Abstract
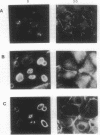
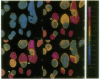
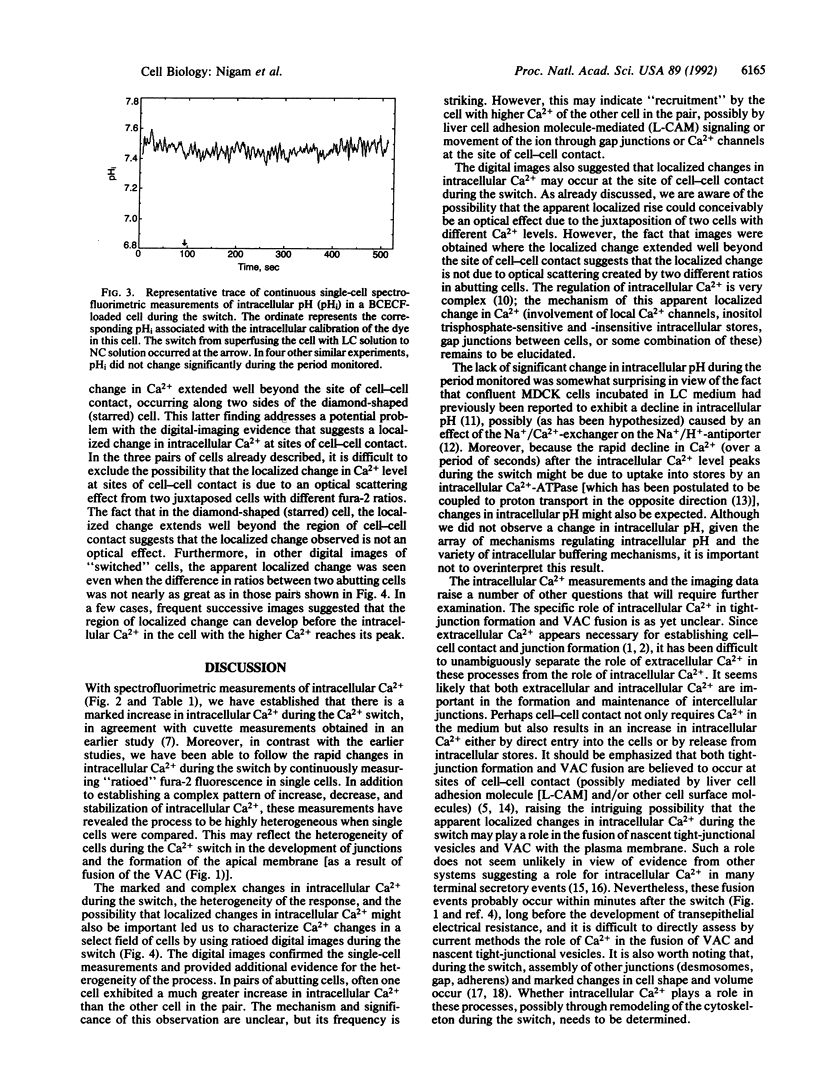
The "Ca2+ switch" model with cultured Madin-Darby canine kidney (MDCK) cells is useful in studying the biogenesis of epithelial polarity and junction formation and provides insight into early steps in the morphogenesis of polarized epithelial tissues. When extracellular Ca2+ in the medium is changed from less than 5 microM to 1.8 mM, MDCK cells rapidly change from a nonpolarized state exhibiting little cell-cell contact (with the apical membrane and junctional proteins largely within the cell) to a polarized state with well-formed tight junctions and desmosomes. To examine the role of intracellular Ca2+ in the development of polarity and junctions, we made continuous spectrofluorimetric measurements of intracellular Ca2+ during the "switch," using the fluorescent indicator fura-2. Intracellular Ca2+ increased greater than 10-fold during the switch and gave a complex pattern of increase, decrease, and stabilization. In contrast, intracellular pH [monitored with 2',7'-bis(2-carboxyethyl)-5(and 6)-carboxyfluorescein (BCECF)] did not change during the period studied. When intracellular Ca2+ curves in several cells were compared, considerable heterogeneity in the rate of increase of intracellular Ca2+ levels and in peak levels was evident, perhaps reflecting the heterogeneity among cells in establishing junctions and polarity. The heterogeneity of the process was confirmed by digital imaging of intracellular Ca2+ and was present even in a "clonal" line of MDCK cells, indicating the heterogeneity was intrinsic to the process and not simply a function of slight genetic variation within the population of MDCK cells. In pairs of cells that had barely established cell-cell contact, often one cell exhibited a much greater increase in intracellular Ca2+ than the other cell in the pair. At the site of cell-cell contact, an apparent localized change (an increase over the basal level) in intracellular Ca2+ was frequently present and occasionally appeared to extend beyond the point of cell-cell contact. Since the region of cell-cell contact is also the site where junctions form and where vesicles containing apical membranes fuse during the development of polarity, we postulate a role for global and local changes in intracellular Ca2+ in these events.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borle A. B., Bender C. Effects of pH on Ca2+i, Na+i, and pHi of MDCK cells: Na(+)-Ca2+ and Na(+)-H+ antiporter interactions. Am J Physiol. 1991 Sep;261(3 Pt 1):C482–C489. doi: 10.1152/ajpcell.1991.261.3.C482. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Borle C. J., Dobransky P., Gorecka-Tisera A. M., Bender C., Swain K. Effects of low extracellular Ca2+ on cytosolic free Ca2+, Na+, and pH of MDCK cells. Am J Physiol. 1990 Jul;259(1 Pt 1):C19–C25. doi: 10.1152/ajpcell.1990.259.1.C19. [DOI] [PubMed] [Google Scholar]
- Brenner B. M. Determinants of epithelial differentiation during early nephrogenesis. J Am Soc Nephrol. 1990 Aug;1(2):127–139. doi: 10.1681/ASN.V12127. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Secretory vesicle-associated proteins and their role in exocytosis. Annu Rev Physiol. 1990;52:647–659. doi: 10.1146/annurev.ph.52.030190.003243. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Chávez de Ramírez B., Cereijido M. Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol. 1985;86(2):113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Contreras R. G., Bolívar J. J., Ponce A., Chávez De Ramirez B., Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Nigam S. K. Intracellular calcium: molecules and pools. Curr Opin Cell Biol. 1992 Apr;4(2):220–226. doi: 10.1016/0955-0674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Meza I., Beaty G., Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1987 Jun;104(6):1527–1537. doi: 10.1083/jcb.104.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S. K., Denisenko N., Rodriguez-Boulan E., Citi S. The role of phosphorylation in development of tight junctions in cultured renal epithelial (MDCK) cells. Biochem Biophys Res Commun. 1991 Dec 16;181(2):548–553. doi: 10.1016/0006-291x(91)91224-z. [DOI] [PubMed] [Google Scholar]
- Pasdar M., Nelson W. J. Regulation of desmosome assembly in epithelial cells: kinetics of synthesis, transport, and stabilization of desmoglein I, a major protein of the membrane core domain. J Cell Biol. 1989 Jul;109(1):163–177. doi: 10.1083/jcb.109.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz I., Thévenod F., Dehlinger-Kremer M. Modulation of intracellular free Ca2+ concentration by IP3-sensitive and IP3-insensitive nonmitochondrial Ca2+ pools. Cell Calcium. 1989 Jul;10(5):325–336. doi: 10.1016/0143-4160(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol. 1988 Nov;107(5):1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J Cell Biol. 1987 May;104(5):1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]