, Dolan RJ, Driver J, Rees G. Reward feedback after correct visual discriminations has both general and specific influences on visual cortex. J Neurophysiol. 2010:104. doi: 10.1152/jn.00870.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase Sensitivity of Synaptic Modifications in Oscillating Cells of Rat Visual Cortex. J Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. JNeurophysiol. 1963;28:1029–1040. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wilson RA. Cosmic Trigger I: The Final Secret of the Illuminati. New Falcon Publications; 1977. [Google Scholar]
- Yang G, Gan WB. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2012;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Sasaki Y, Chan P, Vasios CE, Bonmassar G, Ito N, Nanez JE, Jr, Shimojo S, Watanabe T. Location-Specific Cortical Activation Changes during Sleep after Training for Perceptual Learning. Curr Biol. 2009;19:1278–1282. doi: 10.1016/j.cub.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]