Abstract
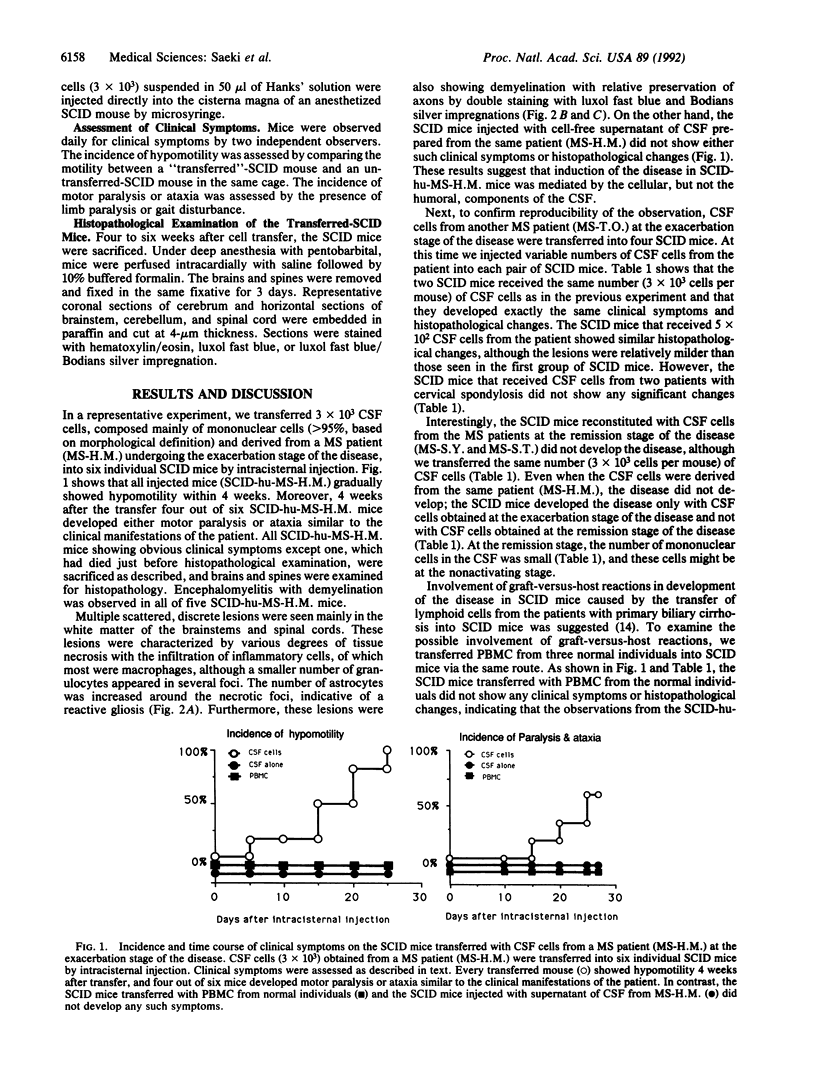
To investigate the mode of the pathogenesis of multiple sclerosis (MS), we transferred cerebrospinal fluid (CSF) cells, predominantly mononuclear cells, from MS patients at both exacerbation and remission stages of the disease into severe combined immunodeficiency mice by intracisternal injection. As controls, (i) CSF cells from patients with cervical spondylosis and (ii) peripheral blood mononuclear cells from normal individuals were transferred. Four to 6 weeks after transfer, most mice transferred with CSF cells from MS patients at the exacerbation stage of the disease developed paralysis and ataxia. The histopathological examination on the sacrificed mice revealed multiple scattered, discrete lesions localized in the white matter of the brainstems and spinal cords. These lesions were characterized by various degrees of tissue necrosis, involving inflammatory-cell infiltration. Most infiltrating cells were macrophages, although a smaller number of granulocytes appeared in several foci. Reactive astrocytic gliosis was also seen around the necrotic foci. Furthermore, these lesions exhibited demyelination. These histopathological changes are similar to those seen in MS. In contrast, none of the severe combined immunodeficiency mice transferred with CSF cells from MS patients at the remission stage of the disease, or with CSF cells from the patients with cervical spondylosis, or with peripheral blood mononuclear cells from normal individuals showed any such histopathological changes. These observations provide convincing direct evidence of encephalitogenicity of mononuclear cells in CSF from MS patients at the exacerbation stage of the disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou Y. K., Henderikx P., Vainiene M., Whitham R., Bourdette D., Chou C. H., Hashim G., Offner H., Vandenbark A. A. Specificity of human T cell clones reactive to immunodominant epitopes of myelin basic protein. J Neurosci Res. 1991 Feb;28(2):280–290. doi: 10.1002/jnr.490280215. [DOI] [PubMed] [Google Scholar]
- Duchosal M. A., McConahey P. J., Robinson C. A., Dixon F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990 Sep 1;172(3):985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Duby A. D., Lee S. J., Benjamin D., Seidman J. G., Weiner H. L. Oligoclonal T lymphocytes in the cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1988 Apr 1;167(4):1313–1322. doi: 10.1084/jem.167.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Weiner H. L. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989 Mar;10(3):104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- Johnson D., Hafler D. A., Fallis R. J., Lees M. B., Brady R. O., Quarles R. H., Weiner H. L. Cell-mediated immunity to myelin-associated glycoprotein, proteolipid protein, and myelin basic protein in multiple sclerosis. J Neuroimmunol. 1986 Nov;13(1):99–108. doi: 10.1016/0165-5728(86)90053-6. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Karuturi S., Chou Y. K., Lafferty J., Forrester J. M., Better M., Nedwin G. E., Offner H., Vandenbark A. A. Preferential T-cell receptor beta-chain variable gene use in myelin basic protein-reactive T-cell clones from patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9161–9165. doi: 10.1073/pnas.88.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams S. M., Dorshkind K., Gershwin M. E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989 Dec 1;170(6):1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Wucherpfennig K. W., Brod S. A., Benjamin D., Weiner H. L., Hafler D. A. Common T-cell receptor V beta usage in oligoclonal T lymphocytes derived from cerebrospinal fluid and blood of patients with multiple sclerosis. Ann Neurol. 1991 Jan;29(1):33–40. doi: 10.1002/ana.410290109. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (first of two parts). N Engl J Med. 1982 Nov 4;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Sherritt M., Begovich A. B., Erlich H. A., Bernard C. C., Cavalli-Sforza L. L., Steinman L. T-cell receptor V alpha and C alpha alleles associated with multiple and myasthenia gravis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):988–992. doi: 10.1073/pnas.86.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Tighe H., Silverman G. J., Kozin F., Tucker R., Gulizia R., Peebles C., Lotz M., Rhodes G., Machold K., Mosier D. E. Autoantibody production by severe combined immunodeficient mice reconstituted with synovial cells from rheumatoid arthritis patients. Eur J Immunol. 1990 Aug;20(8):1843–1848. doi: 10.1002/eji.1830200832. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]